Abstract
Atmospheric contributions of methane from Arctic wetlands during the Holocene are dynamic and linked to climate oscillations. However, long-term records linking climate variability to methane availability in Arctic wetlands are lacking. We present a multi-proxy ~12,000 year paleoecological reconstruction of intermittent methane availability from a radiocarbon-dated sediment core (LQ-West) taken from a shallow tundra lake (Qalluuraq Lake) in Arctic Alaska. Specifically, stable carbon isotopic values of photosynthetic biomarkers and methane are utilized to estimate the proportional contribution of methane-derived carbon to lake-sediment-preserved benthic (chironomids) and pelagic (cladocerans) components over the last ~12,000 years. These results were compared to temperature, hydrologic, and habitat reconstructions from the same site using chironomid assemblage data, oxygen isotopes of chironomid head capsules, and radiocarbon ages of plant macrofossils. Cladoceran ephippia from ~4,000 cal year BP sediments have δ13C values that range from ~−39 to −31‰, suggesting peak methane carbon assimilation at that time. These low δ13C values coincide with an apparent decrease in effective moisture and development of a wetland that included Sphagnum subsecundum. Incorporation of methane-derived carbon by chironomids and cladocerans decreased from ~2,500 to 1,500 cal year BP, coinciding with a temperature decrease. Live-collected chironomids with a radiocarbon age of 1,640 cal year BP, and fossil chironomids from 1,500 cal year BP in the core illustrate that ‘old’ carbon has also contributed to the development of the aquatic ecosystem since ~1,500 cal year BP. The relatively low δ13C values of aquatic invertebrates (as low as −40.5‰) provide evidence of methane incorporation by lake invertebrates, and suggest intermittent climate-linked methane release from the lake throughout the Holocene.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Arctic lakes and wetlands make a substantial contribution to the atmospheric methane budget (Walter et al. 2006). Methane trapped in ice cores records global-scale responses of methane reservoirs (e.g. gas hydrates, wetlands) affected by abrupt climate change (O’hara 2008; Sowers 2006). However, profound knowledge gaps remain regarding past methane emissions from the Arctic and relationships to climate change (IPCC 2001, 2007; Sowers 2010). Understanding how methane emissions will respond to contemporary climate change in the Arctic requires a basic understanding of how the methane cycle and environmental conditions (e.g. temperature and hydrology) have varied in the past. Multi-year studies have assessed seasonal differences in methane emissions from individual lakes (Walter et al. 2006, 2008). On longer time scales (i.e. multi-millennial), Arctic wetlands have been identified as a significant source of atmospheric methane since the last deglaciation (Jones and Yu 2010; Sowers 2006; Walter et al. 2006). The intensity and timing of their contribution have been distinct from the tropics and Southern Hemisphere (Chappellaz et al. 1997; Sowers 2010). Longer temporal records of methane flux from Arctic wetlands in relation to climate change are needed. In this regard, analyses of proxies of past methane release preserved in sediments taken from lakes and wetlands have been proposed (Heiri et al. 2009; Jones and Grey 2011; van Hardenbroek et al. 2010a; Wooller et al. 2009). These proxies are based on the stable carbon isotope composition (expressed as δ13C values) of various components (e.g. chironomid head capsules) preserved in lake sediments (Heiri et al. 2009; van Hardenbroek et al. 2010a, b). Specifically, incorporation of 13C-depleted methane carbon (δ13C typically ≤−40‰, Jones and Grey 2011) into the biomass of aquatic microbes and invertebrates yields δ13C values that are more negative than other primary production sources, such as terrestrial plants in the Arctic that use C3 photosynthesis (~−27‰) (Diefendorf et al. 2010; Kohn 2010; Wooller et al. 2007).
We used a multi-proxy paleoecological reconstruction to examine relative changes in methane production over a multi-millennial time scale from a wetland in Arctic Alaska. Specifically, we analyzed δ13C values of aquatic invertebrate (chironomids and cladocerans with head capsules and ephippia, respectively) remains preserved in a sediment core taken from a lake in Arctic Alaska. δ13C values from some chironomids and cladocerans that are low relative to associated total organic carbon (TOC) have been interpreted as evidence for the incorporation of methane-derived carbon by grazing on methane oxidizing microbes (Jones et al. 1999; Bastviken et al. 2003; Deines et al. 2007, 2009; Eller et al. 2005; Grey and Deines 2005; Grey et al. 2004; Jones and Grey 2004). Notably, significant relationships have been demonstrated between methane fluxes from northern lakes and the δ13C values of some chironomid and cladoceran species, with relatively low δ13C in some invertebrates from lakes with higher methane flux (van Hardenbroek et al. 2011). These findings along with laboratory (Kankaala et al. 2006) and field (Taipale et al. 2007) incubation experiments support the use of these organisms as proxies for methane production in lakes (Jones and Grey 2011; van Hardenbroek et al. 2010a). Similarly, some submerged bryophytes have also been shown to utilize carbon derived from oxidized methane, which can result in relatively low δ13C values from mosses (Kip et al. 2010).
The remains from aquatic organisms in cores of lake sediment retain the signature of the original whole organism (van Hardenbroek et al. 2010a; Perga 2010). Consequently, researchers have proposed using δ13C values from lacustrine biota as a proxy for past incorporation of methane-derived carbon and therein past changes in methane emissions from lakes (Heiri et al. 2009; Jones and Grey 2011; van Hardenbroek et al. 2010a; Wooller et al. 2009). In this study, we applied these principles to examine a ~12,000 year record of the methane cycle in an Arctic lake of Alaska. We applied isotopic mixing models to estimate alterations in the proportional contribution of methane-derived carbon to pelagic (cladocerans) and benthic (chironomids) organisms from a radiocarbon-dated sediment core from which we also describe environmental changes (i.e. temperature, hydrology, and habitat) that occurred at this location during the Holocene. We used changes in the assemblages of chironomids preserved in the sediment core as a proxy of past temperature (Langdon et al. 2011). We also used changes in the past oxygen isotope values (δ18O) of lake water inferred from the δ18O of chironomid head capsules preserved in the sediments as a proxy for hydrologic change (Verbruggen et al. 2010a; Verbruggen et al. 2010b; Wooller et al. 2004, 2008) and plant macrofossils coupled with sediment characteristics (lithological description and elemental composition) as proxies for local habitat change (Jones et al. 2009; Jones and Yu 2010).
Site description
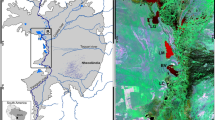
The study site (70°22.739′N, 157°20.861′W) is Qalluuraq Lake, on the North Slope of the Brooks Range, Alaska (Fig. 1), where a methane-seep with a high ebullition rate (~100 kg CH4 per day) currently occurs (Wooller et al. 2009). Chironomids and fingernail clams (Pisideae) are present at higher abundances at the seep compared with locations farther from the seep (Wooller unpublished data). Cladocerans and aquatic beetles are also present in the lake. The lake maximum water depth is about 2 m. The local wetland habitat around the site is characterized by string-and-flark type peatland and a high density of thermokarst lakes with Arctic tundra vegetation in the vicinity. In many respects, including depth and geomorphology, Qalluuraq Lake is typical of the thermokarst lakes in the region, which can in some instances include high ebullition rates. The morphology of the lake basin indicates that Qalluuraq Lake was previously much larger than present (Fig. 1).
Methods
Sample collection
Gases from Qalluuraq Lake were collected using submerged bubble traps into 60 ml glass serum vials, sealed with butyl rubber stoppers, and stored under refrigeration in the dark until analysis in the laboratory (Walter et al. 2008). A 160-cm-long sediment core (core LQ-West) was taken in a polycarbonate tube (7 cm internal diameter) using a hammer corer from the western basin of the lake (Fig. 1). Water samples (n = 4) were taken in May (ice cover) and July (open water) for oxygen and hydrogen isotope analyses (analysis described below). Live chironomids were taken from the site using an Ekman bottom grab sampler, and were allowed to clear their gut contents before they were freeze dried.
Radiocarbon analyses
Plant macrofossils were removed from the core for accelerator mass spectrometry (AMS) radiocarbon dating at National Ocean Sciences Accelerator Mass Spectrometry facility (NOSAMS) and Beta Analytic. We aimed to date terrestrial plant macrofossils where available. All radiocarbon dates were calibrated using the Calib 6.0 software and IntCal04 (Stuiver et al. 2009). AMS radiocarbon analyses were also conducted on chironomid head capsules from some depths that had ample mass for the dating procedure (Table 1). An insufficient amount of material prevented us from dating cladoceran remains from the core. An AMS radiocarbon analysis was also conducted on a live-collected chironomid taken from the lake. Subsamples of CH4 were combusted to CO2, purified, and catalytically reduced to graphite, and the 14C/12C isotopic ratios were measured by accelerator mass spectrometry at the Keck Carbon Cycle AMS Facility at the University of California, Irvine (Walter et al. 2008).
Sediment properties and origins
The magnetic susceptibility of the intact core LQ-West was measured using a Bartington MS2 sensor every 5 cm. The core was split lengthwise and sediment samples were removed from the core at ~5 cm intervals for stable carbon isotope analyses (described below) and elemental analysis of TOC and total nitrogen (TN) at the Alaska Stable Isotope Facility (ASIF). Each sample encompassed 1 cm of core length. To aid the lithological description, plant macrofossils were sieved, identified and quantified from the more organic-rich (peat-like) portions of the core (~80–45 cm and 35–25 cm; Jones et al. 2009). Paleoecological analyses were restricted to the organic-rich portion of the core (0–80 cm sediment depth).
Stable isotope analyses
Between 4.5 and 6.5 mg of sediment were acid fumed to remove carbonate (Harris et al. 2001) and then were freeze dried before δ13CTOC analyses. Briefly, wet subsamples of sediments were placed into open weighing trays in a large desiccator. Approximately 50 ml of 36% w/w HCl was placed in a glass container at the base of the desiccator and the samples were fumigated for about 6 h under vacuum. The samples were then dried in an oven at ~50°C and weighed into tin capsules (~8 mg). Chironomid head capsules were manually separated from 1-cm-thick core sections taken at a mean interval of 3 cm and prepared for isotopic (δ13C and δ18O) analyses using established techniques (van Hardenbroek et al. 2010b; Wang et al. 2008; Wooller et al. 2004, 2008) (~60–100 μg for δ13C and ~100–300 μg for δ18O). Insufficient numbers of chironomid head capsules per sample prevented taxon-specific δ13C analyses (as conducted at some other sites, van Hardenbroek et al. 2011). Our chironomid data therefore represent an integrated chironomid assemblage value. δ13C values were also generated from the analysis of cladoceran ephippia (resting egg cases) where available, and the modern chironomids (n = 10) collected from lake surface sediments. Analytical precisions are expressed as one standard deviation from the mean based on the results from multiple analyses of a standard (details reported below) conducted during the run of samples. A Thermal Conversion Elemental Analyzer (TC/EA) attached via a Conflo III to a Thermo Delta V Plus isotope ratio mass spectrometer (IRMS) was used to determine the δ18O value of the chironomid remains. Analytical precision for these organic δ18O values was based on analyses (n = 15) of benzoic acid (Fisher Scientific, Lot No 947459) and was 0.6‰. The TC/EA-IRMS was also used to generate δD and δ18O values of lake water samples (following the procedure described by Booth et al. 2010). All δD and δ18O values are expressed relative to VSMOW. A Costech ESC 4010 elemental analyzer interfaced via a ThermoConflo III to a Thermo Delta + XP IRMS was used to analyze δ13C and elemental (%C and %N, used to calculate the C:N) content of the sediment. Analytical precision for chironomid δ13C was based on multiple (n = 20) analyses of a peptone standard and was 0.3‰ (%C and %N analytical precisions were 2% and 1% respectively). All δ13C values are expressed relative to VPDB. We determined δ13C of methane using Finnigan MAT Delta V and Delta XP mass spectrometers at Florida State University, and the analytical precision was ±0.1‰.
For δ13C analyses of individual biomarkers, including C27 n-alkane (used here as a terrestrial plant biomarker), and cholesterol (used here as an aquatic biomarker) (Volkman 1986; Yunker et al. 1995; Volkman 2003), 8 g of freeze-dried and homogenized sediment had compounds extracted using an accelerated solvent extractor (ASE 200, DIONEX) with a mixture of dichloromethane (DCM):methanol (MeOH) 3:1 (v/v, four cycles of 5 min each) at 100°C and 7.6 × 106 Pa. Before extraction, known amounts of n-hexatriacontane, behenic acid methylester, n-nonadecanol, and 2-methly-octadecanoic acid were added as internal standards. The obtained total lipid extracts (TLE) were combined and dried using a Turbovap LV (Zymark Corp.) at 35°C under a nitrogen stream. The dried TLEs were separated in DCM-soluble asphaltenes and n-hexane-soluble maltenes. The maltenes were separated by solid phase extraction (Supelco LC-NH2 glass cartridges; 500 mg sorbent). Four fractions of increasing polarity (hydrocarbons, ketones, alcohols, and fatty acids) were obtained by elution with 4 ml n-hexane, 6 ml n-hexane:DCM 3:1 (v/v), 7 ml DCM:acetone 9:1 (v/v), and 8 ml 2% formic acid in DCM (v/v). The resulting hydrocarbon fraction was dissolved in 100 μl n-hexane and spiked with squalane as injection standard prior to gas chromatography isotope ratio mass spectrometry (GC-IRMS) analysis. GC-IRMS was performed using a Thermo Finnigan Trace GC coupled to a Thermo Finnigan Delta plus XP isotope ratio mass spectrometer. The GC was equipped with a 30 m Restek Rxi-5MS fused silica capillary column (0.25 mm ID, 0.25 μm film thickness) using He as carrier gas with a flow rate of 1 ml/min. The GC temperature program used for hydrocarbons was: injection at 60°C, 1 min isothermal; from 60 to 150°C at 10°C/min; from 150 to 310°C at 4°C/min; 30 min isothermal giving a total run-time of 80 min. Peak identification was based on relative retention times in parallel to mass spectrometric identification by GC–MS and stable carbon isotopic analysis were performed in duplicate with a precision of <1‰.
Chironomid assemblage data and environmental reconstruction
Subsamples were taken from core LQ-West for chironomid assemblage analyses following the procedures described by Brooks et al. (2007). Sediment was warmed in 10% KOH and sieved. Chironomid head capsules were then picked from the samples and mounted on microscope slides in Hydromatrix®. Identification and taxonomy followed Brooks et al. (2007). Statistical analyses of the chironomid data were undertaken through ordination analyses and application of a mean July air temperature transfer function (Barley et al. 2006). The model statistics for mean July air temp TF are \( {\text{r}}_{\text{boot}}^{2} = 0.818 \), RMSEP = 1.46°C.
Data analysis
All data from core LQ-West are reported relative to the AMS 14C chronology as calibrated years before present (cal year BP). The δ18O data from the chironomid head capsules were converted into reconstructed lake water δ18O values using the procedures described elsewhere (Wooller et al. 2004, 2008) (unconverted δ18O values available as supplemental data file). Two end-member isotopic mixing models were used to estimate the proportional contributions of methane-derived versus photosynthesis-derived carbon that comprise chironomids and cladocerans:
where F meth is the proportional contribution of methane-derived carbon to the aquatic invertebrates (chironomids or cladocerans), δ13Cinvert is the δ13C value of the chironomids (or cladocerans), δ13Cmeth is that of the methane end-member, and δ13Cphoto is that of the photosynthetic end-member. The measured δ13C value of methane from the site (−57.2 ± 1.2‰) was used for δ13Cmeth. We used the δ13C values of C27 n-alkane (terrestrial biomarker) and cholesterol (aquatic biomarker) to calculate a mean δ13Cphoto end-member value for each sampling interval. Because lipids are depleted in 13C by ~3.5‰ relative to bulk biomass (Meyers 1997; Smallwood et al. 2003), the biomarker δ13C values were increased by that amount to more accurately represent the δ13C of the plant material consumed by the grazing invertebrates. To account for the trophic isotope fractionation experienced by aquatic invertebrates, such as chironomids (Wang et al. 2009), we subtracted 0.7‰ from the chironomid and cladoceran δ13C values. Because the chironomid analyses were conducted at a higher sampling resolution than the biomarker data, corresponding δ13C biomarker values were not always available. In these instances (10 out of 27 chironomid samples) we provided the mixing model with an interpolated biomarker δ13C value by averaging the biomarker δ13C values above and below the chironomid sample depth. We also took into account sample precision (≤0.8‰ based on replicate chironomids and cladocerans measured from the same sample) to determine an error estimate of <15% around our proportional contribution calculations. This same mixing model was applied using the δ13C values from the modern chironomid specimens taken from the lake, and was calculated using their mean δ13C value (−35.2‰), and minimum and maximum values (−40.5 and −29.5‰, respectively).
For the modern chironomid sample and the chironomid head capsules picked from 65.5 cm depth in the core, independent ages of the chironomids were used in a Δ14C-based, two end-member mixing model to estimate F meth. In the case of the modern chironomid, the sample was alive at the time of collection. For the sample from 65.5 cm, wood from the same depth was dated at 12,569 cal year BP. The Δ14C results of the non-methane end-members (modern and -710‰, respectively) were used in the Δ14C-based mixing model along with the Δ14C of the methane at the site (−999‰) and the chironomids (modern = −201‰ and chironomid head capsules = −739‰).
Results
All of the data from Lake Qalluuraq presented in this study are available on-line through the World Data Center for Paleoclimatology (http://www.ncdc.noaa.gov/paleo/pubs/jopl2012arctic/jopl2012arctic.html).
Macrofossil and invertebrate radiocarbon
The methane from the seep at Qalluuraq Lake was radiocarbon-dead (Δ14C = −999‰). We report radiocarbon results from eight plant macrofossils, three samples of fossil chironomids, and one intact (living when collected) chironomid from lake surface sediments (Table 1). Dates from depths 24.5, 40.5, 45.5, 48, 48.5, 65.5, and 79.5 cm sediment depth were used to construct the core chronology (Fig. 2). The dates from closely spaced samples from 45.5, 48.0, and 48.5 cm have similar calibrated ages (Table 1), with a mean of 10,800 cal year BP. Linear interpolations between calibrated ages were used to produce the core chronology (Fig. 2). Whereas the calibrated age for the fossil chironomid remains from 29.5 cm (2,974 cal year BP) is similar to that from graminoids at 24.5 cm (2,958 cal year BP), the two other chironomid ages are older (>1,000 years) than those interpolated from 40.5 cm to the top of the core (Table 1; Fig. 2). The age of the chironomid remains at 65.5 cm is also substantially older (~1,500 years) than both the interpolated age for this depth and the age of a plant macrofossil from the same depth (Table 1). The ‘age’ for the live-collected chironomid is 1,640 cal year BP (Table 1; Fig. 2; δ13C = −35.9‰).
Sediment properties and origins
The sand (160–80 cm) has relatively high magnetic susceptibility compared to the peat (80–45 cm) (Fig. 2). The most distinct lithological transition in the LQ-West core is from the coarse, grey sand to dark peat at ~80 cm sediment depth. The C:N of the peat is relatively consistent (mean = 11 ± 2), and the basal, dark peat layer (80–45 cm) has significantly higher TOC than the sand (Fig. 3). The plant macrofossil assemblage from the lower section of peat is primarily composed of bryophytic material, dominated by Limprichtia revolvens, Fontinalis sp., and Scorpidium scorpioides (Table 2). The age of a relatively clear sedimentary transition at the base of the peat is ~10,500 cal year BP (~45 cm) and is accompanied by a slight decrease in organic content (Fig. 3b). Another marked sedimentary transition from gyttja to a second peat layer with relatively high TOC is evident at ~7,200 cal year BP (~35 cm) (Fig. 3b). This second peat layer is also primarily composed of bryophytic material, although much of this is detrital and the identifiable material is primarily Sphagnum subsecundum, with some Limprichtia revolvens and Warnstorfia exannulata, which contrasts with the lower peat layer (Table 2). The transition from this layer of peat to gyttja occurred at ~3,000 cal year BP (~25 cm) (Fig. 3).
Carbon, oxygen, and deuterium stable isotopes
The δ13C values of modern Chironominae specimens from lake surface sediments range from −40.5 to −29.5‰, with a mean of −35.2‰ (Fig. 3c). The mean δ13CTOC for the core is −25.2‰ and the δ13C values of the C27 n-alkane and cholesterol range between −32‰ at the base of the core to a maximum of −25‰ at 8,000 cal year BP (Fig. 3c), which are typical for C3 photosynthesis. The mean and 1σ values for these two biomarkers are very similar (cholesterol = −30.5 ± 1.6‰ and C27 n-alkane = −29.1 ± 1.5‰). The δ13C values for the other biomarkers down core are also consistent with C3 photosynthetic values (C23 n-alkane = −29.3 ± 1.1‰, sitosterol = −28.4 ± 2.0‰). The δ13C values of the fossil chironomids are consistently lower than the δ13CTOC for the core (Fig. 3c), with the exception of a single value near the base of the core (~10,900 cal year BP) and between 2,400 and 1,200 cal year BP (Fig. 3b). Cladoceran ephippia were only evident in the core from ~6,000 cal year BP, and their δ13Cephippia values are all lower than the δ13CTOC, with the exception of a data point at ~1,800 cal year BP. From ~6,000 to 4,000 cal year BP the δ13Cephippia decreased from −31 to −39‰ (Fig. 3c). The δ13C of the brown mosses (Limprichtia revolvens and Warnstorfia exannulata) and Sphagnum mosses are −26.2 and −25.5‰, respectively, which are typical C3 plant values. The δ13Cephippia values increase by ~10‰ at ~1,800 cal year BP, where they are within analytical precision of the δ13CTOC values, and from 1,800 cal year BP they decreased ~5‰ by ~1,000 cal year BP.
Approximately 10% of the carbon composing the chironomid assemblage is apparently derived from methane between ~12,300 and 8,400 cal year BP, based on the stable carbon isotopic mixing model (Fig. 4a). This F meth estimate is consistent with the Δ14C-based mixing model results for the chironomids analyzed from 65.5 cm depth in the core, which indicated 10.5% carbon from methane.
a Estimated proportional contribution (%) of methane-derived carbon to chironomids and ephippia from the LQ-West core, b δ18O values of modern lake water from Qalluuraq Lake plotted relative to reconstructed δ18O values from analyses of chironomid head capsules (see methods) from the LQ-West core, and c chironomid assemblage, inferred mean July air temperature from the LQ-West core (also plotted relative to inferred temperature from Burial Lake, Alaska, Kurek et al. 2009)
Between ~7,600 and 3,000 cal year BP the average methane contribution to chironomids is ~15%, and between ~2,500 and 1,500 cal year BP it was <10%. The estimates for fossil chironomid contrast with values of the fossil cladocerans, which have a mean methane contribution ~26% (ranging from 12 to 40%) (Fig. 4a), and increases from ~20% at ~6,000 cal year BP (a similar proportion to the chironomids at this time) to a maximum of ~40% at ~4,000 cal year BP. The proportional contribution of methane-derived carbon to cladocerans decreases to <10% at 1,800 cal year BP, and then increases to between ~20 and 30% at ~1,000 cal year BP. The mean contribution of methane-derived carbon for the modern Chironominae is 30% (Fig. 4a), with some individuals apparently obtaining ~50% of their carbon from methane. The F meth estimate using the Δ14C-based mixing model for live-collected Chironominae from the site was 20%, which is within the range based on the δ13C mixing model. The contribution of methane in fossil chironomids (8–24%; mean = 10%) is slightly less than the modern chironomids. However, the fossil chironomid values are estimated based on the total chironomid assemblage rather than individuals of a restricted taxonomic group (unlike the modern sample).
The modern lake water δ18O and δD values of Qalluuraq Lake are −11.9‰ (±0.2) and −111.8‰ (±1.4), respectively, plotting below the global meteoric water line (GMWL) (data not shown) and are significantly higher than the mean modeled (Online Isotopes in Precipitation Calculator, Bowen and Revenaugh 2003) values for precipitation at the site (−19.8 and −155.3‰, respectively). These modern lake-water values also plot along a local evaporation line established from analyses of lakes in the Brooks Range (Clegg and Hu 2010). All of these characteristics indicate that the lake water has likely experienced significant isotopic enrichment due to evaporation.
The δ18O values from analyses of chironomid head capsules from core LQ-West (converted to past δ18O values of lake water, Wooller et al. 2004, 2008) are presented relative to the measured δ18O values of modern lake water from the site (Fig. 4b), indicating that lake-water values between ~12,300 and 10,700 cal year BP were lower than modern lake-water values (Fig. 4b). The values increased (~4‰ from the previous minimum) to values similar to modern between ~10,000 and 7,000 cal year BP. The δ18Ochiron values then decreased slightly (~1‰) below the modern lake value at ~6,000 cal year BP, and increase (4‰) to the maximum at ~3,000 cal year BP, which is higher than the modern lake values. From ~3,000 cal year BP the δ18Ochiron value decreased by ~3‰ to approach modern value, and the most recent δ18Ochiron value is only ~1‰ higher than the modern lake value.
Chironomid assemblage data and environmental reconstruction
The chironomid assemblage data can be used to infer tends in past water temperatures (Fig. 4c). The bottom three samples contain some clear warm stenotherms, such as Microtendipes and Heterotrissocladius marcidus-type, which are then replaced by cold stenotherms, such as increased abundances of Abiskomyia, Paracladius, and Micropsectra, before a return to warmer conditions from 10,000 cal year BP, marked by the relative increase in Chironomini and Tanytarsini. To reconstruct mean July temperature, we used the transfer function of Barley et al. (2006) and grouped some taxa, including one category for Tanytarsina, which included Tanytarsus plus Paratanytarsus. The temperature reconstruction showed significant changes over the last ~12,300 cal year BP (Fig. 4c). The mean July temperature at the site reached the maximum at ~11,500 cal year BP, and then decreased by ~2°C at ~11,000 cal year BP. The July temperature was then ~2°C higher from ~10,500 cal year BP, maintaining a similar temperature until ~7,800 cal year BP. The minimum temperature occurred at ~2,000 cal year BP, followed by a rising but oscillating trend, although the sample resolution is low compared with the variability in the record. Further confidence in the chironomid-inferred temperature reconstruction is given by the comparison with the Burial Lake record (Kurek et al. 2009), which shows similar trends, notably from 10,000 cal year BP to present.
In all, 51 chironomid taxa were identified from 17 samples from the LQ-West core (Fig. 5 shows the major taxa). The chironomid stratigraphy primarily comprised Paratanytarsus and Corynocera ambigua, which fluctuated in dominance throughout the last 12,000 years. Paratanytarsus is often associated with macrophytes (Langdon et al. 2011), whereas Corynocera ambigua is more enigmatic, occurring in cold oligotrophic lakes (Brooks et al. 2007) and mid-thermal-range lakes (~11°C) (Barley et al. 2006). The main change in fauna occurred at ~10,000 cal year BP when the relative dominance of Paratanytarsus switched to an increased abundance of Corynocera ambigua, and coincided with the switch from organic-rich peat to gyttja.
Discussion
Findings from our multi-proxy reconstruction (Fig. 6) illustrate the main environmental changes (inferred temperature, hydrology, and habitat) compared to the relative contribution of methane-derived carbon to the chironomid (benthic) and cladocerans (pelagic) populations. The site was most likely an emergent fen initiated by moist and warm conditions between ~12,300 and 11,500 cal year BP. Our inference for relatively moist conditions comes from the relatively low δ18O values for the habitat water that cannot simply be interpreted as resulting from cold conditions (i.e. the empirical relation between mean annual temperature and δ18O precipitation, Dansgaard 1964), given the relatively warm July temperature inferences from the corresponding chironomid assemblage data. The most parsimonious interpretation for the relatively low δ18O values (relative to modern lake values) is a low proportional loss of lake water to evaporation implying high effective moisture at this time relative to modern conditions (Clegg and Hu 2010). Alternatively, changes in δ18O values of precipitation can result from changes in moisture source area and the lake may also have been temporarily connected to the Meade River, which is presently within 1 km of the modern lake. The emergent wetland that existed at our study location from ~12,300 and 11,500 cal year BP did not include Sphagnum (Table 1) and was not inundated sufficiently to sustain planktonic organisms including cladocerans, although communities of chironomids were established.
During this phase of wetland formation, ≤10% of the carbon in the chironomids was derived from methane. However, most of the δ13Cchiron values were significantly lower than the δ13CTOC values of sediment from the same depth. Chironomid head capsules from this period produced a 14C age that was ~1,500 year older than the age of the enclosing sediment (Fig. 2). This age discrepancy and the low δ13Cchiron values (relative to the δ13CTOC values) might indicate that the chironomids grazed on methanotrophs that used methane produced by methanogens, or that intercepted deeper (older) carbon had migrated up through the peat. Indeed, the results from the Δ14C-based mixing model for the chironomids from the period 12,300 to 11,500 cal year BP also indicate an F meth of 10.5%.
The proportional contribution of methane-derived carbon to chironomids apparently increased following the formation of the lake ecosystem to ~10,000 cal year BP, correlating with an increase in temperature. This increase in temperature may have also initiated thermokarsting at the site. A slight decrease in lake water δ18O (below the modern value) occurred at ~7,000 cal year BP and seems to be synchronous with the development of bryophytic habitat including a rich fen with Sphagnum subsecundum, although permanent water must have been present to maintain the chironomid and cladoceran communities. Sphagnum subsecundum is one of the few Sphagnum taxa that tolerates minerotrophic, submerged conditions (Riis and SandJensen 1997). These lines of evidence indicate the submerged bryophytic habitat evident from ~7,000 to ~3,000 cal year BP likely developed in association with an initial increase in effective moisture, although a substantial evaporative influence on the habitat water δ18O values occurred from ~6,000 to 3,000 cal year BP. The persistence of cladocerans (Fig. 3c) and Sphagnum (evident from the persistence of C23 n-alkane, data not shown), most likely Sphagnum subsecundum, during this period implies that, despite this evaporative loss, sufficient moisture and poor drainage conditions sustained an aquatic ecosystem.
From ~7,000 to ~3,000 cal year BP the strongest apparent connection is evident between the chironomids, cladocerans (benthic and pelagic invertebrates) and methane-derived carbon (Fig. 4a). Cladocerans exhibit the lowest δ13C values down core (Fig. 3c) and the highest estimates of methane-derived carbon, relative to chironomids (Fig. 4a). Some cladocerans are known to graze on planktonic methanotrophs (Jones et al. 1999; Kankaala et al. 2006, 2007; Taipale et al. 2007, 2009). During this interval, methane contributed the highest fraction of carbon to the food web, whereas for other sections this is more uncertain. Our data therefore most likely reflects a strong biological filter of methane by planktonic methanotrophs centered on ~4,000 cal year BP. Interestingly, both brown moss species (L. revolvens, Warnstorfia exannulata) and Sphagnum subsecundum exhibit relatively high δ13C values, despite evidence that submerged mosses can assimilate oxidized methane carbon (Kip et al. 2010). Moreover, the algal biomarker cholesterol also did not show low δ13C values, which suggests the low δ13C values in the invertebrates are not related to carbon isotope fractionation during photosynthesis. Therefore, we suggest this record is a temporal representation of changes in relative contribution of methane-derived carbon to both the benthic (chironomid) and pelagic (cladocerans) components of the lake.
The apparent development of a methane-rich lake ecosystem at ~4,000 cal year BP was associated with a positive δ18O shift from ~6,000 to 3,000 cal year BP. The lack of any marked shifts in temperature during this time implicates an alternative isotopic process rather than purely a temperature driven δ18Oprecip change. Clegg et al. (2010) also observed a peak in δ18O values at ~4,000 cal year BP in sediment from a lake south of the Brooks Range and interpreted the peak values as indicating a decrease in effective moisture, which seems consistent with our record in which the stratigraphy shifts from gyttja to peat and suggests a decrease in lake level and/or increase in clarity of the lake water that would allow for mosses to grow at depth. The strength of this routing of methane-derived carbon to cladocerans at ~4,000 cal year BP appears to have been relatively short lived as the δ13C values of both the chironomids and cladocerans increased between ~2,500 and 1,500 cal year BP, approaching those of the TOC (Fig. 3c).
From ~3,000 to 1,500 cal year BP there appears to have been an increase in effective moisture (evident from the decrease in lake δ18O values), a change in habitat (evident as a change from peat to gyttja), a decrease in temperature, and a decrease in the proportional contribution of methane-derived carbon to the aquatic invertebrates (most notably the cladocerans decreased from 45% to <10%). An increase in δ18O value of lake water at 1,000 cal year BP, which may indicate a decrease in effective moisture, seems to be associated with another increase in the proportion of methane-derived carbon entering both the pelagic and benthic invertebrates (Fig. 6). Indeed, the F meth based on the δ13C data from the modern chironomids ranges from 10 to 50%. The limited range of δ13C values from fossil chironomids indicates that multiple head capsules are required to produce sufficient mass for analysis, rather than that limited methane found its way into the food web in the past. In contrast live organisms are generally large enough to be analyzed individually. Thus, using multiple head capsules dampens the signal derived from methane anyway because not all chironomid taxa use methane. The Δ14C-based mixing model for a modern chironomid individual from the site also indicates a value for F meth of 20%.
Incorporation of methane-derived carbon by aquatic invertebrates
Alternative mechanisms for the incorporation of methane-derived carbon can be invoked to explain relatively low δ13C values from aquatic invertebrates (Jones and Grey 2011). For instance, CO2 emitted from decomposing organic matter in lake sediments can serve as a source of relatively low δ13C and high ∆14C values in cladoceran and chironomid remains. This process would require the aquatic invertebrates to feed on autochthonous primary production that fixed lake-sediment-derived CO2 instead of direct atmospheric CO2 for photosynthesis, causing negative δ13C and older 14C ages. We suggest that the incorporation of methane-derived carbon is the most parsimonious explanation for our paleoecological inferences regarding these relatively low δ13C values.
We argue that our data reflect changes in F meth for three reasons. (1) Some chironomid species and cladocerans are known to graze on methanotrophic bacteria that can have low δ13C values (Jones and Grey 2004; Kankaala et al. 2007, 2006; Taipale et al. 2009, 2007). When δ13C values of chironomids and cladocerans are substantially lower than those of the TOC in lake sediment, the incorporation of methane-derived carbon can be initially suspected (Jones et al. 2008; Jones and Grey 2011). (2) The δ13C values of the TOC in the lake sediments as well as the bryophyte remains are within the range of typical C3 photosynthesis and do not indicate that abnormally depleted algal remains were a major component of the lake at any time. The δ13C of cholesterol analyzed is also within the typical range for photosynthetic produced lipids. (3) The relatively low δ13C values in the chironomids from ~1,500 cal year BP and at 12,569 cal year BP have ages older than expected. This is also most evident by the low δ13C values of the live-collected chironomids (down to −40.8‰) from the site (relative to TOC) that is coupled with an old radiocarbon ‘age’ (1,640 year) (Table 1). Indeed we have shown that lake water and surface sediment from Qalluuraq Lake taken in July 2009 have the potential to oxidize methane and contain an active and diverse consortium of methane oxidizing microbes (including both type I and II methane oxidizers) (He et al. 2012).
Relatively ‘old’ living chironomids deriving carbon from the consumption of methanotrophs provide an alternative explanation for some ‘old’ (1,300 year) diving ducks that seasonally use lakes in northern Alaska and can feed on chironomids (Schell 1983). Other studies have also found evidence for the routing of methane-derived carbon to higher trophic levels (Jones and Grey 2011; Ravinet et al. 2010). We acknowledge that the approach of using isotopic data from aquatic invertebrates to infer past methane availability in lakes is largely in its infancy. However, studies like those conducted by van Hardenbroek et al. (2011) will continue to strengthen the interpretations that can be made using this novel proxy approach.
Like all mixing models, ours is an approximation based on necessary assumptions. For instance, we adopt cholesterol and C27 n-alkane δ13C values as non-methane end-members, even though these two compounds do not encompass the entire range of δ13C values of the bulk organic matter ingested by feeding chironomids. The δ13CTOC values of the bulk sediment are, therefore, an attractive alternate end-member for the model; however, the bulk sediment includes carbon from the methane-altered pools we are investigating (i.e. chironomid headcapsules and cladoceran remains). To avoid such circular reasoning, and to most accurately represent allochthonous and autochthonous inputs into the lake sediments, we use average δ13C values from the C27 n-alkane (a terrestrial plant biomarker) and cholesterol (an aquatic biomarker) as the non-methane end-member in the mixing model calculations (Volkman 1986; Yunker et al. 1995; Volkman 2003). Contributions from planktonic algae and algal biofilms with either positively or negatively variable δ13C values are expressed in cholesterol. Furthermore, we conducted a series of model sensitivity tests using alternative δ13C end-members, including sitosterol (data not shown), a biomarker of land plants and aquatic algae, and found that, no matter what compound we chose, the apparent contribution of methane-derived carbon remained high around ~4,000 cal year BP and in the modern lake.
Conclusions
Our data imply that past methane production and incorporation into aquatic invertebrates at Lake Qalluuraq have changed over the last ~12,300 cal year BP. Low δ13C values for cladoceran and chironomid remains relative to those of TOC are evident intermittently. The site evolved from an emergent fen that developed during relatively warm and moist conditions from 10,000 to 12,000 cal year BP. The most notable period of low δ13C values from cladocerans took place at ~4,000 cal year BP, correlating with a peak in reconstructed lake water δ18O values, which imply significant evaporation from the lake. A plausible explanation for these low δ13C values is an increase in the contribution of methane-derived carbon, which resulted from increased flux of methane from the site concurrent with the development of a submerged Sphagnum-dominated habitat. From our paleoecological model we pose a hypothesis to be tested by future research, that a substantial increase in atmospheric methane may have originated from Arctic lakes as they were colonized by submerged Sphagnum (and other submerged bryophytic/methanogenic/methanotrophic symbiotic habitats) stemming from periods of increased effective moisture following the last glaciation (Charman et al. 2009). Older-than-expected radiocarbon ages from chironomids, relative to the age of the enclosing sediment, coupled with low δ13C values (relative to TOC), imply the intermittent use of old methane by aquatic organisms during the last ~12,300 years and in the lake today.
References
Barley EM, Walker IR, Kurek J, Cwynar LC, Mathewes RW, Gajewski K, Finney BP (2006) A northwest North American training set: distribution of freshwater midges in relation to air temperature and lake depth. J Paleolimnol 36:295–314
Bastviken D, Ejlertsson J, Sundh I, Tranvik L (2003) Methane as a source of carbon and energy for lake pelagic food webs. Ecology 84:969–981
Booth AL, Wooller MJ, Howe T, Haubenstock N (2010) Tracing geographic and temporal trafficking patterns for marijuana in Alaska using stable isotopes (C, N, O and H). Forensic Sci Int 202:45–53
Bowen GJ, Revenaugh J (2003) Interpolating the isotopic composition of modern meteoric precipitation. Water Resour Res 39:15–27
Brooks SJ, Langdon PG, Heiri O (2007) The identification and use of Palaearctic Chironomidae Larvae in Palaeoecology. QRA Technical Guide No. 10, Quaternary Research Association, London, 276 pp
Chappellaz J, Blunier T, Kints S, Dallenbach A, Barnola JM, Schwander J, Raynaud D, Stauffer B (1997) Changes in the atmospheric CH4 gradient between Greenland and Antarctica during the Holocene. J Geophys Res 102:15987–15997
Charman DJ, Barber KE, Blaauw M, Langdon PG, Mauquoy D, Daley TJ, Hughes PDM, Karofeld E (2009) Climate drivers for peatland palaeoclimate records. Quat Sci Rev 28:1811–1819
Clegg BF, Hu FS (2010) An oxygen-isotope record of Holocene climate change in the south-central Brooks Range, Alaska. Quat Sci Rev 29:928–939
Dansgaard W (1964) Stable isotopes in precipitation. Tellus 16:436–468
Deines P, Grey J, Richnow HH, Eller G (2007) Linking larval chironomids to methane: seasonal variation of the microbial methane cycle and chironomid delta C-13. Aquat Microb Ecol 46:273–282
Deines P, Wooller MJ, Grey J (2009) Unravelling complexities in benthic food webs using a dual stable isotope (hydrogen and carbon) approach. Freshw Biol 54:2243–2251
Diefendorf A, Mueller K, Wing S, Koch P, Freeman K (2010) Global patterns in leaf C-13 discrimination and implications for studies of past and future climate. Proc Natl Acad Sci USA 107:5738–5743
Eller G, Deines P, Grey J, Richnow HH, Kruger M (2005) Methane cycling in lake sediments and its influence on chironomid larval partial derivative C-13. Fems Microb Ecol 54:339–350
Grey J, Deines P (2005) Differential assimilation of methanotrophic and chemoautotrophic bacteria by lake chironomid larvae. Aquat Microb Ecol 40:61–66
Grey J, Kelly A, Jones RI (2004) High intraspecific variability in carbon and nitrogen stable isotope ratios of lake chironomid larvae. Limnol Oceanogr 4:239–244
Harris D, Horwath W, Kessel C (2001) Acid fumigation of soils to remove carbonates prior to total organic carbon or carbon- 13 isotopic analysis. J Soil Sci Soc Am 65:1853–1856
He R, Wooller MJ, Pohlman J, Catranis C, Quensen J, Tiedje J, Leigh, M (2012) Identification of functionally active aerobic methanotrophs in sediments from an Arctic lake using stable isotope probing. Environ Microb. doi:10.1111/j.1462-2920.2012.02725
Heiri O, Wooller MJ, van Hardenbroek M, Wang Y (2009) Stable isotopes in chitinous fossils of aquatic invertebrates. PAGES Newsletter 15:22
IPCC (2001) The scientific basis. Cambridge University Press, New York
IPCC (2007) Fourth assessment report. Cambridge University Press, New York
Jones RI, Grey J (2004) Stable isotope analysis of chironomid larvae from some finnish forest lakes indicates dietary contribution from biogenic methane. Boreal Environ Res 9:17–23
Jones RI, Grey J (2011) Biogenic methane in freshwater food webs. Freshw Biol 56:213–229
Jones MC, Yu ZC (2010) Rapid deglacial and early Holocene expansion of peatlands in Alaska. Proc Natl Acad Sci USA 107:7347–7352
Jones RI, Grey J, Sleep D, Arvola L (1999) Stable isotope analysis of zooplankton carbon nutrition in humic lakes. Oikos 86:97–104
Jones RI, Carter CE, Kelly A, Ward S, Kelly DJ, Grey J (2008) Widespread contribution of methane-cycle bacteria to the diets of lake profundal chironomid larvae. Ecology 89:857–864
Jones MC, Peteet DM, Kurdyla D, Guilderson T (2009) Climate and vegetation history from a 14,000-year peatland record, Kenai Peninsula, Alaska. Quat Res 72:207–217
Kankaala P, Taipale S, Grey J, Sonninen E, Arvola L, Jones RI (2006) Experimental delta C-13 evidence for a contribution of methane to pelagic food webs in lakes. Limnol Oceanogr 51:2821–2827
Kankaala P, Eller G, Jones RI (2007) Could bacterivorous zooplankton affect lake pelagic methanotrophic activity? Fundam Appl Limnol 169:203–209
Kip N, van Winden J, Pan Y, Bodrossy L, Reichart G, Smolders A, Jetten M, Damste J, Op den Camp H (2010) Global prevalence of methane oxidation by symbiotic bacteria in peat-moss ecosystems. Nature Geo 3:617–621
Kohn M (2010) Carbon isotope compositions of terrestrial C3 plants as indicators of (paleo)ecology and (paleo)climate. Proc Natl Acad Sci USA 107:19691–19695
Kurek J, Cwynar LC, Ager TA, Abbott MB, Edwards ME (2009) Late Quaternary paleoclimate of western Alaska inferred from fossil chironomids and its relation to vegetation histories. Quat Sci Rev 28:799–811
Langdon PG, Caseldine CJ, Croudace IW, Jarvis S, Wastegard S, Crowford TC (2011) A chironomid-based reconstruction of summer temperatures in NW Iceland since AD 1650. Quat Res 75:451–460
Meyers PA (1997) Organic geochemical proxies of paleooceanographic, paleolimnologic, and paleoclimatic processes. Org Geochem 27:213–250
O’hara K (2008) A model for late Quaternary methane ice core signals: wetlands versus a shallow marine source. Geophys Res Lett 35:5
Perga M (2010) Potential of δ13C and δ15N of cladoceran subfossil exoskeltons for paleo-ecological studies. J Paleolimnol 44:387–395
Ravinet M, Syvaranta J, Jones RI, Grey J (2010) A trophic pathway from biogenic methane supports fish biomass in a temperate lake ecosystem. Oikos 119:409–416
Riis T, SandJensen K (1997) Growth reconstruction and photosynthesis of aquatic mosses: influence of light, temperature and carbon dioxide at depth. J Ecol 85:359–372
Schell D (1983) Carbon-13 and Carbon-14 Abundances in Alaskan aqautic organisms: delayed production from peat in Arctic food webs. Science 219:1068–1071
Smallwood B, Wooller MJ, Jacobson M, Fogel M (2003) Compound specific stable isotope analyses of R. mangle leaves. Geochem Trans 7:14–27
Sowers T (2006) Late quaternary atmospheric CH4 isotope record suggests marine clathrates are stable. Science 20:838–840
Sowers T (2010) Atmospheric methane isotope records covering the Holocene period. Quat Sci Rev 29:213–221
Stuiver M, Reimer PJ, Reimer RW (2009) CALIB 6.0. WWW program and documentation
Taipale S, Kankaala P, Jones RI (2007) Contributions of different organic carbon sources to Daphnia in the pelagic foodweb of a small polyhumic lake: results from mesocosm (DIC)-C-13-additions. Ecosystems 10:757–772
Taipale S, Kankaala P, Hamalainen H, Jones RI (2009) Seasonal shifts in the diet of lake zooplankton revealed by phospholipid fatty acid analysis. Freshw Biol 54:90–104
van Hardenbroek M, Heiri O, Grey J, Bodelier PLE, Verbruggen F, Lotter AF (2010a) Fossil chironomid delta 13C as a proxy for past methanogenic contribution to benthic food webs in lakes? J Paleolimnol 43:235–245
van Hardenbroek M, Heiri O, Lotter AF (2010b) Efficiency of different mesh sizes for isolating fossil chironomids for stable isotope and radiocarbon analyses. J Paleolimnol 44:721–729
van Hardenbroek M, Lotter AF, Bastviken D, Duc NT, Heiri O (2011) Relationship between δ13C of chironomid remains and methane flux in Swedish lakes. Freshw Biol 57:166–177
Verbruggen F, Heiri O, Reichart GJ, De Leeuw JW, Nierop KGJ, Lotter AF (2010a) Effects of chemical pretreatments on delta O-18 measurements, chemical composition, and morphology of chironomid head capsules. J Paleolimnol 43:857–872
Verbruggen F, Heiri O, Reichart GJ, Lotter AF (2010b) Chironomid delta O-18 as a proxy for past lake water delta O-18: a Lateglacial record from Rotsee (Switzerland). Quat Sci Rev 29:2271–2279
Volkman JK (1986) A review of sterol markers for marine and terrigenous organic matter. Org Geochem 9:83–99
Volkman JK (2003) Sterols in microorganisms. Appl Microbiol Biotechnol 60:495–506
Walter KM, Zimov SA, Chanton JP, Verbyla D, Chapin FS (2006) Methane bubbling from Siberian thaw lakes as a positive feedback to climate warming. Nature 443:71–75
Walter KM, Chanton JP, Chapin FS, Schuur EAG, Zimov SA (2008) Methane production and bubble emissions from Arctic lakes: isotopic implications for source pathways and ages. J Geophys Res 113:15–27
Wang Y, Francis DR, O’Brien DM, Wooller MJ (2008) A protocol for preparing subfossil chironomid head capsules (Diptera: Chironomidae) for stable isotope analysis in paleoclimate reconstruction and considerations of contamination sources. J Paleolimnol 40:771–781
Wang Y, O’Brien DM, Jenson J, Francis D, Wooller MJ (2009) The influence of diet and water on the stable oxygen and hydrogen isotope composition of Chironomidae (Diptera) with paleoecological implications. Oecologia 160:225–233
Wooller MJ, Francis D, Fogel ML, Miller GH, Walker IR, Wolfe AP (2004) Quantitative paleotemperature estimates from delta O-18 of chironomid head capsules preserved in Arctic lake sediments. J Paleolimnol 31:267–274
Wooller M, Zazula GD, Edwards M, Froese DG, Boone RD, Parker C, Bennett B (2007) Stable carbon isotope compositions of Eastern Beringian grasses and sedges: Investigating their potential as paleoenvironmental indicators. Arct Antarct Alp Res 21:318–331
Wooller M, Wang YM, Axford Y (2008) A multiple stable isotope record of late quaternary limnological changes and chironomid paleoecology from northeastern Iceland. J Paleolimnol 40:63–77
Wooller M, Ruppel C, Pohlman J, Leigh M (2009) Permafrost gas hydrates and climate change: lake-based seep studies on the Alaska north slope. Fire Ice 4:6–9
Yunker MB, Macdonald RW, Veltkamp DJ, Cretney WJ (1995) Terrestrial and marine biomarkers in a seasonally ice-covered Arctic estuary—integration of multivariate and biomarker approaches. Mar Chem 49:l–50
Acknowledgments
We thank Nathan Stewart (UAF), Doug Whiteman (Atqasuk), Wanda Kippi (Atqasuk), Thomas Itta (Atqasuk), Robert Vagnetti (DOE-NETL), Laurel McFadden (UAF), Peter Anthony (UAF), and Monica Heintz (UCSB) for field assistance; Tim Howe and Norma Haubenstock for assistance with laboratory analyses; Hayley Essex and Madeleine Velupillai for picking chironomid samples; the community in Atqasuk for kindly hosting us, and the Mayor and members of the city council; Kimberly Brent and the Meade River School in Atqasuk for allowing us to share our work with high school students; Carolyn Ruppel (USGS) for generating the site map; Darrell Kaufman, three anonymous reviewers, and Ben Jones (USGS) for their constructive comments on our manuscript. This work was conducted under BLM permit (FF095556), and North Slope Borough permits (NSB 09-0478 and NSB 10-018). This work was supported by the US Department of Energy National Energy Technology Laboratory (DE-NT000565) and US National Science Foundation (ARC-0909523 to Wooller). Funding for M. Elvert, K. Becker, and K.-U. Hinrichs was provided by the “Deutsche Forschungsgemeinschaft” through DFG-Research Center/Excellence Cluster “The Ocean in the Earth System”. K. Walter Anthony received additional support for methane-flux measurements and isotope analyses from the DOE Climate Change Prediction Program (Grant DE-PS02-08ER08-05). Any use of trade, product, or firm names is for descriptive purposes only and does not imply endorsement by the US Government.
Author information
Authors and Affiliations
Corresponding author
Additional information
This is one of 18 papers published in a special issue edited by Darrell Kaufman and dedicated to reconstructing Holocene climate and environmental change from Arctic lake sediments.
Rights and permissions
About this article
Cite this article
Wooller, M.J., Pohlman, J.W., Gaglioti, B.V. et al. Reconstruction of past methane availability in an Arctic Alaska wetland indicates climate influenced methane release during the past ~12,000 years. J Paleolimnol 48, 27–42 (2012). https://doi.org/10.1007/s10933-012-9591-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10933-012-9591-8