Abstract
Cotton microdust (CMD) is an abundantly generated waste from textile industry with no commercial value. It is composed of cellulose as the predominant component along with other non-cellulosic components like lignin. The conversion of CMD to value-added materials would further improve the economics of the cotton mills. In this study, the CMD was converted into high value cellulose membrane through thermo-chemical pretreatment and spin coating methods. The thermal pretreated CMD was solubilized in 68 wt% zinc chloride solution prior to preparation of membrane and then the solubilized cellulose was spread on a plate and spin coated with 22% (w/v) sodium chloride. During the spin coating process, the cellulose membrane was formed with the simultaneous separation of lignin. The present method is a novel approach since a separate process for lignin removal from cotton wastes is not required to produce cellulose membrane. The XRD and FTIR analyses showed significant changes in the crystallinity and the composition of the cellulose membrane. The SEM picture of the membrane revealed uniform surface morphology. Moreover, the membrane was associated with good mechanical properties as indicated by rheology and tensile studies. The cellulose membrane had antibacterial activity against E. coli and efficient absorption and release of antibiotics. Therefore, the results would be useful for further development of a process for the production of cellulose membrane as a high value byproduct from the CMD in cotton mills.
Graphic Abstract
Development of simple and green process for the valorization of cotton microdust waste from spinning mills into Zn loaded cellulose membrane

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The excessive dependence on petroleum based plastics caused irreversible damage to the environment due to its non-biodegradability, eco-toxicity and water pollution [1]. To mitigate the critical impact of plastic based products on the environment, substitution towards renewable, sustainable and environment friendly resources is an impelling solution [2]. Considering its widespread availability and abundance, cellulose is an attractive polymer for producing versatile products. Cellulose is a linear homopolymer composed of glucose monomeric units linked by β-(1-4)-glycosidic linkages and exists in the form of nanofibrils. In turn, the cellulose nanofibrils associate by intermolecular and intramolecular hydrogen bonds to produce tough fibers owing to the highly ordered structures. Moreover, cellulose is associated with profound characteristics like high mechanical strength, hydrophilicity, durability, biodegradability, biocompatibility and other tunable properties [3]. Interestingly, the presence of crystalline and amorphous domains in cellulose provides the flexibility to tailor its mechanical properties. As a result of promising attributes of cellulose, extensive studies have been reported to produce different cellulose based products like thin film, membrane, sponges, hydrogels, flat sheets etc. For the preparation of cellulose based high value membranes, the solubilisation of cellulose is the primary and crucial step. However, the recalcitrant structure of cellulose formed by multiple hydrogen bonds poses challenge for the efficient solubilisation of cellulose [4, 5]. The conventional cellulose solvents like lithium chloride/dimethylacetamide, sodium hydroxide/urea, dimethyl sulfoxide are associated with limitations like process cost, solvent recovery, toxicity and environmental security. Eventhough ionic liquids are potential cellulose solvents with high dissolution capacity and recyclability, the cost involved in the solvent production limits its commercial viability [6, 7]. Alternative approach is the cellulose solubilisation using molten inorganic salts, which is considered to be simple, cost-effective and environmental friendly. Moreover, the inorganic salts can be efficiently recovered making the process recyclable and economical. Zinc chloride is the most commonly used inorganic salt for preparing cellulose solutions and cellulose based products like regenerated cellulose film, fibers etc. [7,8,9,10].
Most of the reported studies on the preparation of cellulose membrane used purified cellulose like microcrystalline cellulose. The purified cellulose is obtained from lignocellulosic biomass through its fractionation into cellulose, lignin and other components. The production of N-methyl morpholine N-oxide mediated regenerated cellulose film from lignocellulosic sugarcane bagasse has been reported through the organosolv extraction of the cellulose [11]. Similarly, the production of cellulose film from corn stalk pith has been reported using sodium chlorite bleaching and zinc chloride solubilization [10]. Though lignin is removed to produce cellulose membrane, the presence of lignin in trace amounts has been reported to improve the mechanical properties and cytocompatibility of the cellulose hydrogel films [12]. A composite based on bacterial cellulose and lignin has been reported for dressing for chronic wound healing [13]. However, the presence of higher amount of lignin reduced the homogeneity of cellulose film and the fabrication process necessitated partial lignin removal using chemical methods [10, 14].
Alternative cellulosic sources are cotton wastes, which can be utilized for cellulose based membranes. As cotton is rich in cellulose, the wastes generated in processing of cotton are promising feedstock for the production of cellulose membranes. Though there are a few reports on valorization of cotton processing wastes to fuels like ethanol, there is lack of studies on conversion of the cotton wastes to high value biomaterials like membranes [15, 16]. Recently, Haque et al. has reported the production of film from cotton gin trash, where the whole cotton gin trash has been converted into film due to difficulties associated with the separation of cellulose [17]. However, the production of cellulose film with high homogeneity from the cotton processing waste would be advantageous for value addition and specific biomedical applications. The cotton microdust (CMD) is one such processing waste generated in cotton processing industries. The CMD are dust particles of cotton fiber released during the spinning process in the textile industry. It is a complex mixture mainly comprised of microparticles of cotton fiber and cotton plant materials. The generation of cotton microdust in cotton mills is enormous, as it accounts for 10% of the processed cotton. The CMD is a serious air particulate pollutant, which affects the respiratory system. The common method of its disposal involves mixing with soil to increase the soil carbon content [15]. The valorization of the CMD as a high value product is a critical step of waste management in cotton spinning mills. More importantly, cotton microdust is a renewable feedstock chiefly composed of cellulose [18]. However, the presence of non-cellulosic components like lignin in the cotton microdust reduces the cellulose accessibility and contributes to its recalcitrant nature. For the effective conversion of cotton microdust in to cellulose based product, the pretreatment step altering the structural, composition and physicochemical characteristics is indispensible [15, 17].
In this study, a novel process for the production of cellulose membrane from CMD is reported. CMD was pretreated with alkali and acid to improve the cellulose solubilisation in zinc chloride solution. The cellulose membrane was prepared using spin coating along with addition of simple salt sodium chloride leading to simultaneous removal of lignin. The present method is simple and novel for the production of cellulose based membrane from CMD. As cellulose membrane is applied in different fields including biochemical, biomedical, pharmaceutical and drug delivery, the present results will be useful for further development of low cost cellulose based membranes from cotton wastes.
Materials and Methods
Materials
The CMD was kindly donated by Ayyan Textiles & Ltd, Coimbatore, India. The zinc chloride with purity of 95% was purchased from Merck (India) and the sodium chloride (99.9% pure) was obtained from Fischer Scientific (India). All reagents and chemicals used were of analytical grade.
Composition Analysis of CMD
The compositions of the raw CMD, pretreated CMD and cellulose membrane were analyzed according to the National Renewable Energy Laboratory (NREL) procedure [19]. The cellulose membrane with dimension of 50 mm × 50 mm was used for the analysis. The CMD and cellulose membrane were subjected to initial hydrolysis process using 72% (w/w) sulphuric acid at 30 °C for 60 min followed by dilute acid hydrolysis using 4% (w/v) sulphuric acid at 120 °C for 60 min. The insoluble solids in the hydrolyzates were filtered and dried at 105 °C for 6 h and measured to find the acid insoluble lignin. The acid soluble lignin in the hydrolyzates was photometrically measured at 240 nm. The sugars present in the hydrolyzates were estimated by HPLC system connected to a refractive index detector and Aminex HPX-87H column. The mobile phase used was 5 mM sulphuric acid and the operating conditions included flow rate of mobile phase as 0.6 mL/min and column temperature as 60 °C. HPLC grade sugars were used as standards for quantification.
Pretreatment of CMD
The CMD was treated with alkali and acid in two-stages sequentially under the conditions previously optimized in the lab. The two-stage pretreatment was performed at a solid to liquid ratio of 1:10 in a stainless steel reactor (Amar Equipments, Mumbai). The first-stage was carried out by soaking the CMD in 0.1% (w/v) sodium hydroxide solution at 120 °C for 30 min. The alkali-pretreated solids were washed in water to neutral pH and further soaked in 0.5% (v/v) sulphuric acid at 163 °C for 30 min. The two-stage pretreated solids were washed with water until the pH was neutral, air dried overnight in fume hood and stored in zip lock bags for further use.
Preparation of Cellulose Membrane
The pretreated CMD was used for the preparation of cellulose membrane. The pretreated CMD was weighed based on the cellulose content. The CMD corresponding to 3% cellulose was mixed with different concentrations of zinc chloride solution (66–68 wt %) at 65 °C for 30 min with shaking at 250 rpm. The mixture was centrifuged at 8000 rpm for 30 min at room temperature to remove the insoluble solids. The supernatant was poured on a petri plate and incubated further at 65 °C for 60 min. The content was spin coated with simultaneous drop by drop addition of 22% (w/v) sodium chloride at 200 rpm for 10 min to produce cellulose membrane. The addition of sodium chloride facilitated the separation of lignin which was accumulated as dark precipitate at the corner of the plate. After the removal of lignin, the cellulose membrane was coagulated in 70% ethanol for 30 min. The coagulation step was repeated with fresh 70% ethanol for another 30 min. The cellulose membrane was washed in deionized water for 30 min to remove excess salt. Finally, the membrane was soaked in 5% (v/v) glycerol for 30 min and air dried overnight. Among the various concentration of zinc chloride used, the cellulose membrane prepared using 68% was optimum and hence was used for further experiments. The cellulose membrane prepared using 68% (w/v) zinc chloride solution was only taken for further characterization studies.
Characterization
The surface structure and morphology of the cellulose membrane was investigated using scanning electron microscopic technique. The samples were sputter coated with gold and imaged at a magnification of 2000× under a Zeiss Supra scanning electron microscope (SEM) at an accelerating voltage of 10 kV. The elemental analysis was carried using energy dispersive X ray spectroscopy (EDAX). The compositions of the CMD and cellulose membrane were studied using Fourier transform infrared spectroscopy (FTIR). FTIR spectra of the samples were observed under attenuated total reflectance (ATR) mode in the scan range from 600 to 4000 cm−1 at a resolution of 4 cm−1 with 32 scans per sample. The crystallinity index of the CMD and cellulose membrane samples were determined using X-ray diffraction (XRD) analysis with a Rigaku D8 Advance equipped with the sealed tube of CuKα source. The recorded region of 2θ was collected from 10° to 60° with scanning rate of 12°/min. The crystallinity index (CrI) of the sample was determined according to Vanitjinda et al. [14] as given below.
where, I002 is intensity of the diffraction from the 002 plane at 2θ = 21.7° and Iam is intensity of amorphous region at 2θ = 16°.The viscoelastic measurements of the cellulose membrane were carried out using Anton Paar Rheometer at 25 °C. Changes in the storage modulus and loss modulus of elasticity were measured through a sweep of strain in the frequency range of 1 to 10 Hz at room temperature. The measurements were done in duplicate and the average values were reported. The tensile strengths of the cellulose membrane were measured using Instron Universal testing systems equipped with 50 N load cell at a crosshead speed of 2 mm/min. The membrane was cut into rectangular strip with dimension of 80 mm × 10 mm and the gap between the grips was adjusted to 60 mm. All the measurements were carried out in triplicates according to the ASTM standards and the average values were reported. Water contact angle of the cellulose membrane were measured using Optical Tensiometer. The strips (20 mm × 60 mm) of membrane were put on glass sample stage and adjusted horizontally. A drop of 2 µL of distilled water was placed on the surface of the membrane using a micro-syringe. Initial contact angle (after 2 s) was measured in a conditioned room by recording contact angle values.
Antibacterial Activity of the Antibiotic Loaded Membrane
The antibacterial activity of the cellulose membrane against E. coli (Gram negative bacteria) was studied according to standard antibacterial test [20]. Nutrient agar medium was prepared and the E. coli cells were spread on the agar plates. The testing samples of cellulose membranes were cut into discs of 5 mm diameter. The discs of cellulose membrane were sterilized by UV irradiation for 60 min and loaded with 10 µL of sterile solution of antibiotic kanamycin (50 µg/mL). The positive control discs were prepared using sterile Whatmann No.1 filter paper. The sterile filter paper discs were also loaded with the same concentration of sterile kanamycin. The sterile cellulose membrane discs loaded with sterile MilliQ water was also used as the control. The test and control discs were air dried aseptically. The discs were placed over the agar plates pre-inoculated with E. coli cells and incubated at 37 °C for 12 h. The zones of inhibition formed around the discs were measured using a Vernier caliper. All the experiments were carried out in triplicates and the mean values were reported.
Results and Discussion
Pretreatment of the CMD
Though the cotton wastes are primarily composed of cellulose, the non-cellulosic component lignin is present in significant amount [16]. The extractives are also present in the cotton wastes. These non-cellulosic components in the cotton wastes are attributed to the mixing of cotton fibers with cotton seed covers and plant materials coming from the ginning processes [15, 16]. The production of heterogenic thick and opaque cellulose film from cotton gin trash has been reported through the dissolution of whole biomass in formic acid [17]. The study indicated that removal of non-cellulosic components is essential for producing cellulose film of desirable homogeneity. In the case of CMD, there are additional issues such as aggregation and poor dispersibility in aqueous media. As a result, the untreated CMD did not dissolve in ZnCl2 solution. Therefore, the CMD was pretreated with alkali and acid to facilitate its dissolution in ZnCl2 for further preparation of cellulose membrane. The pretreatment not only improved the solubilization of CMD in ZnCl2, but also increased the cellulose content by removing extractives. The cellulose content in the pretreated CMD was increased to 69% from 37% in the untreated sample (Table 1). However, the lignin content (28%) was not altered after the pretreatment. The preparation of cellulose membrane with lignin has been reported by covalently linking lignin to the azide modified microcrystalline cellulose [21]. Whereas, the present result indicates the possibility of production of cellulose-lignin composite membrane using the pretreated CMD itself. However, to prepare cellulose rich membrane, the lignin in the CMD needs to be removed. Therefore, the pretreated CMD containing both cellulose and lignin can be used to prepare cellulose membrane with varying concentration of lignin through partial delignification. The presence of lignin in the cellulose based membrane has the functions like antimicrobial property and UV ray filtration [13, 21].
The pretreatment of CMD in the present study resulted in considerable reduction in the length of cellulose fibers. In the case of cellulose film production from sugarcane bagasse, the mechanical disintegration of cellulose in to cellulose nanofibrils was carried out by grinding followed by high pressure homogenization [22]. On the contrary, in the present study, the soaking step was performed using dilute acid at moderate temperature for reducing the degree of polymerization of cellulose in CMD. The single stage soaking of CMD in either alkali or acid was not effective, as the CMD treated with either alkali or acid formed aggregates in the ZnCl2 solution. The CMD waste consisted of densely packed cellulose fibers associated with randomly distributed lignin as seen in Fig. 1a. However, the alkali treatment of CMD using 1% NaOH resulted in partial removal of lignin and the cellulose fibers were swollen and thick (Fig. 1b). In the case of single stage acid pretreatment of CMD, there was a marginal decrease in the length (10–50 µm) of cellulose fibers (Fig. 1c). On the contrary, the sequential treatment of cotton microdust by alkali and acid contributed to substantial reduction in the length (< 10 µm) of cellulose chains (Fig. 1d). Therefore, the sequential alkali and acid treatments yielded discrete particles of CMD, which resulted in efficient solubilization in ZnCl2 solution. Similarly, cellulose solubilization using various solvents has been reported to be associated with the reduction of the length of cellulose chains [5, 22]. Thus, the alkali acid soaking of CMD caused change in its morphology that favored cellulose solubilization using zinc chloride solution.
Production of Cellulose Membrane
The solubilization of pretreated CMD was carried out using concentrated ZnCl2 solution and CaCl2 was added for the preparation of cellulose membrane by manual plating method. The role of ZnCl2 involved the breakdown of O3-H and O5-H hydrogen bonds in cellulose and CaCl2 cross linked the Zn-cellulose to produce cellulose membrane as reported by Xu et al. [7]. However, the addition of CaCl2 to the Zn-CMD solution resulted in rapid gelation and uneven formation of cellulose membrane. To produce a homogeneous cellulose membrane, the spin coating process was used. However, spin coating of Zn-CMD in various optimizing trails like CaCl2 concentrations and spin coater rotating speed did not produce a proper cellulose membrane. This is due to the strong cross linking between Zn-cellulose chains and CaCl2 leading to instantaneous formation of gel and further membrane fracture and damage. Hence to improve the membrane formation, the common monovalent salt NaCl was used by replacing CaCl2. The interaction between NaCl and cellulose has been reported. Bellesia et al. [23] showed the existence of strong interactions between Na+ ions and O2, O3, O6 hydroxyl groups in cellulose by molecular dynamics simulation. When spin coating of Zn-CMD solution with NaCl was performed in the present study, the rate of gelation was slow and resulted in uniform membrane formation (Fig. 2). Interestingly, the addition of NaCl produced cellulose membrane with simultaneous removal of lignin. As seen in Fig. 2, a black mass accumulated at the edge of the plate. Jiang et al. [24] reported the breakdown of bond between cellulose and lignin in corn cob by the addition of NaCl-water system. In the case of CMD, there was no strong association between cellulose and lignin due to the physical mixing of cellulose fibers with lignin from other cotton plant materials like cotton seeds, hulls [16]. As a result, spin coating of Zn-cellulose with NaCl facilitated the separation of lignin in the present study. Moreover, speed of spin coating also contributed to lignin removal. Wu et al. [25] has reported the effect of shear on disruption of interaction between cation and alginate resulting in low viscosity during the coating process. Likewise during the formation of cellulose membrane in the present study, the shear induced by the spin coating process in the presence of NaCl influenced the separation of lignin by altering the inter- and intra-molecular association of cellulose, lignin and zinc. Consequently, the slower membrane formation at high shear rate of 200 rpm facilitated the removal of lignin. Therefore, the spin coating process using sodium chloride was an additional refinement step for the separation of lignin to produce a homogeneous cellulose membrane from CMD. The solubilization of cellulose using zinc chloride solution was observed to be efficient. In the present study, dissolution of pretreated CMD was carried out in ZnCl2 at varying concentration (66 to 68%) and further increase ZnCl2 concentration showed reduction in solubility, which was in accordance with the reported result by Xu et al. [7]. Moreover, the maximum cellulose solubilization was observed using 68% ZnCl2 solution. Therefore, the cellulose membrane prepared using 68% ZnCl2 was further evaluated by SEM, FTIR, XRD, rheology, wettability and tensile studies for analyzing the surface morphology, composition, crystallinity and mechanical properties.
Characterization of Cellulose Membrane
The composition of cellulose membrane is given in Table 1. The lignin present in the cellulose membrane was 8% and it was substantially lower as compared to CMD. The cellulose content in the membrane increased to 89%, as a result of lignin removal achieved during the spin coating. The surface structure of the cellulose membrane is shown in Fig. 3a. The surface of the membrane was observed to be homogeneous with few random distributions of acid insoluble lignin particles. Matovic et al. [13] has reported the production of composite containing bacterial cellulose and lignin (DHP). The roughness of the film has been attributed to the presence of lignin globules on the surface of cellulose microstructure [13]. A similar surface structure of the cellulose membrane was observed in the present study. As the partial lignin removal was achieved in the membrane fabrication process, the membrane was associated with reduced surface roughness. In contrast, Haque et al. [17] produced cellulose film from cotton gin trash with enhanced surface roughness. The surface roughness of film has been related to the amount of insoluble particles. Moreover in their study, the cellulose film was associated with substantial amount of insoluble solids from the cotton gin trash [17]. The observed high homogeneity and smoothness of the cellulose membrane using ZnCl2 concentration is due to absence of insoluble particles from CMD. The elemental composition of the cellulose membrane was analyzed by Energy dispersive X-ray spectroscopic technique (EDS) and shown in Fig. 3b. The major elements present in the membrane were carbon (C), oxygen (O), zinc (Zn) and chloride (Cl) with trace amounts of sodium (Na). These results confirmed the association of cellulose molecules with Zn and Na ions in the membrane. Similarly, the interactions of cellulose molecules with Zn and Na ions have been reported. These cations disrupt the crystallinity of cellulose fibers and facilitating their dissolution [7, 23, 24].
The FTIR spectra of CMD and cellulose membrane are shown in Fig. 4a. The broad band located between 3000 and 3700 cm−1 represented hydrogen bonded OH stretching in cellulose, which included intramolecular hydrogen bonds for O2H…O6, O3H…O5 and O6H…O3 respectively. Additional peak at 1633 cm−1 represented the OH bending vibration of sorbed water [26]. The peaks representing cellulose were present between 800 to 1200 cm−1. In the case of CMD, the peak at 1030 cm−1 corresponded to cellulose intense polysaccharide. As the cellulose present in the CMD was more crystalline, the peak at 1094 cm−1 representing amorphous cellulose was less intense [27]. However in the cellulose membrane, there was augmentation of peak at 1094 cm−1 showing the interaction between Zn2+ and OH groups of cellulose. Moreover, the presence of Zn2+ in the membrane was indicated by peaks at 995 cm−1 and 663 cm−1. Xu et al. [7] has reported the occurrence of similar peaks representing the bonding between Zn2+and OH in the cellulose film. The peaks at 1375 cm−1, 1315 cm−1, 1200 cm−1 and 1157 cm−1 corresponded to CH bending, CH2 wagging, C–O stretching and asymmetric bridge C–O–C stretching in cellulose [27]. There was an enhancement in the cellulose content in the membrane, as there were marked increases in the peaks for C–O stretching and asymmetric bridge C–O–C stretching. The existence of lignin in CMD was indicated by the peak at 1508 cm−1 corresponding to aromatic skeletal vibration and C=C stretching [12]. In the preparation of cellulose hydrogel with trace amount of lignin from the sugarcane bagasse, the signature peak for lignin in the FTIR spectra has been reported at 1515 cm−1 corresponding to the aromatic skeletal vibration of lignin [12]. In the case of regenerated cellulose film produced from sugarcane bagasse, the peak at 1462 cm−1 was identified as aromatic C–H bending in lignin [14]. Likewise, the peaks at 1508 cm−1, 1456 cm−1 corresponding to aromatic ring vibration and aromatic C=C in plane stretching were present in the cellulose membrane. However, the intensity of peak at 1508 cm−1 was reduced for the cellulose membrane indicating the lignin removal achieved during the membrane preparation process.
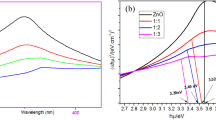
The XRD spectra of CMD and cellulose membrane are shown in Fig. 4b. In the case of CMD, peak at 22° corresponding to the lattice plane (1 0 1) of crystalline cellulose was prominent. The intensity of the peak for amorphous cellulose at 16° was considerably low as compared to the crystalline cellulose. As a result, the crystallinity index of the CMD was observed to 35.18%. In contrast, the crystallinity of pure cotton is as high as 73% [15]. The reduced crystallinity of the CMD was attributed to the complex association of cellulose with other amorphous components like lignin. The preparation of cellulose membrane from CMD resulted in two fold reduction in the crystallinity of the cellulose, which was attributed to the transition of cellulose from its crystalline form (Cellulose I) to amorphous form (Cellulose II) [28]. During the process of formation of cellulose membrane, the initial solubilisation of CMD using zinc chloride solution disrupted the crystallinity of cellulose and further the rearranged cellulose present in the membrane majorly existed as cellulose II (amorphous) [29]. As observed in the XRD spectrum for the cellulose membrane, the peak at 22° representing crystalline cellulose was broad and shifted to lower degree. Moreover, the cellulose crystalline peak corresponding to the lattice plane (1 0 1) was significantly reduced indicating the breakdown of hydrogen bonds in cellulose by Zn2+ ions. In contrast, the peak at 18° corresponding to amorphous cellulose was more prominent. Moreover, the transition of cellulose from crystalline to amorphous form was dependent on the dissolution capacity of the cellulose solvent. The cellulose membrane was associated with the crystallinity index of 17% indicating the highly efficient cellulose solubilisation achieved using zinc chloride solution. Further in the cellulose membrane, there were additional peaks representing zinc located at 31.6° and 47.7°. The peaks for zinc corresponded to planes (1 1 1) and (1 0 2) respectively, which were similar to the observations by Bindu et al. [30] for ZnO nanoparticles. The peak intensities were proportional to the zinc available in the cellulose membrane. As the zinc was directly involved in the interaction with cellulose, the higher amount of zinc correlates with the increased presence of cellulose in the membrane material. This observation was in accordance with the result obtained from FTIR in the present study. Therefore, the reduction in the cellulose crystallinity was more favorable for the production of cellulose membrane.
To measure the modulus of elasticity of the cellulose membrane, the rheology study was carried out using mechanical rheometer. Elasticity of the membrane was proportional to the degree of extension at different frequencies. The flexibility of the cellulose membrane was studied based on the modulus of elasticity restored when the membrane was subjected to extensional strain. The cellulose membrane showed enhanced storage modulus of 175 MPa at 10 Hz, as seen in Fig. 5a. Moreover, there was a substantial increase in the storage modulus of the membrane in the frequency range of 1–10 Hz. As a result, the membrane was more flexible and extensional stress resistant. However, the loss modulus due to extension increased along with stress and consequently the loss factor due to extension of the membrane was constant in the tested frequency range. Nakasone and Kobayashi [12] have reported the influence of lignin in the cellulose hydrogel film on the enhancement of elasticity modulus of the film. Similar to the above reported result, the cellulose membrane was observed to be associated with improved elasticity and deformation stress resistance due to the presence of trace amount of lignin in the membrane.
The mechanical strength of the cellulose membrane was determined by measuring its tensile strength. The study was performed by applying stress on one end of the membrane by fixing the other end and the force was applied upto its breakpoint. Therefore, tensile study indicated the maximum stress which the membrane could withstand. As seen in Fig. 5a inset, there was a linear increase in the load upto 3 N. Moreover, the membrane was cut (break point) beyond the load of 3 N. The tensile strength of the membrane was observed to be 1550 kN/m2. The production of a cellulose film with a tensile strength of 88.2 kN/m2 has been reported from Zn-cellulose solution using calcium chloride as a cross linker [7]. However, the reported tensile strength was lower than the present cellulose membrane prepared using sodium chloride. Moreover, low tensile strength cellulose membranes have been used as a drug carrier in dentistry and a transdermal patch in wound healing application [31, 32].
The hydrophobicity/hydrophilicity of the membrane surface was measured by contact angle meter. A small drop of water (1 µL) was placed on the surface of the cellulose membrane using a microsyringe and the shape of the droplet was recorded. The interaction between the water droplet and the membrane was indicated by the contact angle. For the material to be hydrophobic, the contact angle should be greater than 90° [17]. However, in the present study the contact angle for the cellulose membrane was observed to be 36°, indicating the hydrophilic nature of the membrane as seen in Fig. 5b. The surface property of the cellulose membrane is dependent on many factors like composition, porosity, heterogeneity, surface roughness etc. [10]. The hydrophilicity of the cellulose membrane could be attributed to the zinc, which is derived from the hygroscopic salt zinc chloride during the fabrication process. Similarly, Zhang et al. [10] reported lower contact angle for the regenerated cellulose film produced from corn stalk pith using zinc chloride solution. However, Haque et al. [17] showed the hydrophobic nature of the cellulose film to the modification of cellulose by formic acid and the accumulation of insoluble particles from cotton gin trash. However in the present study, the cellulose membrane was produced by partial removal of lignin. Therefore, the hydrophilic nature of the cellulose membrane produced in the present study was related to the increased presence of zinc and cellulose. Moreover, hydrophilicity is an important characteristic of a potential carrier for drug delivery applications.
Antibacterial Activity of Cellulose Membrane
The antibacterial activity of the cellulose membrane was measured by disc diffusion method. It was evaluated against the growth of Gram negative bacteria (E. coli). The cellulose membrane was also tested for the loading of kanamycin. The concentration of kanamycin loaded onto the cellulose membrane was 50 µg/mL and the dosage of kanamycin used in the present study was slightly higher than the minimum inhibitory concentration reported by Canil et al. [33] against E. coli K12. For testing the effect of kanamycin against E. coli, the Whatmann No.1 filter paper dipped in the kanamycin solution was used. The filter paper was chosen for the study as it was not associated with antibacterial activity. The antibacterial activity of the membrane and the effect of loading kanamycin against E. coli are shown in Table 2. In the case of control containing cellulose membrane without kanamycin, the inhibition zone was prominent. This result showed that the antibacterial activity cellulose membrane was attributed to the concentration of zinc. The presence of higher amount of zinc was observed in the cellulose membrane as indicated by FTIR and XRD spectra in the present study. As a result of increased zinc available in the cellulose membrane, the agglomeration of zinc ions was responsible for the enhanced antibacterial resistance of the cellulose membrane. Moreover, the largest zone of inhibition was seen for the cellulose membrane loaded with kanamycin. However, the inhibition zone observed for the kanamycin alone was smaller as compared to that of kanamycin loaded onto the cellulose membrane. The enhanced inhibition zone obtained for the cellulose membrane showed its increased affinity towards kanamycin as compared to that for filter paper. In few reports on lignin based nanomaterials, studies investigated the potential application of lignin for the controlled release of drugs like trans-resveratrol and polyphenols [34, 35]. In the present study, the cellulose membrane was fabricated by spin coating process which facilitated partial lignin removal. Therefore, the residual lignin in the cellulose membrane was anticipated to be involved in the binding and release of kanamycin. Xie et al. [36] produced an antibacterial nanocomposite film by blending bacterial cellulose with graphene oxide-copper oxide (GO–CuO) nanohybrid. The preparation of GO–CuO nanohybrid involved mixing reaction of graphene oxide nanosheets with copper chloride at high temperature. However, the current study employed a facile route through dissolution in ZnCl2 solution for the production of cellulose membrane with the incorporation of antibacterial property. Zinc ion was observed to be cytotoxic to bacteria due to the induction of oxidative stress in the microbial cell for shutting down the vital cellular functions. More importantly, the zinc ion was predicted to be involved in the alteration of cell membrane permeability thereby causing lipid peroxidation and disruption of plasma membrane [37]. Wahid et al. [38] developed an antibacterial nanocomposite film from bacterial cellulose by incorporating zinc oxide nanoparticles. Wu et al. [25] reported improved resistance of cellulosic paper against the growth of E. coli and S. aureus when the coating of zinc oxide particles was applied. The cytotoxicity of zinc ions was shown to be proportional to its availability. In the current study, the cellulose membrane prepared using 68% zinc chloride solution showed significant antibacterial effect against E. coli due to the elevated levels of zinc associated in the membrane. More importantly, the cellulose membrane could be used for drug delivery application, as it showed high affinity to the binding and release of antimicrobial drug kanamycin.
Conclusion
The valorization of CMD was carried out for the production of cellulose membrane. The CMD was not suitable for any application, as it formed aggregates in aqueous solution. The cellulose solubilisation of the pretreated CMD in ZnCl2 is a promising approach for conversion of the CMD into value added materials. The novel aspect of the process is the simultaneous removal of lignin and formation of cellulose membrane during spin coating of Zn-CMD solution with NaCl. The roles of NaCl and shear force facilitating the lignin removal in the spin coating process are interesting aspects for the further development of the process. The mechanical and antimicrobial properties of the cellulose membrane holds promise for low cost membrane production for biomedical, pharmaceutical and drug delivery applications.
References
Rodrigues MO, Abrantes N, Gonçalves FJM, Nogueira H, Marques JC, Gonçalves AMM (2019) Impacts of plastic products used in daily life on the environment and human health: what is known? Environ Toxicol Pharmacol 72:103239
Haider TP, Völker C, Kramm J, Landfester K, Wurm FR (2019) Plastics of the future? The impact of biodegradable polymers on the environment and on society. Angew Chem Int Ed 58(1):50–62
Rol F, Belgacem MN, Gandini A, Bras J (2019) Recent advances in surface-modified cellulose nanofibrils. Prog Polym Sci 88:241–264
Sharma A, Thakur M, Bhattacharya M, Mandal T, Goswami S (2019) Commercial application of cellulose nano-composites–a review. Biotechnol Rep 21:e00316
Swensson B, Larsson A, Hasani M (2020) Dissolution of cellulose using a combination of hydroxide bases in aqueous solution. Cellulose 27(1):101–112
Brehm M, Pulst M, Kressler J, Sebastiani D (2019) Triazolium-based ionic liquids: a novel class of cellulose solvents. J Phys Chem B 123(18):3994–4003
Xu Q, Chen C, Rosswurm K, Yao T, Janaswamy S (2016) A facile route to prepare cellulose-based films. Carbohyd Polym 149:274–281
Bi Z, Lai B, Zhao Y, Yan L (2018) Fast disassembly of lignocellulosic biomass to lignin and sugars by molten salt hydrate at low temperature for overall biorefinery. ACS Omega 3(3):2984–2993
Lara-Serrano M, Morales-delaRosa S, Campos-Martín JM, Fierro JL (2020) High enhancement of the hydrolysis rate of cellulose after pretreatment with inorganic salt hydrates. Green Chem. https://doi.org/10.1039/D0GC01066A
Zhang H, Chen K, Gao X, Han Q, Peng L (2019) Improved thermal stability of regenerated cellulose films from corn (Zea mays) stalk pith using facile preparation with low-concentration zinc chloride dissolving. Carbohyd Polym 217:190–198
Wang X, Zhou J, Pang B, Zhao D (2019) Rapid microwave-assisted ionothermal dissolution of cellulose and its regeneration properties. J Renew Mater 7(12):1363–1380
Nakasone K, Kobayashi T (2016) Cytocompatible cellulose hydrogels containing trace lignin. Mater Sci Eng, C 64:269–277
Zmejkoski D, Spasojević D, Orlovska I, Kozyrovska N, Soković M, Glamočlija J, Dmitrović S, Matović B, Tasić N, Maksimović V, Sosnin M (2018) Bacterial cellulose-lignin composite hydrogel as a promising agent in chronic wound healing. Int J Biol Macromol 118:494–503
Vanitjinda G, Nimchua T, Sukyai P (2019) Effect of xylanase-assisted pretreatment on the properties of cellulose and regenerated cellulose films from sugarcane bagasse. Int J Biol Macromol 122:503–516
Ranjithkumar M, Ravikumar R, Sankar MK, Kumar MN, Thanabal V (2017) An effective conversion of cotton waste biomass to ethanol: a critical review on pretreatment processes. Waste Biomass Valor 8(1):57–68
McIntosh S, Vancov T, Palmer J, Morris S (2014) Ethanol production from cotton gin trash using optimised dilute acid pretreatment and whole slurry fermentation processes. Biores Technol 173:42–51
Haque ANMA, Remadevi R, Wang X, Naebe M (2020) Physicochemical properties of film fabricated from cotton gin trash. Mater Chem Phys 239:122009
Liu W, Liu S, Liu T, Liu T, Zhang J, Liu H (2019) Eco-friendly post-consumer cotton waste recycling for regenerated cellulose fibers. Carbohyd Polym 206:141–148
Sluiter A, Hames B, Ruiz R, Scarlata C, Sluiter J, Templeton D, Crocker D (2010) Determination of structural carbohydrates and lignin in biomass. Lab Anal Proced 1617:1–16
Wahid F, Wang HS, Zhong C, Chu LQ (2017) Facile fabrication of moldable antibacterial carboxymethyl chitosan supramolecular hydrogels cross-linked by metal ions complexation. Carbohyd Polym 165:455–461
Sadeghifar H, Venditti R, Jur J, Gorga RE, Pawlak JJ (2017) Cellulose-lignin biodegradable and flexible UV protection film. ACS Sustain Chem Eng 5(1):625–631
Santucci BS, Bras J, Belgacem MN, da Silva Curvelo AA, Pimenta MTB (2016) Evaluation of the effects of chemical composition and refining treatments on the properties of nanofibrillated cellulose films from sugarcane bagasse. Ind Crops Prod 91:238–248
Bellesia G, Gnanakaran S (2013) Sodium chloride interaction with solvated and crystalline cellulose: sodium ion affects the cellotetraose molecule and the cellulose fibril in aqueous solution. Cellulose 20(6):2695–2702
Jiang Z, Yi J, Li J, He T, Hu C (2015) Promoting effect of sodium chloride on the solubilization and depolymerization of cellulose from raw biomass materials in water. Chemsuschem 8(11):1901–1907
Wu W, Liu T, He H, Wu X, Cao X, Jin J, Sun Q, Roy VA, Li RK (2018) Rhelogical and antibacterial performance of sodium alginate/zinc oxide composite coating for cellulosic paper. Colloids Surf B 167:538–543
Abidi N, Cabrales L, Haigler CH (2014) Changes in the cell wall and cellulose content of developing cotton fibres investigated by FTIR spectroscopy. Carbohyd Polym 100:9–16
Plácido J, Capareda S (2014) Analysis of alkali ultrasonication pretreatment in bioethanol production from cotton gin trash using FT-IR spectroscopy and principal component analysis. Bioresour Bioprocess 1(1):23
Yue Y, Han J, Han G, Zhang Q, French AD, Wu Q (2015) Characterization of cellulose I/II hybrid fibers isolated from energycane bagasse during the delignification process: morphology, crystallinity and percentage estimation. Carbohyd Polym 133:438–447
Tan X, Chen L, Li X, Xie F (2019) Effect of anti-solvents on the characteristics of regenerated cellulose from 1-ethyl-3-methylimidazolium acetate ionic liquid. Int J Biol Macromol 124:314–320
Bindu P, Thomas S (2014) Estimation of lattice strain in ZnO nanoparticles: X-ray peak profile analysis. J Theor Appl Phys 8(4):123–134
Weyell P, Beekmann U, Küpper C, Dederichs M, Thamm J, Fischer D, Kralisch D (2019) Tailor-made material characteristics of bacterial cellulose for drug delivery applications in dentistry. Carbohyd Polym 207:1–10
Khamrai M, Banerjee SL, Paul S, Ghosh AK, Sarkar P, Kundu PP (2019) A mussel mimetic, bioadhesive, antimicrobial patch based on dopamine-modified bacterial cellulose/rGO/Ag NPs: a green approach toward wound-healing applications. ACS Sustain Chem Eng 7(14):12083–12097
Canil A, Saleh N, Chan J (2019) Knockout of OmpF and OmpC does not affect kanamycin susceptibility in two different strains of Escherichiacoli K-12. UJEMI 24:1–7
Ciolacu D, Oprea AM, Anghel N, Cazacu G, Cazacu M (2012) New cellulose–lignin hydrogels and their application in controlled release of polyphenols. Mater Sci Eng C 32(3):452–463
Liu R, Dai L, Zou Z, Si C (2018) Drug-loaded poly (L-lactide)/lignin stereocomplex film for enhancing stability and sustained release of trans-resveratrol. Int J Biol Macromol 119:1129–1136
Xie YY, Hu XH, Zhang YW, Wahid F, Chu LQ, Jia SR, Zhong C (2020) Development and antibacterial activities of bacterial cellulose/graphene oxide-CuO nanocomposite films. Carbohyd Polym 229:115456
Siddiqi KS, ur Rahman A, Husen A (2018) Properties of zinc oxide nanoparticles and their activity against microbes. Nanoscale Res Lett 13(1):1–13
Wahid F, Duan YX, Hu XH, Chu LQ, Jia SR, Cui JD, Zhong C (2019) A facile construction of bacterial cellulose/ZnO nanocomposite films and their photocatalytic and antibacterial properties. Int J Biol Macromol 132:692–700
Acknowledgements
The authors greatly acknowledge IIT Madras for awarding half time teaching assistantship to Vignesh Natarajan. The authors acknowledge the Director, PSG Institute of Advanced Studies for the support rendered in characterization studies.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Vignesh, N., Suriyaraj, S.P., Selvakumar, R. et al. Facile Fabrication and Characterization of Zn Loaded Cellulose Membrane from Cotton Microdust Waste and its Antibacterial Properties—A Waste to Value Approach. J Polym Environ 29, 1651–1662 (2021). https://doi.org/10.1007/s10924-020-02021-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10924-020-02021-1