Abstract
Stable oxygen and hydrogen isotope analyses of fossil aquatic organisms, such as the chitinous head capsules of chironomid larvae (Chironomidae: Diptera), are promising proxies for inferring paleoecological conditions. In order for analyses of stable oxygen (δ18O) and hydrogen isotope ratios (δ2H) of fossil chironomid head capsules to be used effectively in paleoecological research, it is necessary to understand the factors controlling their stable oxygen and hydrogen composition. We cultured chironomid larvae in two isotopically distinct waters under controlled, replicated laboratory conditions. Chironomid larvae were fed on identical diets, to examine the degree to which water and diet influence the δ18O and δ2H of these organisms. We used a two-end member mixing model to determine the proportional contributions of oxygen and hydrogen from water to the oxygen and hydrogen of chironomid larvae. Our experiment demonstrated that 69.0 ± 0.4% of oxygen and 30.8 ± 2.6% of hydrogen in chironomid larvae are derived from habitat water. Our results show that oxygen isotopes from chironomid remains can better constrain past habitat water isotopic changes compared to hydrogen, due to 69% of the chironomid oxygen being influenced by habitat water. Our data add to a small but growing suite of comparative data on the sources of oxygen and hydrogen in animal tissues, and provide the first such analyses from aquatic insects.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Stable oxygen and hydrogen isotope data derived from analyses of fossilized animal remains have been used as proxies of past environmental change (Cormie et al. 1994a, b; Ehleringer et al. 2008; Grocke et al. 2006; Hobson 1999; Levin et al. 2006; Miller et al. 1988, 1993; Wooller et al. 2004). Two assumptions inherent to all these studies are that organisms record the isotopic composition of source water in organic compounds, and that the source water is primarily derived from precipitation (Crowley 2000; Ehleringer et al. 2008; Hobson 1999; O’Brien and Wooller 2007). The second assumption has been well validated: storm-track trajectories, moisture origins, seasonality, and climate conditions can result in substantial temporal gradients in the 18O and 2H composition of precipitation (Bowen and Revenaugh 2003; Bowen et al. 2005b; Bowen and Wilkinson 2002; Gat 1996; Gat and Alrey 2006). These variations have been examined using teeth, bones and the chitinous exo-skeletons of animal remains (Ambler et al. 1999; Cormie et al. 1994a, b; Fogel et al. 1997; Levin et al. 2006; Macko et al. 1999). However, the first assumption, that these materials faithfully record the isotope composition of precipitation, has not been examined for most animal remains in the fossil record.
Stable isotope analyses of subfossil chironomid head capsules preserved in lake sediments are a recent addition to the suite of tools used to reconstruct the stable isotopic composition of past lake water (Wang et al. 2008; Wooller et al. 2004, 2008), a proxy for assessing past precipitation patterns and hydrological history. Chironomid head capsules, which are composed primarily of chitin, are well preserved in lake sediments (Walker 2001). The stable isotope composition of chitin (carbon, nitrogen, oxygen and hydrogen) is not significantly altered by biological and thermal degradation (Schimmelmann and DeNiro 1986a, b). The family Chironomidae is one the most abundant and widespread of aquatic insects, and chironomids are even able to thrive in extreme environments such as Antarctica and the bottom of Lake Baikal (Gullan and Cranston 2000; Oliver 1971). Changes in chironomid faunal assemblages over time have been used to infer past environmental changes (i.e., temperature, salinity and oxygen availability) based on modern calibration data sets and transfer functions (Brodersen et al. 2004; Brooks 2000; Heinrichs et al. 1999; Langdon et al. 2006). The stable oxygen and hydrogen isotope analyses of chironomid chitin can provide supplemental information to reconstruct past environmental conditions using the same organisms (Wooller et al. 2004, 2008).
For data on ratios of stable oxygen (δ18O) and hydrogen isotopes (δ2H) from analyses of fossil chironomid chitin to be used effectively in paleoecological research, it is necessary to understand the factors controlling the stable oxygen and hydrogen composition of insect chitin (Miller et al. 1988; Wang et al. 2008). However, such fundamental knowledge is lacking. The first step to improving our understanding of how oxygen and hydrogen are derived in chitin is to determine what happens in bulk tissues. Although the δ18O of habitat water seems to have a strong influence on the δ18O of chironomids (Wooller et al. 2004), diet may also play a role in influencing 18O composition (Grocke et al. 2006; Schimmelmann and DeNiro 1986a, c). The degree to which water and diet influence the δ18O and δ2H of aquatic organisms has not yet been quantified experimentally.
A handful of previous studies have demonstrated that the proportional contribution of hydrogen derived from water and diet to the composition of organisms is fairly consistent (Ehleringer et al. 2008; Hobson et al. 1999; Kreuzer-Martin et al. 2003; O’Brien and Wooller 2007). In contrast, the proportional contribution of oxygen derived from water and diet to the composition of organisms varies greatly among different organisms (Ehleringer et al. 2008; Hobson et al. 1999; Kreuzer-Martin et al. 2003; O’Brien and Wooller 2007). For example, the contribution of drinking or habitat water to hydrogen in human hair, quail feathers, and microbial biomass was found to vary between 26% and 36%. In contrast, the contribution of oxygen from drinking water to organic compounds in these organisms varied from 27% in humans to 70% in microbes (Ehleringer et al. 2008; Hobson et al. 1999; Kreuzer-Martin et al. 2003; O’Brien and Wooller 2007; Sharp et al. 2003). It is possible that aquatic organisms like chironomids may be more strongly influenced by the isotopic signatures of their habitat water. However, the influences of water and diet on the stable oxygen and hydrogen isotope composition of aquatic organisms are largely unexplored and controlled experiments are necessary to understand the factors influencing the stable isotopic composition of organisms. Chironomids provide an excellent model for examining the influence of environmental parameters on their isotopic composition because they can be cultured under controlled laboratory conditions (Walker 1995).
Here we present the results of a controlled, replicated growth experiment designed to quantify how the δ18O and δ2H of water and diet influence the δ18O and δ2H of chironomid larvae. We grew chironomid larvae on an isotopically homogeneous diet, in two waters of contrasting δ18O and δ2H signatures. We then calculated the proportional contribution of oxygen and hydrogen from water and diet to larval tissue.
Materials and methods
Study species and culture setup
Chironomus dilutus were supplied as egg masses by the Environmental Protection Agency (EPA Mid-continent Ecology Division, Duluth, Minn.). This species has been used for toxicity studies by the U.S. Fish and Wildlife Service, National Fisheries Contaminant Research Center, and the EPA, and its rearing requirements are well known. The life cycle of C. dilutus is between 4 and 6 weeks at 20–23°C under ad libitum feeding conditions. Our experiment was conducted in an environmental chamber (Smith, San Diego, Calif.) at the Water & Environmental Research Center (WERC) at the University of Alaska Fairbanks (UAF). Our culture operation was based on the EPA (Mid-continent Ecology Division) standard operating procedures (EPA 1997).
C. dilutus were cultured in clear plastic aquaria (28 × 16 × 20 cm), each containing 5 l of water. Aquaria were maintained at 23°C with a photoperiod of ~16-h light:8-h dark. A ~2-cm layer of fine silica sand (<0.2-mm grain size), pre-combusted for 24 h at 200°C, was provided as a substrate. The aquaria stood in a water bath (23°C) leveled with gravel to help buffer any fluctuations in environmental temperature. Each aquarium was aerated using an air-stone to maintain high levels of dissolved oxygen (DO) throughout the entire experiment (DO levels were >90% in the aquaria measured on a calibrated YSI 556 DO meter). One liter of water was replaced each day via a gravity feed system from a 1-l Nalgene bottle, and drained through a drainage hole 11 cm from the bottom of the aquarium. This minimized the concentration of ammonium and the decomposition of excessive food in the aquaria. The pH, ammonium levels, and ionic content of the water in each aquarium were monitored daily throughout the experiment to make sure they remained constant.
Experimental design
The experiment ran for ~8 weeks, determined by the larval development time. Two and one half egg masses were placed into each aquarium, and chironomid larvae were allowed to develop from eggs to fourth instar larvae (determined by body length measurements following EPA guidelines) (EPA 1997). Once the larvae had reached their fourth instar, they were harvested using a pair of fine forceps and stored in a freezer until their analysis. Larvae from all of the aquaria were terminated at the same time. The numbers of chironomid larvae harvested from each aquarium were not the same, which may have resulted from variability in hatching success rate for each aquarium due to inadequate egg mass fertilizations (Maier et al. 1990).
Two water treatments with different stable isotopic compositions [natural abundance (W1) and isotopically labeled (W2)] were used in this experiment, with three aquaria for each treatment. Water was collected from the Fox spring outside of Fairbanks, Alaska in March 2007 in two 200-l drums, and kept at 4°C throughout the experiment. W1 consisted of natural abundance Fox spring water in one of the drums and was used for aquaria 1, 2, and 3. W2 consisted of Fox spring water in the second drum that was isotopically labeled using water enriched in 18O (>97 atom percent) (Iso-Solution, Ottawa, CA) and deuterium oxide (2H > 99.9 atom %) (Cambridge Isotope Laboratories, Andover, Mass.). The labeled water was used for aquaria 4, 5, and 6. Ten milliliters of 18O enriched water and 6.5 ml enriched deuterium oxide were added to W2.
The diet for each treatment was constant throughout the experiment and consisted of fine powdered Spirulina algae (Aquatic Eco-Systems). Ten grams of dry Spirulina algae was mixed with 500 ml of water from each treatment and food mixes were stored in the refrigerator for use during the experiment. A final concentration of 0.02 mg/ml was achieved for each aquarium each day. Feeding stopped a week before harvesting to minimize larval gut contents.
Isotope sampling and analysis
Stable isotope ratios are expressed in δ notation in per mil (‰): \( \delta \;(\permille) = (R_{\text{sample}} /R_{\text{standard}} - 1) \times 1000\permille \), where R is the ratio of heavy to light isotopes (e.g., 2H/1H, 18O/16O). The isotope compositions of our results are expressed relative to international standards: Vienna Pee Dee belemnite for carbon, atmospheric nitrogen (air) for nitrogen and Vienna standard mean ocean water (VSMOW) for oxygen and hydrogen.
We sampled water from each aquarium at 2-day intervals throughout the experiment to check that the isotopic composition of the growth water remained constant. The aquaria did not have lids, and some evaporation did occur during the experiment. Water samples were collected into a 2-ml glass vial by pipette and were then crimped shut with no headspace in preparation for stable isotopic (oxygen and hydrogen) analysis. The vials were loaded into an autosampler (CTC Analytics A200SE liquid autosampler) and 0.2 μl of each sample was injected into an on-line pyrolysis, thermochemical reactor elemental analyzer (TCEA) (Finnigan ThermoQuest) coupled to a continuous flow (Conflo III) isotope ratio mass spectrometer (IRMS) (Finnigan MAT Delta V) at the Alaska Stable Isotope Facility (ASIF) at the UAF. δ18O and δ2H of gases (carbon monoxide and H2) from each sample were measured relative to calibrated reference gases (carbon monoxide and H2). The quality control scheme involved analyzing laboratory working standards after every seventh sample. Laboratory working standards were internally calibrated Duckering Building Millipore water (DMW), National Institute of Standards and Technology (NIST ref. 8535 VSMOW), Greenland ice sheet precipitation (GISP 8536) and standard light Antarctic precipitation (SLAP 8537) and measured versus expected had an R 2 of >0.99. Multiple (n = 15) δ18O and δ2H analyses of DMW conducted during the sample sequence yielded 1σ = 0.4‰ and 1.7‰, respectively. Each sample and standard was analyzed in triplicate. Triplicate δ18O and δ2H analyses of separate DMW and water samples yielded 1σ of ≤0.3‰ and 1.6‰, respectively.
The δ18O and δ2H of larvae and diet were also measured at the ASIF. Between 0.1 and 0.3 mg of individual freeze-dried whole chironomid larvae and diet samples was weighed into tin capsules. Acknowledging the possibility of exchangeable hydrogen in organic samples (Kreuzer-Martin et al. 2003, 2006; Miller et al. 1988; Schimmelmann and DeNiro 1986a, c), we adopted a method consistent with that outlined by Wassennar and Hobson (2002), where samples were air equilibrated with ambient laboratory air moisture in the ASIF at room temperature for >96 h to minimize the influence of exchangeable oxygen and hydrogen. After equilibration, samples were freeze dried for >6 days, as recommended for organic samples such as keratin (Bowen et al. 2005a). We treated all of our samples in the same manner, allowing relative comparisons between samples. After comparative equilibration and freeze drying, all samples were loaded into a zero-blank autosampler that was purged with research grade helium. Measurements of the δ18O and δ2H of these organic samples were made using the same instrumentation as the water samples. δ18O and the δ2H of sample gases were then calibrated relative to internally calibrated organic standards, bowhead whale baleen keratin (BWBII) (Wassenaar and Hobson 2002) and international, calibrated standards [Australian National University sucrose (ANU sucrose), National Bureau of Standards 22 and 30 (NBS-22, NBS-30) and polyethylene foil (PEF-1)] (measured versus expected R 2 of >0.99). The δ18O and δ2H analyses of laboratory working organic standards, benzoic acid (lot no. 947459; Fisher Scientific), were also conducted throughout the run (n = 10) and yielded 1σ of 0.4‰ and 1.7‰, respectively. Blank tin capsules were also analyzed among the samples.
To assess whether chironomid larvae had any additional sources of available food, we analyzed the δ13C and δ15N of food (Spirulina) and chironomid larva in an initial experiment in 2006. These larvae were freeze dried before weighing and analyzed for δ13C and δ15N. Microorganisms were observed growing on the inner wall of the aquarium and these were also collected and analyzed for δ13C and δ15N. The δ13C and δ15N values of the samples were determined using a Costech ECS4010 EA attached via a Conflo III to a continuous flow IRMS (Thermo Finnigan Deltaplus XL) at the ASIF. Multiple (n = 7) analyses of our laboratory working standard (peptone, lot no. 76F-0300; Sigma) yielded 1σ of 0.1‰ for both δ13C and δ15N. The second and fourth larval instars were also measured for δ18O and δ2H using the procedure described above to examine whether the isotopic composition varied between larval stages. We chose second and fourth instars to conduct our stable isotopic analyses to examine whether different instars had different isotopic values. These two instars were largely chosen arbitrarily, although first instars were not considered since they were very small and did not possess sufficient sample mass to conduct individual larvae isotopic measurements.
Data analysis
The oxygen isotope composition of chironomid larvae is determined by the isotopic composition of its oxygen sources, weighted by their proportional contribution (Schwarcz 1991) and potentially offset by fractionation (Boutton et al. 1983):
where C = chironomid, D = diet, W = water, and p = the proportional contribution of water to chironomid tissues. The terms εD and εW are fractionation effects associated with diet and water uptake, respectively. Because δ18OD and εD are the same for both treatments, we can solve for p by using the two experimental water types as follows:
where W1 is the natural abundance water and W2 is the labeled water. The same equations can also be used to calculate the proportional contributions of hydrogen from diet and water to that of chironomids by substituting H into the equations where O is used. The possible contribution of atmospheric oxygen to the oxygen isotope composition of the chironomid larvae in both treatments were assumed constant for all aquaria. This contribution, if present, would be confounded with the proportional contribution of dietary O (1 − p).
Statistical analyses
All statistical analyses were performed using JMP IN 5.2.1 (JMP; SAS Institute, Cary, N.C.). Data from the two treatments were analyzed separately. Values of δ18OC (δ2HC) and δ18OW (δ2HW) were analyzed using ANOVA to compare among tanks within two treatments. We assessed the differences among chironomid larvae and water from different aquaria with Tukey honest significant difference (HSD) contrasts. Because calculations of p required δ18OC (δ2HC) to be compared with δ18OW (δ2HW), we used the mean of the δ18OC (δ2HC) compared with the δ18OW (δ2HW) (Eq. 2) of each tank. All these measurements are reported as the mean ± 1 SD. Brown and Forsythe’s tests were performed to check the constant variances of δ18OC and δ2HC within each treatment. Isotope differences in two larval stages (2nd and 4th instar larvae) were assessed using a Student t-test. Unless otherwise stated, statistical significance is assessed at P < 0.05.
Results
Stable isotopic composition of water and diet
Labeled water treatments were heavily enriched relative to the natural abundance treatments throughout the entire experiment. There were no significant differences in δ18OW and δ2HW among the three natural abundance aquaria (P = 0.211 for δ18O and P = 0.235 for δ2H) (Table 1). However, δ18OW and δ2HW were not identical among isotopically labeled aquaria, which may have been due to slight differences in evaporation levels from each aquarium (ANOVA, P = 0.007 for both δ18O and δ2H). The δ18O and δ2H of the diet was 28.0 ± 0.9‰ and −152.3 ± 2.6‰, respectively.
Stable isotopic composition of chironomid larvae
Similar to the water, the δ18O and δ2H of chironomid larvae from the aquaria were distinctly different between the natural abundance (W1) and enriched water treatments (W2) (Table 1; Fig. 1). However, the magnitude of the difference between the two treatments is much smaller compared to that of their growth water (Fig. 1). This is a clear indication that diet also influenced the δ18OC and δ2HC of the larvae. Variation in δ18OC and δ2HC among aquaria were also observed within the same treatment; however, it was much smaller than the variability between the two growth water treatments. Differences in the larvae grown in W1 aquaria were significant for δ18O but not for δ2H (P = 0.01 and P = 0.33 for δ18O and δ2H, respectively). The larvae grown in the W2 did not show significant differences for their δ18O and δ2H (P = 0.34 for δ18O and P = 0.53 for δ2H) and variances among aquaria were also constant (P = 0.09 and P = 0.63, for δ18O and δ2H, respectively) (Table 1). The δ18O and δ2H analyses of second (n = 3) and fourth larval instars (n = 3) from an initial experiment showed that there was no significant difference between these life stages (P = 0.781 and P = 0.744 for δ18O and δ2H, respectively).
The δ18O and δ2H of natural water (white triangle), isotopically labeled water (black triangle), chironomid larvae from both types of water (white circle and black circle, respectively), and the larval diet (black square) expressed as mean ± SD. The isotope compositions are expressed relative to the international standard, Vienna standard mean ocean water
Proportional contributions of oxygen and hydrogen from water versus diet to chironomid larvae
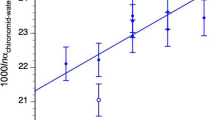
We used two approaches to calculate the proportional contributions of oxygen and hydrogen from water to the organic composition of chironomid larvae. In the first approach, by neglecting the slight differences among replicate aquaria within the same treatment, we calculated the mean δ18O and δ2H of all water samples measured for each treatment (W1 and W2) and the mean δ18O and δ2H of chironomids associated with each treatment (Table 1). By using these data to solve Eq. 2, we found that 72% of chironomid oxygen was derived from water whereas 32% of their hydrogen is derived from water. This result is represented graphically in Fig. 2, where the slopes of the relationship between water and chironomid isotope values represent p. However, because there were small but significant isotopic differences between the water in replicate aquaria 4 and 5 and also among the δ18O for chironomid larvae in replicate aquaria 2 and 3 (Tukey HSD), we also solved Eq. 2 for each individual aquarium. In order to do this, we calculated the proportional contribution (p) of oxygen and hydrogen from water for every possible paired combination of natural abundance (three) and enriched aquaria (three) (a total of nine combinations). We then solved Eq. 2 and calculated the p for both oxygen and hydrogen from the nine combinations and the mean p from these nine combinations (Table 2). Using this approach we estimated that 69 ± 4% of oxygen in chironomid larvae was derived from the water they lived in, whereas 31 ± 3% of hydrogen in chironomid larvae was derived from water. In both approaches, we calculated the proportional contribution using the mean chironomid δ18O and δ2H for each aquarium. Because more than 100 chironomid heads are used for a single isotope value in paleoresearch (Wang et al. 2008), each aquarium is analogous to a single sample collected from sediment. These two approaches yielded very similar results: that only ~30% of oxygen in chironomid larvae originated from diet while ~70% of hydrogen in the larvae was derived from diet.
a The δ18O values of chironomid larvae versus their growth water δ18O (black circle). b The δ2H of chironomids versus δ2H of their growth waters (black circle). The δ18O and δ2H of chironomids versus water from an independent culture trial (white circle) is superimposed on the line. The regression slope (solid line, y 1 equation) changes in b when a data point from an independent culture trial is added (dotted regression line, y 2 equation)
Diet of chironomid larvae from the initial experiment
Spirulina (n = 3) (i.e., diet) yielded a mean δ13C and δ15N of −25.8 ± 0‰ and −1.4 ± 0.5‰, respectively, while the chironomid larvae (n = 3) yielded a mean δ13C and δ15N of −25.1 ± 0.2‰ and 2.0 ± 0.1‰, respectively. Thus, the larvae had mean δ13C and δ15N values that were 0.7‰ and 3.4‰ higher than the diet, consistent with the diet being their sole food source (Fig. 3). The microorganisms collected from the walls of the aquarium had a distinctly different δ13C and δ15N values of −34.4 ± 2.1‰ and −6.0 ± 1.5‰.
The δ15C and δ15N of chironomid larvae (black circle, n = 3), their diet of Spirulina algae (black square, n = 3), and the microorganism (white circle, n = 4) growing on the aquarium walls, mean ± SD. The isotope compositions are expressed relative to the international standard, Vienna Pee Dee belemnite
Discussion
The δ18O and δ2H of chironomid larvae grown from two water treatments were distinctly different from each other, despite having been fed the same diet. These differences are consistent with the δ18O and δ2H of their growth water. However, the magnitude of differences between chironomid larvae from the two treatments is smaller than that of two types of growth water. These results demonstrate that both water and diet affect the oxygen and hydrogen isotope composition of chironomid larvae. By providing a diet with constant isotopic composition and water with two different isotopic compositions, we were able to quantify the proportional contributions of oxygen and hydrogen from water to chironomid larvae. Our data show that ~70% of the oxygen in the total organic composition of the chironomid larvae is derived from the water of the larval habitat. In contrast, diet dominates the hydrogen isotope ratios of chironomid larvae (~70%), because only 30% of hydrogen in the chironomid larvae was derived from water. Interestingly, our findings for both oxygen and hydrogen are identical to the proportional contributions of oxygen and hydrogen from water and growth substrate supplied to microbial spores, where 70% of oxygen and 30% of hydrogen of microbial spores were found to derive from water, whereas the remainder was derived from the organic compounds supplied as substrate (Kreuzer-Martin et al. 2003).
Our finding that ~30% of chironomid total organic hydrogen derives from water is consistent with the handful of published studies of animals, both observational and experimental. Despite differences in tissues, the influence of water is remarkably consistent. Estimates of the drinking water contribution to hair hydrogen in humans range from 27% to 35% (Ehleringer et al. 2008; O’Brien and Wooller 2007; Sharp et al. 2003). Similarly, the contribution of water to hydrogen in bird feather keratin was between 26% and 32% (Hobson et al. 1999). These results confirm that the majority of the hydrogen in lipids, protein and keratin are derived from diet rather than water.
Our estimate that ~70% of total organic oxygen in chironomid larvae derived from water is about twice as high as has been reported in two previous human studies (Ehleringer et al. 2008; O’Brien and Wooller 2007). It is not clear why proportional contributions of oxygen from water in aquatic organisms [chironomids (this study) and microbes (Kreuzer-Martin et al. 2003)] should be different from humans, and it would be interesting to assess whether this pattern holds over a broader sample of aquatic and terrestrial organisms. We also note that neither this study nor previous studies have directly assessed the contribution to tissue oxygen from dissolved or bound O2, which may make a small but measurable contribution to tissue oxygen. Because chironomid larvae vary in whether they possess hemoglobin, the magnitude of this contribution could vary taxonomically. Subsequent investigations in a broader sample of organisms will help to resolve these questions.
To assess the consistency of our results, we plotted the mean δ18O and δ2H values of chironomid larvae and growth water from both our preliminary culture trial and the main experiment (Fig. 2). The preliminary δ18O data did not change the slope of the regression line from the main experiment (Fig. 2a). However, the preliminary δ2H data shifted the slope from 0.31 to 0.35 (Fig. 2b) and while not exactly identical, the data are consistent with our estimated proportional contribution of water to chironomid total organic hydrogen (31 ± 3%).
It was important for our study that the chironomid larvae consumed only the experimental diet provided to them. The experimental diet to tissue fractionation for C and N found in this study (εδ13C = 0.7‰ and εδ15N = 3.4‰) (Fig. 3) is consistent with a classic trophic level fractionation (εδ13C = ~1‰, εδ15N = ~3‰) (Fry and Sherr 1984; Peterson and Fry 1987). In contrast, the δ13C and δ15N values of trace microorganisms found growing on walls of the aquarium were distinctively different from those of the diet, which shows that the microorganisms were not likely to have contributed significantly to the diet. Thus our calculations of the contributions of oxygen and hydrogen from diet and water were not affected by alternate diet sources.
Further studies are needed to establish the relationships between specific compounds (e.g., chitin) from chironomids. Previous studies have shown that hydrogen in different tissues (such as muscle, lipids and feathers) in birds can derive from different proportions of hydrogen from water (Hobson et al. 1999). The proportional contribution of oxygen and hydrogen to whole chironomid larvae, which include lipids and proteins, may differ from those of chitin, the predominant material preserved in the fossil record (Walker 1987). Furthermore, fractionation factors (ε) associated with both water and diet in our experiment cannot be determined using Eq. 1 because there are three unknowns (p, εW, εD). A labeling study of nutritionally identical diets with different isotope ratios would make it possible to solve all the unknowns (Sessions and Hayes 2005) including the p, εW and εD.
Our findings support that the stable isotopic composition of chironomid subfossils can be used to explain changes in the oxygen and hydrogen isotope values of past environments in concert with other geological evidence (Wang et al. 2008; Wooller et al. 2008). The stable oxygen isotope composition of chironomid larvae provides a stronger marker of habitat water isotopic values compared with hydrogen isotopic data derived from chironomids since a greater proportion (~70%) of chironomid larvae oxygen is derived from water compared with hydrogen (~30%). Conversely, hydrogen isotope analyses of chironomid larvae better constrain the hydrogen isotopic composition of diet. In our experiment, the magnitude of changes in δ18OC and δ2HC is based on the mean value of chironomid larvae within each aquarium instead of individual larvae. The systematic variation in each aquarium resembles paleoecological research, where one measurement is based on more than 100 fossil chironomid head capsules. Our research is the first of its type on aquatic invertebrates to calculate the proportional contribution of oxygen and hydrogen from water versus diet. Our findings add to the growing suite of comparative data available on the proportional contribution of oxygen and hydrogen deriving from water and diet available to organisms.
References
Ambler RP, Macko SA, Sykes B, Griffiths JB, Bada J, Eglinton G (1999) Documenting the diet in ancient human populations through stable isotope analysis of hair—discussion. Philos Trans R Soc Lond Ser B Biol Sci 354:75–76
Boutton TW, Arshad MA, Tieszen LL (1983) Stable isotope analysis of termite food habits in East African grassland. Oecologia 59:1–6
Bowen GJ, Revenaugh J (2003) Interpolating the isotopic composition of modern meteoric precipitation. Water Resour Res 39(10):1299. doi:1029/2003WR002086
Bowen GJ, Wilkinson B (2002) Spatial distribution of delta O-18 in meteoric precipitation. Geology 30:315–318
Bowen GJ, Chesson L, Nielson K, Cerling TE, Ehleringer JR (2005a) Treatment methods for the determination of delta H-2 and delta O-18 of hair keratin by continuous-flow isotope-ratio mass spectrometry. Rapid Commun Mass Spectrom 19:2371–2378
Bowen GJ, Wassenaar LI, Hobson KA (2005b) Global application of stable hydrogen and oxygen isotopes to wildlife forensics. Oecologia 143:337–348
Brodersen KP, Pedersen O, Lindegaard C, Hamburger K (2004) Chironomids (Diptera) and oxy-regulatory capacity: an experimental approach to paleolimnological interpretation. Limnol Oceanogr 49:1549–1559
Brooks SJ (2000) Late-glacial fossil midge stratigraphies (Insecta: Diptera: Chironomidae) from the Swiss Alps. Palaeogeogr Palaeoclimatol Palaeoecol 159:261–279
Cormie AB, Schwarcz HP, Gray J (1994a) Determination of the hydrogen isotopic composition of bone-collagen and correction for hydrogen-exchange. Geochim Cosmochim Acta 58:365–375
Cormie AB, Schwarcz HP, Gray J (1994b) Relation between hydrogen isotopic-ratios of bone-collagen and rain. Geochim Cosmochim Acta 58:377–391
Crowley TJ (2000) Causes of climate change over the past 1,000 years. Science 289:270–277
Ehleringer JR, Bowen GJ, Chesson LA, West AG, Podlesak DW, Cerling TE (2008) Hydrogen and oxygen isotope ratios in human hair are related to geography. Proc Natl Acad Sci USA 105:2788–2793
EPA (1997) Standard operating procedures (SOP): culture of Chironomonus tentans. EPA, Duluth
Fogel ML, Tuross N, Johnson BJ, Miller GH (1997) Biogeochemical record of ancient humans. Org Geochem 27:275–287
Fry B, Sherr EB (1984) Delta-C-13 measurements as indicators of carbon flow in marine and fresh-water ecosystems. Contrib Mar Sci 27:13–47
Gat JR (1996) Oxygen and hydrogen isotopes in the hydrologic cycle. Annu Rev Earth Planet Sci 24:225–262
Gat JR, Alrey PL (2006) Stable water isotopes in the atmosphere/biosphere/lithosphere interface: scaling-up from the local to continental scale, under humid and dry conditions. Glob Planet Chang 51:25–33
Grocke DR, Schimmelmann A, Elias S, Miller RF (2006) Stable hydrogen-isotope ratios in beetle chitin: preliminary European data and re-interpretation of North American data. Quat Sci Rev 25:1850–1864
Gullan PJ, Cranston PS (2000) Aquatic insects, vol 2. Blackwell Science, Guthrie
Heinrichs ML, Walker IR, Mathewes RW, Hebda RJ (1999) Holocene chironomid-inferred salinity and paleovegetation reconstruction from Kilpoola Lake, British Columbia. Geog Phys Quat 53:211–221
Hobson KA (1999) Tracing origins and migration of wildlife using stable isotopes: a review. Oecologia 120:314–326
Hobson KA, Atwell L, Wassenaar LI (1999) Influence of drinking water and diet on the stable-hydrogen isotope ratios of animal tissues. Proc Natl Acad Sci USA 96:8003–8006
Kreuzer-Martin HW, Lott MJ, Dorigan J, Ehleringer JR (2003) Microbe forensics: oxygen and hydrogen stable isotope ratios in Bacillus subtilis cells and spores. Proc Natl Acad Sci USA 100:815–819
Kreuzer-Martin HW, Lott MJ, Ehleringer JR, Hegg EL (2006) Metabolic processes account for the majority of the intracellular water in log-phase Escherichia coli cells as revealed by hydrogen isotopes. Biochemistry 45:13622–13630
Langdon PG, Ruiz Z, Brodersen KP, Foster IDL (2006) Assessing lake eutrophication using chironomids: understanding the nature of community response in different lake types. Freshw Biol 51:562–577
Levin NE, Cerling TE, Passey BH, Harris JM, Ehleringer JR (2006) A stable isotope aridity index for terrestrial environments. Proc Natl Acad Sci USA 103:11201–11205
Macko SA, Engel MH, Andrusevich V, Lubec G, O’Connell TC, Hedges REM (1999) Documenting the diet in ancient human populations through stable isotope analysis of hair. Philos Trans R Soc Lond Ser B Biol Sci 354:65–75
Maier KJ, Kosalwat P, Knight AW (1990) Culture of Chironomus-Decorus (Diptera, Chironomidae) and the effect of temperature on its life-history. Environ Entomol 19:1681–1688
Miller RF, Fritz P, Morgan AV (1988) Climatic implications of D/H ratios in beetle chitin. Palaeogeogr Palaeoclimatol Palaeoecol 66:277–288
Miller RF, Vossfoucart MF, Toussaint C, Jeuniaux C (1993) Chitin preservation in quaternary coleoptera—preliminary-results. Palaeogeogr Palaeoclimatol Palaeoecol 103:133–140
O’Brien DM, Wooller MJ (2007) Tracking human travel using stable oxygen and hydrogen isotope analyses of hair and urine. Rapid Commun Mass Spectrom 21:2422–2430
Oliver DR (1971) Life history of the Chironomidae. Annu Rev Entomol 16:211–230
Peterson BJ, Fry B (1987) Stable isotopes in ecosystem studies. Annu Rev Ecol Syst 18:293–320
Schimmelmann A, DeNiro MJ (1986a) Stable Isotopic studies on chitin, measurements on chitin/chitosan isolates and d-glucosamine hydrochloride from chitin. In: Muzzarelli RAA, Jeuniaux C, Gooday GW (eds) Chitin in nature and technology. Plenum, New York, pp 357–364
Schimmelmann A, DeNiro MJ (1986b) Stable isotopic studies on chitin. 2. The C-13-C-12 and N-15-N-16 ratios in arthropod chitin. Contrib Mar Sci 29:113–130
Schimmelmann A, Deniro MJ (1986c) Stable isotopic studies on chitin. 3. The D/H and O-18/O-16 ratios in arthropod chitin. Geochim Cosmochim Acta 50:1485–1496
Schwarcz HP (1991) Some theoretical aspects of isotope paleodiet studies. J Archaeol Sci 18:261–275
Sessions AL, Hayes JM (2005) Calculation of hydrogen isotopic fractionations in biogeochemical systems. Geochim Cosmochim Acta 69:593–597
Sharp ZD, Atudorei V, Panarello HO, Fernandez J, Douthitt C (2003) Hydrogen isotope systematics of hair: archeological and forensic applications. J Archaeol Sci 30:1709–1716
Walker IR (1987) Chironomidae (Diptera) in paleoecology. Quat Sci Rev 6:29–40
Walker IR (1995) Chironomids as indicators of past environmental change. In: Armitage PD, Cranston PS, Pinder LCV (eds) The Chironomidae: the biology and ecology of non-biting midges. Chapman & Hall, London, pp 403–422
Walker IR (2001) Midges: Chironomidae and related diptera. In: Smol JP, Birks HJB, Last WM (eds) Tracking environmental change using lake sediments. Volume 4: zoological indicators. Kluwer, Dordrecht, pp 43–66
Wang Y, Francis DR, O’Brien DM, Wooller MJ (2008) A protocol for preparing subfossil chironomid head capsules (Diptera: Chiornomidae) for stable isotope analysis in paleoclimate reconstruction and consideration of contamination sources. J Paleolimnol 40:771–781
Wassenaar LI, Hobson KA (2002) Comparative equilibration and online technique for determination of non-exchangeable hydrogen of keratins for use in animal migration studies. Isot Environ Health Stud 39:211–217
Wooller MJ, Francis D, Fogel ML, Miller GH, Walker IR, Wolfe AP (2004) Quantitative paleotemperature estimates from delta O-18 of chironomid head capsules preserved in arctic lake sediments. J Paleolimnol 31:267–274
Wooller MJ, Wang Y, Axford Y (2008) A multiple stable isotope record of Late Quaternary limnological changes and chironomid paleoecology from northeastern Iceland. J Paleolimnol 40:63–77
Acknowledgements
This research was supported by a National Science Foundation grant (ESH-0317766) and a National Institute for Water Research grant (no. G-3077) awarded to Dr Matthew Wooller. This publication is also the result, in part, of research sponsored by the Cooperative Institute for Arctic Research (project CIPY-01) with funds from the National Oceanic and Atmospheric Administration under cooperative agreement NA17RJ1224 with the University of Alaska. We thank the Wilson Environmental Laboratories and EPA Mid-continent Ecology Division for supplying chironomid egg masses. We thank the WERC at UAF for accommodating our growth experiment. Special thanks to the WERC laboratory manager Shane Billing for numerous suggestions to modify our growth chamber setup and also for helping us fetch water. Our thanks also go to Tim Howe and Norma Haubenstock at the ASIF for laboratory support. We also thank Dr Jonathan Grey and an anonymous reviewer for their very constructive and positive reviews of our paper.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Craig Osenberg.
Rights and permissions
About this article
Cite this article
Wang, Y.V., O’Brien, D.M., Jenson, J. et al. The influence of diet and water on the stable oxygen and hydrogen isotope composition of Chironomidae (Diptera) with paleoecological implications. Oecologia 160, 225–233 (2009). https://doi.org/10.1007/s00442-009-1303-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-009-1303-3