Abstract
A novel biosorbent yttrium/sodium alginate (Y/SA) hydrogel was prepared by the crosslinking of sodium alginate (SA) and yttrium (Y) ions and characterized by Scanning Electron Microscopy (SEM) and Energy Dispersive Spectroscopy (EDS), X-ray Diffraction (XRD), Fourier Transform-Infrared (FTIR), UV visible diffuse reflectance spectroscopy (UV–Vis DRS) and Thermogravimetric Analysis (TGA), respectively. The important parameters affecting the adsorption of Congo Red (CR) and Weak Acid Brilliant Blue RAW (WABB RAW) onto Y/SA were investigated systematically, respectively. The results show that Y/SA gel beads have super-strong adsorption performance for CR and WABB RAW dyes in a wide pH range of 2.0–10.0. The equilibrium data fitted by Langmuir and Freundlich models were found to completely follow Langmuir model where the obtained maximum adsorption capacities of 1567 and 1087 mg/g for CR and WABB RAW were very close to the experimental adsorption capacities, respectively, showing the adsorption of monomolecular layer. The adsorption kinetic data of two dyes by Y/SA fully accorded with the pseudo-second-order rate equation and adsorption processes were mainly controlled by intraparticle diffusion. The results of thermodynamic study indicate the feasible, spontaneous and exothermic nature of adsorption reaction. The further study revealed that electrostatic adsorption, hydrogen bonding and ion exchange reaction were the predominant adsorption for dye removal from aqueous solution by Y/SA gel beads. As a highly efficient, green and conducive to solid–liquid separation biosorbent, Y/SA hydrogel has great potential-application prospects for the purification of high-concentration dyestuff effluent.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
A large number of synthetic dyes have been widely used in various industrial production, such as textile printing and dyeing, dyeing of leather, paper, plastic, printing ink, cosmetics, food, etc., but more than 15% of them are lost as waste liquid [1]. The dyes with complex aromatic ring structure are difficult to be degraded, and have potential toxicity and side effects. When the industrial waste liquid containing these high-concentration dyes is discharged into nearby water body, it will not only seriously affect the photosynthesis of aquatic organisms due to its deep color, disturb and damage to the normal aquatic environment, but also cause harm to human health [2]. As an anionic dye with diazo structure, Congo red (CR) has carcinogenicity and mutagenicity [3, 4]. Weak acid brilliant blue RAW (WABB RAW) is also an anionic dye with anthraquinone structure, which is commonly used for dyeing wool, silk, nylon and their blends, etc. Due to the environmental side of their structure, color and concentration, the effluent liquor containing these dyes must be effectively treated before discharge. In common wastewater treatment technologies, adsorption technology is considered one of the most economical and effective methods owing to its simple process, convenient operation and high efficiency. So a large number of related studies have been reported in recent years [5, 6]. The key of adsorption method depends on the selection of adsorbent. High-effect adsorbent can not only reduce the investment and operation cost of treatment process, but also usefully improve the treatment efficiency. Traditional adsorbents, activated carbon, zeolite and so on, have disadvantages of high treatment cost and limited adsorption capacity, which will limit their popularization and application [7, 8]. Therefore, based on the characteristics of different dye-discharged wastewater, the development projects about adsorbents with abundant sources and low costs like some industrial wastes, agricultural by-products and biosorbents, have been widely performed for removing dyes from aqueous solutions at present [9,10,11,12].
Sodium alginate (SA) is a natural polysaccharide ionic polymer from algae, which has many advantages of biosafety, biocompatibility, no-toxicity, regeneration and abundant resources. SA has strong hydrophilicity and can react with polyvalent cations to form polymer gel beads [13, 14]. This property makes SA an ideal framework for preparing alginate gel beads and alginate-based composite adsorbents [15]. A large number of –OH and –COOH groups on the linear molecular chain of SA can adsorb metal ions and organic pollutants from industrial effluent through coordination, ion exchange and electrostatic interaction. Hence, the preparation and adsorption application of environmentally-friendly alginate-based gel composites have been extensively studied in recent years [16,17,18].
Based on the special electronic layer structure of rare-earth elements, the high charge of their ions and coordination to oxygen, and the coagulation effect of rare-earth salts, the research and application of rare earths in the preparation of water treatment materials have attracted more and more attention in recent years. The exchange reactions between rare-earth ions and ligands are very fast in solution. Therefore, the combination of rare-earth ions with organic polymers and rare-earth metal-based composite adsorbents can play a synergistic role and maximize the adsorption and coagulation effect [19,20,21].
In this study, biopolymer yttrium(III)/alginate (Y/SA) gel beads were prepared and used to remove Congo Red (CR) and Weak Acid Brilliant Blue RAW (WABB RAW) dyes in wastewater respectively. The effects of some important factors on the adsorbent performance were explored through a series of adsorption experiments. The performance and adsorption mechanism of the adsorbent were evaluated by the characterization of materials, adsorption kinetics and isothermal studies. We hope that this environment-friendly biosorbent can have superb adsorption capacities for anionic dyes in high- concentration industrial wastewater.
Materials and Methods
Materials
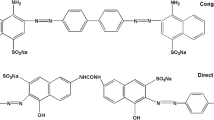
Sodium alginate (SA, A.R.) and Yttrium nitrate hexahydrate (Y(NO3)3·6H2O, A.R.) were purchased from Shandong Xiya Chemical Industry Co., Ltd. and Tianjin Guangfu Fine Chemical Research Institute, China, respectively. Congo Red (CR, C32H22N6O6S2Na2, relative molecular mass Mr = 696.68 g/mol) and Weak Acid Brilliant Blue RAW (WABB RAW, C22H28N2O8S2Na2, Mr = 678.68 g/mol) dyes were bought from Shanghai Jiaying Chemical Co., Ltd., China. The maximum adsorption wavelength (λmax) of CR and WABB RAW are at 498 nm and 566 nm, respectively. Their molecular structures are presented in Fig. 1.
Preparation and Characterization of Y/SA Biopolymer
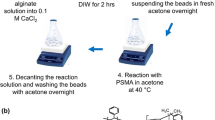
The optimum process for preparation of Y/SA gel beads was as follows (in Fig. 2): the uniformly stirred 2 g/100 mL SA solution was dripped slowly into 4 g/100 mL Y(NO3)3 solution through the syringe at 25 °C, and the Y/SA gel beads were gradually generated. After crosslinking reaction for 2 h, the milk-white gel beads were removed, washed and dried, finally the beige Y/SA biosorbent was obtained.
The surface feature and elemental distribution of SA and Y/SA gel beads were characterized using scanning electron microscopy and energy dispersive spectroscopy (SEM–EDS, SU8010, Hitachi Inc., Japan). The molecular structures and crystallinity of materials were analyzed by a Nicolet Nexus 6700-type Fourier transform infrared spectrometer (FTIR, KBr tabletting method, Thermo Fisher Scientific Inc., U.S.A), UV-2550 ultraviolet visible diffuse reflection spectrometer (UV–Vis DRS, Shimadzu Inc., Japan) and a PW-1830 X-ray diffractometer (XRD, Philips, Holland) by applying monochromated Cu Ka radiation (λ= 0.154056 nm) in the 2θ range of 5–80°. Thermal stability of materials was investigated by DTG-60H Thermogravimetric analyzer (TGA, Shimadzu Inc., Japan).
Adsorption Tests
In order to investigate the adsorption efficiency of dyes onto Y/SA in detail, a series of adsorption experiments were carried out including optimization of adsorption conditions, adsorption kinetics and isothermal adsorption tests. A 0.05 g adsorbent was added to 25 mL dye solution with a certain concentration and oscillated to the adsorption equilibrium at 160 rpm and a fixed temperature. The dye concentration in a solution after adsorption was determined at the maximum wavelength (498 nm and 566 nm) of dye on an UV/Vis spectrophotometer (U-2900E, Japan), respectively. The linear regression equations of the standard curves used for the determination of dye concentration are as follows: A = 0.0223C + 0.0027 and the correlation coefficient R2 = 0.9998 for CR, and A = 0.0148C + 0.068 and R2 = 0.9999 for WABB RAW, respectively. Through 11 parallel blank determinations, the obtained detection limits of CR and WABB RAW are 0.27 and 0.20µ g/mL, respectively. The adsorption capacity (Qe, mg/g) and removal efficiency (R, %) of dyes by adsorbent are calculated from the following formulas, respectively.
where C0 and Ce are the dye concentration (mg/L) in a solution before and after adsorption, respectively. V (L) is the volume of each dye solution and m (g) is the adsorbent dosage. For each sample, three parallel determinations were completed. The mean value, whose relative standard deviation was controlled below 0.2%, was used as the result for analysis.
Determination on Point of Zero Charge (pHpzc)
To further understand the charged properties of adsorbent surface, the point of zero charge (pHpzc) of Y/SA was measured according to the following method. A 0.01 mol/L NaCl solution of 10 mL was added to a series of 50 mL conical flasks. The pH value of each solution was adjusted in the range of 2.0–12.0 (named as pH0), and 0.05 g Y/SA biosorbent was added in each solution. After 24 h of continuous oscillation at 25℃, the series of mixed solution were withdrawn and the pH of each solution was again determined (named as the pH*). The relation curve of ∆pH (pH0–pH*) versus pH0 was plotted, and the intersection point between ∆pH and pH0 was the point of zero charge (pHpzc) of Y/SA adsorbent.
Results and Discussion
Characterizations of SA and Y/SA Materials
The surface morphology and element distribution of SA powder and Y/SA gel beads were analyzed by SEM and EDS (in Fig. 3). It is seen from Fig. 3a that SA is composed of irregular particles with different sizes. Figure 3b shows that the wet Y/SA gel beads synthesized in the solution are milky white translucent spheres with a diameter of about 3 mm. After drying, Y/SA gel beads turned to beige spheres with a diameter of about 1 mm. The surface of each gel ball emerges irregular deep grooves and wrinkles. This is due to the ion exchange between Y(III) ions and a large number of Na+ in SA molecules, and further crosslinking of SA molecular chain with Y(III) ions, resulting in the formation of polymer gel beads with three-dimension network structure and unique surface morphology. The polymer adsorbent with such structure is very conducive to the improvement of its adsorption performance and solid–liquid separation. It is found from the EDS analysis of Fig. 3d that the content of Na element in Y/SA is significantly lower than that in SA (Fig. 3c). There is not Y element in SA (Fig. 3c), but its content in Y/SA increases significantly, indicating that most of Na+ ions in SA molecules was exchanged by Y(III) ions and Y/SA biosorbent was successfully synthesized.
XRD Patterns
The crystal structures of SA and Y/SA biopolymer analyzed by XRD are given in Fig. 4a. The characteristic diffraction peaks of SA were respectively at 2θ = 13.5° and 23°, and there was a large steamed bread peak in the 2θ range of 10°–30°. Similar results was also been reported in relevant literature [22]. Compared with the XRD patter of SA, the two broad new characteristic peaks appearing around 2θ = 17.5° and 38.3° represented the formation of yttrium alginate in the XRD diagram of Y/SA, further confirming that the formation of Y/SA gel beads completely changed the crystalline structure of SA.
FTIR Analysis
Figure 4b shows the FTIR spectra of SA and Y/SA. The characteristic absorption bands for SA are assigned as follows: 3440 cm−1 (–OH stretching vibration), 2930 cm−1 (–CH stretching vibration), 1631 and 1404 cm−1 (the asymmetric and symmetric stretching peaks of –C=O and –COO−), 1033 cm−1 (C–O–C stretching vibration) [21, 23]. Compared with FTIR spectra of SA, the characteristic peak of –COO− group disappeared in Y/SA spectra, but sharper absorption peak appeared near 1384 cm−1. The stretching vibration peak of C–O–C was moved from 1033 to 1038 cm−1. Other absorption peaks also changed in intensity [21]. The variation of these characteristic peaks further confirmed that Y(III) ions reacted with the –COO− groups on SA molecules and formed water-borne gel beads, and the oxygen between the rings on SA molecular chain also participated in the polymerization reaction.
UV–Vis DRS Analysis
It can be observed from the UV–Vis DRS spectra of Fig. 4c that the spectrum of SA has no absorption peak in the range of 200–800 nm, while that of Y/SA has two absorption peaks at 283 and 330 nm, respectively. This fully indicated that a new compound, namely Y/SA, was synthesized by the reaction of SA and Y ion(III).
Thermogravimetric Analysis (TGA)
To know the thermal stability of SA and Y/SA better, the thermogravimetric analysis of SA and Y/SA was carried out and the results are given in Fig. 4d. With the rise of temperature, the change of Y/SA mass mainly underwent three stages of decline. The rate of mass decline was slow before 180 °C, and the Y/SA mass decreased by 21% due to the evaporation of free water and bound water. The sample underwent a rapid decomposition stage from 200 to 350 °C, and the mass reduced by 31%. The thermal decomposition rate slowed down gradually in the range of 350 to 600 °C and then entered a relatively stable state after 600 ℃. However, the decomposition rate of SA was very fast before 100 °C. The results of TGA analysis illustrate that the stability of Y/SA is significantly better than that of SA, and Y/SA adsorbent with good thermal stability before 150 °C is more convenient to store and use in ordinary temperature.
Effect of Solution pH and Adsorbent Dosage
The water acidity is one of the most important factors affecting the adsorbent performance. The effect of initial solution pH on the adsorption of CR and WABB RAW by Y/SA is shown in Fig. 5a. When the pH of dye solution was 2.00, the adsorption capacity (Qe) and removal rate (R%) reached the maximum values with 1500 mg/g and 100% for CR and 980 mg/g and 98% for WABB RAW, respectively. When the pH value increased from 2.0 to 10.0, the values of Qe and R only decreased slightly. The removal rates of CR and WABB RAW could still reach 95% and 91% at pH = 10.0, respectively, suggesting that Y/SA has excellent adsorption performance in a wide range of pH and can be used for highly efficient treatment of dye effluent with different acidity and alkalinity. After the solution pH was greater than 10.0, the values of Qe and R decreased rapidly. The highly pH-dependent adsorption is primarily related to the surface properties of Y/SA polymer and species of dyes. CR and WABB RAW dye molecules exist as anions in solution because of containing two −SO3− groups in each molecule. The positive charge density on the surface of Y/SA increases with the reduction of solution pH owing to the protonation of rich –OH groups and the existence of Y(III) ions with high positive charge on Y/SA gel beads. The point of zero charge (pHpzc) of Y/SA was determined to be around 6.15, indicating that the surface of Y/SA is positively charged at pH < pHpzc, which resulted in strongly electrostatic adsorption between Y/SA and dye anions. In contrast, the surface of gel ball is negatively charged at pH > pHpzc which generated electrostatic repulsion between adsorbent and the dye anions and reduced the adsorption capacity. However, the Qe values of two dyes decreased significantly after pH > 10.0, which indicated that there were other adsorption effects such as hydrogen bonding and ion exchange in the pH range of 6.15–10.0. The pH values of actual CR and WABB RAW dye solutions are about 9.5 and 7.0, respectively, so the acidity of dye solutions does not need to be adjusted and can be directly treated with Y/SA adsorbent, making the adsorption operation easier, faster and more efficient.
The effect of Y/SA dosage on the removal of CR and WABB RAW dye with 3000 and 2000 mg/L initial concentrations respectively is presented in Fig. 5b. For dye solutions with fixed mass concentration, the dosage of adsorbent directly affects the adsorption capacities and removal rates of dyes. Figure 6b shows that the adsorption capacities of two dyes on Y/SA increase first and then diminish with the increase of adsorbent dosage, but the removal rates of dyes increase rapidly at first and then slowly, so the platforms appear at the upper parts of the curves. When the adsorbent dosage was 0.04 g, the adsorption capacity of Y/SA for CR reached the maximum value of 1478 mg/g, but the removal rate was only 79%. When the adsorbent dosage was 0.05 g, the adsorption capacity for WABB RAW reached the maximum value of 953 mg/g and the corresponding removal rate can exceed 95%, and the removal rate of CR can also exceed 94%. Considering the adsorption efficiency and the convenience of practical operation, the dosage of Y/SA adsorbent was chosen to be 0.05 g.
Adsorption Kinetic and the Effect of Temperature
The process of dye adsorption is mainly accomplished in two steps: the first step is the diffusion of dye anions from solution to adsorbent surface, and the second step is the migration and adsorption reaction from the outer surface to the inner surface of adsorbent particles. However, the change of adsorption rate given by different processes is totally different, which is also related to the adsorption temperature. In order to investigate the adsorption kinetics and the effect of temperature on it, the adsorption amount was plotted as a function of time for CR and WABB RAW, respectively, as shown in Fig. 6a and b. It can be seen clearly from Fig. 6a and b that the adsorption rates of two dyes were very fast, and the adsorption amounts reached 1326 mg/g and 880 mg/g in 30 min and accounted for more than 88% of the total dye removal rates. Afterwards the adsorption rate slowed down obviously. The fast adsorption kinetics of dye-Y/SA system is very beneficial to the actual wastewater treatment because of the shortening of adsorption equilibrium time, the improvement of treatment efficiency and the reduction of operation cost. The adsorption of two dyes was in kinetic equilibrium after 90 min.
In addition, It was found from Fig. 6a and b that the increase in temperature from 298 k to 328 K caused a significant decrease in the adsorption rate of CR, especially after 30 min of adsorption. However, the adsorption of WABB RAW evidently increased with the increase of temperature before 30 min as shown in Fig. 6b, thereafter, the influence of temperature was very little, even the value of Qt decreased slightly, especially after adsorption equilibrium. It shows that room temperature is beneficial to the effective equilibrium adsorption of two dyes. Considering the adequacy of adsorption, 120 min of adsorption equilibrium time were selected for further study, and the adsorption capacities of CR and WABB RAW dyes can reach 1439 and 943 mg/g with removal rates of 95.9 and 94.3%, respectively.
Moreover, the adsorption capacity of CR by Y/SA was significantly higher than that of WABB RAW in equilibrium (Fig. 6a). This difference may be mainly due to the fact that CR molecule contains more active groups, such as two –NH2 groups and –N = N– groups, than WABB RAW molecule, which made the interaction between CR anion and adsorbent stronger. At the same time, it was also related to dimensions of dye organic chain [24].
The experimental data at different temperatures were processed using the pseudo-first-order, pseudo-second-order rate models and intraparticle diffusion model respectively to clarify the kinetic mechanism of adsorption process, and the corresponding expressions are as follows [25,26,27], respectively.
where k1 and k2 are the rate constants of pseudo-first-order and pseudo-second-order models, min−1 and g/(mg⋅min), respectively. Kp is the intraparticle diffusion rate constant, mg/(g⋅min1/2). Some parameters, the correlation coefficients (R2) and the error values including sum of squared errors (SSE) and root mean squared error (RMSE) [2, 28] obtained by pseudo-first-order and pseudo-second-order kinetic models are listed in Table 1. It can be known from Table 1 that the pseudo-second-order rate model for the adsorption of two dyes shows a better fit (R22 > 0.999) to the experimental data than the pseudo-first-order model (R12 > 0.854) at different temperatures (298–328 K). The pseudo-first-order model is suitable for the initial fast adsorption stage due to a large concentration difference between the adsorbent surface and the solution. This also elucidates that the whole adsorption processes of two dyes onto Y/SA are not only controlled by external mass transfer. However, the adsorption of both fast and slow processes fits well with the pseudo-second-order model, which indicates that the adsorption of two dyes occurs through internal and external mass transfer mechanism. The equilibrium adsorption capacities (Qe,2) are close to the experimental values, showing that the adsorption of CR and WABB RAW onto Y/SA occurred by the mechanisms of liquid film diffusion, pore migration and monolayer buildup of adsorption [29].
To identify the rate-limiting steps for the whole adsorption process of CR and WABB RAW onto Y/SA, according to Eq. (5), fitted data of Qt versus t1/2 and the Weber and Morris plots present the three distinct linearity in Fig. 6c and Table 1, respectively. All regression lines do not pass the origin. The values (R12 > 0.939 and R22 > 0.983) of the correlation coefficient in the initial part (2–10 min) and the second part (10–30 min) indicate that the intraparticle diffusion is a rate-limiting step, but not the only one. This further elucidates that both liquid film diffusion and intraparticle diffusion occur simultaneously. The third part is a dynamic adsorption equilibrium stage after 60 min as shown in Fig. 6c.
Adsorption Isotherms
The relationship between adsorption capacity (Qe) and equilibrium concentration (Ce) of dyes between solid and liquid phases at adsorption equilibrium is given in Fig. 6d. The adsorption capacities of two dyes on Y/SA decrease with increasing temperature from 298 to 328 K, especially in the high concentration regions in Fig. 6d. This is consistent with the effect of temperature on adsorption kinetics, illustrating that room temperature is more conducive to the highly efficient adsorption. The adsorption behavior has been commonly described using two models, namely Langmuir and Freundlich models, whose linear equations are expressed as follows:
where Qm is the maximum adsorption capacity, mg/g; KL is the Langmuir adsorption coefficient, L/mg; KF and n are the Freundlich empirical constants, respectively; RL is called a separation factor defined by Langmuir model. The fitting results, correlation coefficients (R2) and the values of sum of squared error (SSE) and root mean squared error (RMSE) of all equilibrium data of CR and WABB RAW adsorption on Y/SA fitted by the two models are listed in Table 2. The Langmuir model was in good agreement with the equilibrium data for the CR and WABB RAW adsorption (R2 > 0.99) and the values of Qe,exp were very close to those of Qm, suggesting the dye adsorption of monomolecular layer and the existence of chemical adsorption. The values of KL diminished with rise in temperature, affirming the exothermic nature of dye adsorption which agreed with the kinetic results. The maximum adsorption capacities of CR and WABB RAW on Y/SA can reach 1567 and 1000 mg/g at 298 K, respectively.
Some studies on adsorbing performance of various materials for CR and the corresponding maximum adsorption capacities have been reported [3, 30,31,32,33,34,35,36,37,38,39,40,41], which are listed in Table 3. Compared with various adsorption materials presented in Table 3, Y/SA biosorbent shows more significant advantages and higher cost-effectiveness for CR adsorption, and has simpler preparation methods and greater potential for popularization and application as a superior green adsorbent.
The value of separation factor RL can judge whether the adsorption type is favorable (0 < RL < 1) or unfavorable (RL > 1). It is observed from Fig. 6e that all values of RL for the adsorption of two dyes were in the range of 0.0024–0.112, confirming to be favorable for the adsorption of CR and WABB RAW on Y/SA. However, absorption data of two dyes on Y/SA did not have a good fit for Freundlich model (R2 > 0.629). This may be because the Freundlich adsorption model is an empirical model, which refers to multi-layer adsorption. Nevertheless, the values of n less than 10 from Freundlich model (in Table 2) also show the advantage of adsorption reaction.
Adsorption Thermodynamics
According to van’t Hoff equation and the dependence of Langmuir adsorption coefficient (KL) on temperature (T, K), the standard Gibbs free energy change (ΔG°, kJ/mol), standard enthalpy change (ΔH°, kJ/mol) and entropy change (ΔS°, kJ/mol⋅K) can be calculated by the Eqs.(9) and (10) [42].
The linear curve of lnK versus 1/T is presented in Fig. 6f and the values of ΔH° and ΔS° calculated by the slope and intercept and ΔG° are given in Table 2. The negative values of ΔG° and ΔH° for CR and WABB RAW dyes revealed the spontaneous and exothermic nature of adsorption reaction. The positive values of ΔS° indicated the increase of confusion degree on solution-gel beads interface. This might be owing to the release of large amounts of water molecules hydrated on the gel beads surface through substitution of dye macromolecules.
Discussion on Adsorption Mechanism
To explore the interaction between Y/SA adsorbent and dye molecules, the FTIR and UV–Vis DRS spectra of Y/SA before and after adsorbing CR and WABB RAW are presented in Fig. 7. The main changes in the spectra of Y/SA after dye adsorption in Fig. 7a are that the sharp stretching vibration peaks at 1384 cm−1 almost disappear, while the new absorption peaks appear at 1404 cm−1 and 1410 cm−1, respectively. The intensity or position of other characteristic peaks also varies slightly. These changes fully explain the interaction between Y/SA gel beads and dye molecules. The significant changes of the UV–Vis DRS spectra of Y/SA after dye adsorption were shown in Fig. 7b. The original two absorption peaks disappeared and two new absorption peaks appeared at 349 and 504 nm for Y/SA-CR and at 312 and 603 nm for Y/SA-WABB RAW, respectively, and the absorption peaks became wider, further proving the interaction between Y/SA and dye molecules.
Combined with the results of material characterization and adsorption studies, it might be considered that the adsorption of CR and WABB RAW dyes onto Y/SA gel beads was mainly accomplished by strong electrostatic adsorption at pH < pHpzc (around 6.15) as follows:
RSO3Na (aq., dye molecule) → R-SO3− + Na+
SA–Y–OH + H+ → SA–Y–OH2+ (Under acidic conditions).
SA–Y–OH2+ + R–SO3− → SA–Y–OH2+ −O3S–R.
When the solution pH was in the range of 6.15–10.0, the adsorption of dye anions onto Y/SA gel beads was first related to the ion-exchange reaction between hydroxyl groups bonded to yttrium (Y–OH) and dye anions (R–SO3−) as shown in Fig. 8 [21], then to the hydrogen bonding of multiple sites between dye anions with –NH2, –N = N– and –SO3− functional groups and Y/SA polymer with rich –OH and –COOH groups (in Fig. 8) [3, 31]. In addition, the adsorption capacity of Y/SA adsorbent for CR was larger than that for WABB RAW. This may be due to the fact that there are still two –NH2 groups and –N = N– groups in CR molecules, which can have more binding sites to interact with the adsorbent than WABB RAW, except for two sulfonic groups in both dye molecules.
Conclusions
The biosorbent Y/SA gel beads synthesized by droplet polymerization using SA as raw material and Yttrium ions as crosslinker exhibited a superior adsorption performance for direct dye CR and acidic dye WABB RAW from aqueous solutions. Through the study of some important factors affecting the adsorption, it is found that the adsorbent Y/SA has ultrahigh adsorption capacity and removal efficiency for two dyes in a wide pH range of 2.0–10.0. The analysis of experimental kinetic data revealed that the adsorption processes of two dyes nicely followed the pseudo-second-order rate model (R2 > 0.999) and were significantly controlled by the intraparticle diffusion in the fast adsorption stage. The good fitting of equilibrium data for Langmuir model in the given adsorption systems (R2 > 0.998) indicated the monolayer formation of dye adsorption onto Y/SA gel beads, and the obtained maximum adsorption capacities were 1567 mg/g for CR and 1087 mg/g for WABB RAW at 298 k, respectively. The results of thermodynamics study interpreted the spontaneous and exothermic properties of dye adsorption. The results of spectral characterization of the adsorbent before and after dye adsorption and the adsorption research indicated that the adsorption between Y/SA adsorbent and dye molecules mainly included electrostatic adsorption, hydrogen bonding and ion exchange reaction. As a very promising and important alternative material, the novel high-efficiency biosorbent Y/SA gel beads with ultrahigh adsorption capacity would be used for the treatment of high-concentration dyes effluent.
References
Tharaneedhar V, Senthil Kumar P, Saravanan A, Ravikumar C, Jaikumar V (2017) Sustain Mater Technol 11:1
Anitha T, Senthil Kumar P, Sathish Kumar K (2016) J Water Process Eng 13:127
Waheed A, Mansha M, Kazi IW, Ullah N (2019) J Hazard Mater 369:528
Senthil Kumar P, Ramalingam S (2013) Int J Ind Chem 4:17
Sienkiewicz A, Kierys A, Goworek J (2019) J Disper Sci Technol 40:1396
Grace Pavithra K, Senthil Kumar P, Jaikumar V, Sundar Rajan P (2019) J Ind Eng Chem 75:1
Rashidi NA, Yusup S (2017) Chem Eng J 314:277
Hadi P, Xua M, Ning C, Lin CSK, McKay G (2015) Chem Eng J 260:895
De Gisi S, Lofrano G, Grassi M, Notarnicola M (2016) Sustain Mater Technol 9:10
Liu QM, Li YY, Chen HF, Lu J, Yu GS, Möslang M, Zhou YB (2020) J Hazard Mater 382: https://doi.org/10.1016/j.jhazmat.2019.121040
Zhang L, Sellaoui L, Franco D, Dotto GL, Bajahzar A, Belmabrouk H, Bonilla-Petriciolet A, Oliveira MLS, Li Z (2019) Chem Eng J 372: https://doi.org/10.1016/j.cej.2019.122952
Senthil Kumar P, Sivaranjanee R, Vinothini U, Raghavi M, Rajasekar K, Ramakrishnan K (2013) Desalin Water Treat 51:1
Cho E, Kim J, Park CW, Lee K-W, Lee TS (2018) J Hazard Mater 360:243
Eskhan A, Banat F (2018) J Polym Environ 26:2901
Chiew CSC, Poh PE, Pasbakhsh P, Tey BT, Yeoh HK, Chan ES (2014) Appl Clay Sci 101:444
Agnihotri S, Singhal R (2019) J Polym Environ 27:372
Benhouria A, Islam MA, Zaghouane-Boudiaf H, Boutahala M, Hameed BH (2015) Chem Eng J 270:621
Kumar M, Dosanjh HS, Singh H (2018) J Inorg Organomet P 28: 1688
Xu QY, Chen ZB, Wu ZS, Xu F, Yang DX, He Q, Li G, Chen Y (2019) Bioresour Technol 289:1
Zhang SY, Lyu Y, Su XS, Bian YY, Yu BW, Zhang YL (2016) Environ Earth Sci 75:401
He JS, Cui AN, Ni F, Deng SH, Shen F, Yang G (2018) J Colloid Interface Sci 531:37
Fabryanty R, Valencia C, Soetaredjo FE, Putro JN, Santoso SP, Kurniawan A, Ju YH, Ismadji S (2017) J Environ Chem Eng 5:5677
Wei L, Hong TQ, Liu HB, Chen TH (2017) J Cryst Growth 113:60
Cestari AR, Vieira EFS, Santos AGP, Mota JA, Almeida VP (2004) J Colloid Interface Sci 280:380
Lagergren S (1898) K Sven Vetenskapsakad Handl 24:1
Ho YS, McKay G (1999) Process Biochem 34:451
Weber JW, Morris JC (1963) J Sanit Eng Div 89:31
Jothirani R, Senthil Kumar P, Saravanan A, Narayan Abishek S, Abhishek D (2016) J Ind Eng Chem 39:162
Dotto GL, Pinto LAA (2011) J Hazard Mater 187:164
Zheng Y, Zhu B, Chen H, You W, Jiang C, Yu J (2017) J Colloid Interface Sci 504:688
Zhong L, Tang A, Yan P, Wang J, Wang Q, Wen X, Cui Y (2019) J Colloid Interface Sci 537:450
Lorenc-Grabowska E, Gryglewicz G (2007) Dyes Pigm 74:34
Golder A, Samanta A, Ray S (2006) Chem Eng J 122:107
Vimonses V, Lei S, Jin B, Chow CWK, Saint C (2009) Chem Eng J 148:354
Namasivayam C, Arasi D (1997) Chemosphere 34:401
Wang L, Wang A (2007) J Hazard Mater 147:979
Dawood S, Sen TK (2012) Water Res 46:1933
Sayǧili H, Güzel F (2015) Chem Eng Res Des 100:27
Dawood S, Sen TK, Chi P (2014) Water Air Soil Pollut 225:1818
Li J, Ng DHL, Song P, Kong C, Song Y, Yang P (2015) Biomass Bioenerg 75:189
Lei C, Zhu X, Zhu B, Jiang C, Le Y, Yu J (2017) J Hazard Mater 321:801
Tellinghuisen J (2006) Biophys Chem 120:114
Acknowledgements
This study was funded by the National Natural Science Foundation of China (21167011), the Inner Mongolia Natural Science Foundation (2015MS0226) and the Inner Mongolia Normal University Science Research Foundation (112129K18ZZYF006).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Li, B., Yin, H. Superior Adsorption Property of a Novel Green Biosorbent Yttrium/Alginate Gel Beads for Dyes from Aqueous Solution. J Polym Environ 28, 2137–2148 (2020). https://doi.org/10.1007/s10924-020-01757-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10924-020-01757-0