Abstract
Novel magnetic sodium alginate-based biopolymer Fe3O4@SA-Fe was successfully prepared by the cross-linking reaction of sodium alginate (SA) and Fe(III) ions and adding magnetic ferric oxide (Fe3O4), and characterized by Scanning Electron Microscopy (SEM), Energy Dispersive Spectroscopy (EDS), UV-visible diffuse reflectance spectroscopy (UV-Vis DRS), Fourier Transform-Infrared (FTIR), X-ray Photoelectron Spectroscopy (XPS) and Vibrating Sample Magnetometer (VSM), respectively. The effects of preparation and adsorption conditions on the adsorbent were investigated by adsorbing Congo red (CR) and Direct red 23 (DR 23) dyes. The results showed that the synthesized Fe3O4@SA-Fe polymer gel beads exhibited super-high adsorption property and good stability when the mass concentrations of SA, Fe(III) ions and Fe3O4 are 16, 12.5 and 7 g/L at room temperature respectively. The adsorption rates of two dyes by Fe3O4@SA-Fe were very fast at 298 K, and the time required to reach the equilibrium was very short, which was 30 min for CR and 60 min for DR 23. Moreover, the dye removal efficiencies were over 99.4% and 95.2% in a wide pH range of 2.0 ~ 9.0 for CR and 2.0 ~ 10.0 for DR 23, respectively. The adsorption process could be accurately described by the pseudo-second-order rate model, which were mostly controlled by intra-particle diffusion. The fitting results of isothermic data by Langmuir, Freundlich and Dubinin-Radushkevich (D-R) models revealed that the equilibrium data completely obeyed the Langmuir model and the obtained maximum adsorption capacities of CR and DR 23 were 3333 and 1429 mg/g at 298 K, respectively. FTIR, UV-Vis and XPS analysis indicated that the electrostatic adsorption, hydrogen bonding and ligand exchange promoted the interaction between dye molecules and Fe3O4@SA-Fe. A green magnetic biosorbent Fe3O4@SA-Fe gel beads with simple preparation method and high-cost performance can be used for super-efficient purification of high-concentration dye effluent and quickly separated from the aqueous phase and recovered, which would have a good application prospect.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Over the years, a large amount of dye wastewater has been produced due to the wide application of various dyes in food, textile printing and dyeing, leather, paper, plastic and other fields [1]. Without treatment or incomplete treatment, the colored dye wastewater discharged into the environmental water body can bring serious water environmental pollution and harm to human health. In particular, azo dyes are one of the most widely used synthetic dyes, accounting for more than half of the total dyes. Under special conditions, azo dyes can decompose to produce a variety of carcinogenic aromatic amines, which may cause human diseases and cancer after activation [2,3,4,5,6,7]. Therefore, azo-dye wastewater must be treated innocuously before discharge. As two frequently-used water-soluble anionic dyes, direct dyes congo red (CR) and direct red 23 (DR 23) are azo dyes used widely due to their molecular linear structure and the good coplanarity of aromatic ring structure. Therefore, efficient treatment methods must be selected for the purification of this kind of wastewater. Among the common water treatment technologies, including various physical, chemical and biological methods [8,9,10], the research and application of adsorption technology has been paid wide attention due to its convenient operation, high efficiency of wastewater treatment and low energy consumption. Especially in recent years, more and more attraction has been focused on the development of natural biomaterials such as alginate, chitosan and cellulose as adsorbents for the removal of heavy metals and organic pollutants from various industrial wastewater [11,12,13,14]. These biological materials have been widely used as the matrix to prepare various environmental-friendly adsorption materials with better adsorption properties by chemical reaction or composite action with other materials [15,16,17]. However, there are still some problems in the application of adsorbents, such as the high cost of adsorbent, the difficulty of separating powder adsorbents from aqueous phase after adsorbing pollutants and the risk of secondary pollution. Accordingly, the traditional adsorbents can be made into macroparticles and magnetized by adding magnetic Fe3O4 particles to realize rapid separation and recovery by the action of external magnetic field after adsorption, which will make them have better practical application value [18,19,20,21,22]. A novel magnetic/activated charcoal/β-cyclodextrin/alginate (Fe3O4/AC/CD/Alg) polymer nanocomposite was prepared and used to adsorb cationic dye. The experimental results indicated that the removal efficiency and adsorption capacity of methylene blue onto Fe3O4/AC/CD/Alg were 99.53% and 10.63 mg/g in 90 min, respectively [23]. Ge et al. [24] used magadiite–magnetite (MAG-Fe3O4) nanocomposite for removal of methylene blue from aqueous solutions and the adsorption capacity and removal efficiency of methylene blue onto MAG-Fe3O4 were 93.7 mg/g and 96.2%, respectively. The magnetic zeolite-alginate-polyanetholesulfonic acid gel beads (m-ALG/PESA) were applied to cationic dyes adsorption and the maximum adsorption capacities of methylene blue and malachite green onto m-ALG/PESA gel beads were 400 mg/g and 164 mg/g, respectively [25].
Sodium alginate (SA) is a copolymer composed of consecutive sequence of GM、MM and GG blocks formed by β-D-1,4-mannanic acid (M-unit) and α-L-1,4-guluronic acid (G-unit) through the link of 1,4-glycosidic bond. There are abundant carboxyl and hydroxyl groups in the molecular chains of SA. As a natural polysaccharide, SA has many advantages, such as strong hydrophilicity, biosafety, biodegradability, non-toxic side effects, low cost and rich sources, so it is widely used in food industry, pharmaceutical industry and other industries [26,27,28,29]. The occurrence of ionic crosslinking reaction by replacing sodium ions with polyvalent metal ions can make SA form the gel spheres with three-dimensional structure [30], which will turn into an ideal matrix for the preparation of SA-based composite materials with stronger adsorption performance and better separation property [31,32,33].
In the current study, novel magnetic alginate-Fe(III) polymer gel spheres were prepared by selecting Fe(III) ions as crosslinking ions and adding magnetic Fe3O4 particles, and used for the ultra-efficient removal of CR and DR 23 dyes from aqueous solutions. The related research has not been reported. The important conditions of preparation and adsorption affecting the properties of magnetic composite were investigated. The adsorption efficiency and magnetic separation performance of the magnetic polymer were evaluated, and the adsorption mechanism was discussed by adsorption study and material characterization. It would be expected to obtain a green and pollution-free magnetic biopolymer adsorbent with low-cost performance and ultra-high adsorption capacity for direct dyes, and achieve rapid separation and recovery of the magnetic gel beads from aqueous phase after adsorption.
Materials and Methods
Materials and Reagents
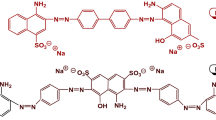
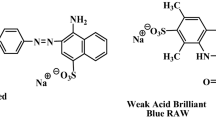
SA, Fe3O4 and ferric chloride hexahydrate (FeCl3·6H2O) were purchased respectively from Tianjin Damao Chemical Reagent Factory, Institute of Guangfu Fine Chemicals and Windboat Chemical Reagent Technology Co., Ltd., China, which are of analytical pure grade. CR and DR 23 dyes with the wavelength corresponding to absorption maximum (λmax) of 498 nm and 502 nm were purchased from Shanghai Jiaying Chemical Co., Ltd., China. The chemical structures of CR (molecular formula C32H22N6Na2O6S2, relative molar mass 696.68 g/mol) and DR 23 (C35H25N7Na2O10S2, relative molar mass 790.69 g/mol) are shown in Fig. 1.
Preparation and Characterization of Magnetic Polymer Fe3O4@SA-Fe Gel Beads
0.40 g SA was dissolved in deionized water with magnetic stirring for 1 h at 298 K to obtain the uniformly dispersed solution, then 0.175 g Fe3O4 was added into SA solution and further stirred and ultrasonically dispersed to a uniform mixed solution. The mixed solution was slowly dropped into 12.5 g/L FeCl3 solution with slow stirring and the gel beads were continuously generated. After 4 h of cross-linking polymerization, the brown black gel beads were removed, washed with deionized water to neutral and dried. The magnetic biosorbent Fe3O4@SA-Fe was successfully synthesized.
The surface morphology and elemental distribution of SA, SA-Fe and Fe3O4@SA-Fe polymer gel beads were characterized by scanning electron microscopy and energy dispersive spectroscopy (SEM-EDS, S-4800, Hitachi, Japan). The functional structures of materials were analyzed by a Nicolet Nexus 6700-type Fourier transform infrared spectrometer (FTIR, KBr tableting method, Thermo Fisher Scientific Inc., U.S.A) and U-2900 ultraviolet visible diffuse reflection spectrometer (UV-Vis DRS, BaSO4 tableting method, Hitachi Inc., Japan). The elemental status was studied by an Axis Ultra type X-ray Photoelectron Spectroscopy (XPS, Shimadzu Inc., Japan). The magnetic hysteresis loops of samples were determined by JDAW-2000D type vibrating sample magnetometer (VSM, Yingpu Magnetoelectric Technology Development Co., Ltd., China) at room temperature.
Adsorption Experiment
The adsorbent Fe3O4@SA-Fe with fixed mass (0.030 g for CR, 0.025 g for DR 23) was added to a series of conical flasks containing certain-concentration dye solutions and shaken for 2 h in a water bath thermostatic oscillator at 298 K. The mixture was then filtered through 0.45 μm microfiltration membrane and the dye concentration in each filtrate was measured by means of spectrophotometry at λmax of each dye. The equilibrium adsorption capacity qe (mg/g) and removal rate η (%) of dyes were calculated by use of the following equations.
where C0 (mg/L) and Ce (mg/L) are the concentrations of the dye solutions before and after the adsorption, respectively, V (L) is the dye solution volume, m (g) is the adsorbent mass.
Results and Discussion
Preparation Conditions of Fe3O4@SA-Fe Polymer
The adsorption properties of SA-based polymer hydrogel depend strongly on the choice of SA solution concentration during preparation, which determines the pelletizing property, mechanical properties and stability of gel beads. Accordingly, the effect of SA solution concentration on the polymer properties was discussed. As shown in Fig. 2a, when the concentration of SA solution increased from 14 to 17.2 g/L, the removal efficiencies of two dyes by Fe3O4@SA-Fe were only slightly reduced from 99.6 to 92.8% for CR and from 99.7 to 97.8% for DR 23, and then decreased significantly with the increase of SA concentration at 298 K. It was found simultaneously that when the concentration of SA solution was lower than 14 g/L, the spheroidization of magnetic polymer was poor, however, when the SA concentration was higher than 18 g/L, it was difficult to produce uniform crosslinking polymerization because of the high viscosity of SA solution. Therefore, a 16 g/L concentration of SA solution was chosen to prepare composite gel beads.
Fe(III) ions as a crosslinking agent is the key factor to make SA hydrogel polymerized into spheres. It can be clearly seen from Fig. 2b that the adsorption capacities and removal efficiencies of two dyes onto prepared Fe3O4@SA-Fe polymer increase significantly with the increase of Fe(III) ion concentration from 5 to 12.5 g/L, and then remain basically unchanged as the Fe(III) ion concentration continues to increase from 12.5 to 30 g/L. This indicates that the 12.5 g/L of Fe(III) concentration can synthesize the magnetic gel beads with the best adsorption property through perfect crosslinking with SA, so it is selected for the preparation of magnetic polymer.
The purpose of adding Fe3O4 in polymer gel beads is to make the polymer adsorbent has magnetism to realize the rapid separation of solid and liquid phases through the external magnetic field, and also change the adsorbent performance at the same time. The concentrations of SA and Fe(III) ion solutions were fixed at 16 and 12.5 g/L respectively, the effect of Fe3O4 concentration on the properties of synthesized magnetic polymer is given in Fig. 2c. Although the prepared Fe3O4@SA-Fe had the best adsorption effect for two dyes when the concentration of Fe3O4 was 3 g/L, its magnetic property was too weak to realize magnetic separation. Hence, considering comprehensively, the choice of 7 g/L Fe3O4 concentration was most suitable for the preparation of magnetic polymer Fe3O4@SA-Fe with better performance.
The surface morphology of SA and polymer Fe3O4@SA-Fe gel beads was characterized through SEM. It is displayed in Fig. 3a that SA primarily consists of massive and schistose fragment in various ways. The brown black Fe3O4@SA-Fe polymer beads obtained by crosslinking SA with Fe(III) ions and Fe3O4 are shown in Fig. 3b, c. Each gel bead with a diameter of about 1.36 mm emerges a “cauliflower like” surface which is composed entirely of seemingly ordered grooves and wrinkles. This surface structure is very conducive to the adsorption of pollutants. Meanwhile, there are scattered white spots on the bead surface, which may be the magnetic Fe3O4 particles in Fig. 3d. After further EDS analysis, it was found from Table 1 that the higher contents of Fe and Cl elements were found in the polymer, but not in SA. The Na content in the polymer decreased significantly compared with that in SA. These changes further confirmed that the exchange reaction between Fe(III) ions and Na+ ions in SA molecules occurred, and continued to crosslink with SA chains to form magnetic Fe3O4@SA-Fe polymer gel beads with three-dimensional structure.
FTIR spectra of SA and Fe3O4@SA-Fe polymer are presented in Fig. 3e. The broad peak for SA at 3284 ~ 3612 cm−1 emanates from stronger intermolecular hydrogen bonding [34]. The other absorption bands at 2914, 1629, 1414 and 1041 cm−1 should be attributed to the stretching vibration of C-H, asymmetric and symmetric stretching vibrations of -COO− and the stretching vibration of C-O-C, respectively [35]. In the spectrum of Fe3O4@SA-Fe polymer, the three peaks of -COO− and C-O-C groups in the spectrum of SA were shifted to 1593, 1424 and 1029 cm−1, respectively, illustrating that the occurrence of crosslinking polymerization between Fe(III) ions and -COO− and C-O-C groups in SA molecules. Meanwhile, the new peak appeared at around 586 cm−1 should be assigned to the Fe-O bond, suggesting that Fe3O4 was also involved in polymerization.
The measured saturation magnetization value of Fe3O4@SA-Fe was 13.79 emu/g (Fig. 3f). In the presence of an external magnetic field near the bottle wall, the magnetic polymer gel beads after adsorption of two dyes can be quickly gathered onto the bottle wall, indicating that the magnetic properties of the polymer gel beads can achieve the separation of the adsorbent quickly and effectively from the treated wastewater, which would be very important for the recovery of adsorbent and the avoidance of the secondary pollution in practical application. Meanwhile, the coercivity of Fe3O4@SA-Fe was 101.5 Oe, which can be used as a permanent magnetic material [36].
Effect of Important Adsorption Factors
When the dosage of Fe3O4@SA-Fe adsorbent was 0.030 and 0.025 g for CR and DR 23 adsorption respectively, the change of dye adsorption capacity and removal rate with the initial concentrations of dyes was shown in Fig. 4a. The adsorption capacity increased first and then decreased with the increase of initial concentration of each dye, while the removal efficiencies reached the maximum values with 99.8% and 99.4% for CR and DR 23 at 2000 mg/L and 1000 mg/L concentrations respectively, and then decreased gradually with the increase of dye concentration. This was because there were enough active sites on the polymer surface to adsorb a number of dye molecules, but the dye-adsorbed amount per the unit mass adsorbent could only decrease with increasing dye concentration when the adsorption sites were saturated.
The solution pH is a key parameter affecting the adsorption owing to the changes of the charge on the adsorbent surface and the existing forms of dyes with the solution acidity. The influence of solution pH value on the CR and DR 23 adsorption by Fe3O4@SA-Fe composite was studied in the range of pH 2.0 ~ 11.0 as shown in Fig. 4b. The adsorption capacities and removal efficiencies of CR and DR 23 onto Fe3O4@SA-Fe at pH 2.0 reached the maximum values with 3166 mg/g, 1395 mg/g, 100% and 99.6%, respectively, and then almost unchanged with pH increase from 2.0 to 9.0, and finally reduced rapidly with the further increase of pH. Similar results have also been reported in literature [17, 37]. However, the removal rate of DR 23 could still reach 95.2% at pH 10.0. The electrostatic interaction between the adsorbent surface with positive charge via protonation and dye anions increased with the decrease of pH in acidic solution, leading to the significant increase of adsorption capacities. Under alkaline conditions, the electrostatic repulsion between dye anions and adsorbent by deprotonation should be enhanced with the increase of pH, resulting in the decrease of adsorption capacities. But in fact, the adsorption capacity and removal efficiency for two dyes did not change significantly under the alkaline conditions of pH ≤ 9.0 (pH ≤ 10.0 for DR 23), suggesting that there should be other adsorption mechanisms, such as hydrogen bonding or chemical adsorption except electrostatic adsorption. The rich -OH, -COOH and -SO3− groups on Fe3O4@SA-Fe surface readily formed hydrogen bonds with direct dye molecules containing -NH2, -OH, -N=N- groups and phenyl. Therefore, the natural acidity of each dye solution (pH 8.0 for CR solution and pH 9.0 for DR 23 solution respectively) was selected to perform the following adsorption study, which was more convenient for practical operation. The results show that the Fe3O4@SA-Fe polymer with ultra-high adsorption capacity, nearly 100% dye removal efficiency and wide pH application range has greater advantages than the adsorption materials reported in references [38, 39], and can realize the rapid separation and recovery from liquid phase.
The effect of contact time and temperature on dye adsorption by Fe3O4@SA-Fe is presented in Fig. 4c. The adsorption rates of CR and DR 23 were exceedingly fast and the basic adsorption equilibrium can be reached in 20 min at 298 K, and the corresponding adsorption capacities prominently rose to 2442 mg/g and 929 mg/g, respectively, which was very beneficial to the practical application of dye wastewater treatment because of the reduction of investment cost and the significant improvement of treatment efficiency. In addition, it was found from Fig. 4c that the adsorption rates of two dyes onto Fe3O4@SA-Fe gel beads decreased with the rise of temperature from 298 K to 328 K, but the dynamic adsorption equilibrium could still be reached within 30 min, proving that the two adsorption reactions were all of exothermic nature and the room temperature would be more conducive to the operation of the adsorption system.
Adsorption Kinetics
Pseudo-first-order, pseudo-second-order and intraparticular diffusion models were used to fit all kinetic experimental data at different temperatures (298, 313 and 328 K) to investigate mechanism of adsorption process and rate determining step. The concrete expressions of three models are as follows [40, 41]:
where k1 (min−1), k2 [g/(mg·min)] and kp (mg/g·min1/2) are the first-order, second-order and intraparticular diffusion rate constants, respectively; qt and qe (mg/g) are the amount of dye adsorbed at time t (min) and equilibrium, correspondingly.
The fitting parameters and correlation coefficient (R2) obtained by kinetic models and the error analysis (sum of squared error, SSE; root mean squared error, RMSE) by using origin software [42] are listed in Table 2. The R2 values (R2 > 0.999) fitted by the pseudo-second-order rate model were nearly 1.000 at different temperatures, which were significantly better than those obtained by the pseudo-first-order model (R2 > 0.362). The values of SSE and RMSE calculated for the pseudo-second-order model fitting were remarkably lower than those for pseudo-first-order model fitting. Meanwhile, the values of qe,2 calculated by the pseudo-second-order model approached the values of actual equilibrium adsorption capacities (qexp), confirming that the whole adsorption process of CR and DR 23 onto Fe3O4@SA-Fe polymer can be formulated by the pseudo-second-order rate model. In addition, the k2 values of two dye sorption decrease with increasing temperature, demonstrating the exothermic nature of adsorption reaction.
The fitting results of the experimental data by the intraparticle diffusion model at different temperatures are presented in Fig. 5a, b and Table 2, respectively. The occurrence of dye adsorption processes could be considered as two aspects. The adsorption data of first rapid stages from 3 to 20 min for CR and from 3 to 10 min for DR 23 had good goodness-of-fit for the intraparticle diffusion model based on the values of correlation coefficient (R2), illustrating that the adsorption rate of the process was mainly controlled by intraparticle diffusion. The last stages were basically in the dynamic adsorption equilibrium processes of two dyes (the upper platforms of the curves), which were also affected by the intraparticle diffusion, but not the only rate-limiting step.
Adsorption Isotherms
The adsorption isotherms drawn from the equilibrium data obtained by isothermal experiments at different temperatures are presented in Fig. 5c, d. The adsorption capacity of each dye onto Fe3O4@SA-Fe polymer decreased with the increase of temperature from 298 K to 328 K. Three isotherm models known as the Langmuir, Freundlich and Dubinin-Radushkevich (D-R) models were used to discuss the adsorption behavior of dyes on the magnetic polymer adsorbent, and expressed as follows [43, 44], respectively:
Langmuir:
Freundlich:
Dubinin-Radushkevich:
where qmax (mg/g) represents the the maximum adsorption capacity; b (L/mg) is the Langmuir adsorption coefficient; kF and n are Freundlich constants related to adsorption; β (mol2/J2) is the constant associated with adsorption energy; ε and E (kJ/mol) are the Polanyi potential and the mean energy of adsorption. In addition, the separation factor RL given by Langmuir, which is equal to 1/(1 + bc0), was applied to illustrate the difficult and easy nature of adsorption [45, 46].
The important parameters and error analysis obtained by fitting the equilibrium data of CR and DR 23 sorption onto Fe3O4@SA-Fe using three isothermal models at various temperatures are listed in Table 3. Based on the comparison of the analytical results in Table 3, the linear fitting of Langmuir model gave the highest R2 values (R2 ≥ 0.999) and the lower values of SSE and RMSE, and the maximum adsorption capacities (qmax) of CR and DR 23 were 3333 mg/g and 1429 mg/g at room temperature (298 K), respectively. The Langmuir adsorption coefficients (b) decreased with the increase of temperature. The values of qmax for two dyes on Fe3O4@SA-Fe were also close to the experimental values (qe,exp) at three temperatures (298 K ~ 328 K). These revealed that the Langmuir model was most suitable to explain the dye adsorption behavior with a monomolecular layer and the exothermic nature of the adsorption systems, which were consistent with the kinetic results. This confirmed that the room temperature would be the most favorable for the ultra-efficient removal of CR and DR 23 dyes and also very convenient for the actual operation of dye wastewater treatment. Compared with the reported literature values of maximum adsorption capacity (qmax) of CR and DR 23 onto other different types of adsorbents [29, 47,48,49,50,51,52,53,54,55,56,57,58,59,60,61] in Table 4, the magnetic polymer Fe3O4@SA-Fe revealed a greater advantage because it has greater adsorption capacities for two dyes, and could quickly recover from the aqueous phase after adsorption and did not produce secondary pollution. Hence, polymer Fe3O4@SA-Fe gel beads may be considered a promising green adsorbent for super-efficient removal of direct dyes. In addition, the values of separation factor RL with the range of 0.0028 ~ 0.0319 in Fig. 5e suggested the easy adsorption reaction for two dyes on Fe3O4@SA-Fe.
Meanwhile, the equilibrium data were in good agreement with D-R model (R2 ≥ 0.903), and the values of the obtained mean energy (E, kJ/mol) of adsorption were in the range of 19.3 ~ 27.7 kJ/mol for CR and 22.7 ~ 24.1 kJ/mol for DR 23 in Table 3, indicating the occurrence of chemical adsorption and hydrogen bonding between the adsorbent and dye molecules [62].
In addition, the adsorption results showed that the adsorption capacity of CR by Fe3O4@SA-Fe was significantly higher than that of DR 23. This should be mainly because the relative molar mass of DR 23 (790.69 g/mol) is much larger than that of CR (696.68 g/mol) and the molecular chains of DR 23 are markedly longer than those of CR, which will lead to a smaller number of molecules adsorbed on the unit surface area of adsorbent, so its adsorption capacity will be relatively lower.
Discussion on Adsorption Mechanism
The UV-Vis spectra of Fe3O4@SA-Fe before and after adsorption of two dyes were displayed in Fig. 6a. The wavelength corresponding to absorption maximum of the adsorbent at λmax = 407 nm were moved to 412 nm, 415 nm and 478 nm after CR and DR 23 adsorption, respectively, and the two absorption bands became wider. These changes confirmed the interaction between the adsorbent and each dye molecules.
In the FTIR spectrum of Fe3O4@SA-Fe polymer (Fig. 6b), the changes of different characteristic peaks after dye adsorption are as follows: the asymmetric and symmetric stretching vibrations of -COO− groups were shifted from 1593 and 1424 cm−1 to 1622 and 1416 cm−1 for Fe3O4@SA-Fe-CR and 1616 cm−1 and 1413 cm−1 for Fe3O4@SA-Fe-DR 23, respectively. The absorption peaks of C-O-C were moved from 1029 to 1046 cm−1 and 1036 cm−1 for CR and DR 23 adsorption, respectively. The peak of Fe-O bond has changed concurrently from 586 cm−1 to 616 cm−1 and 612 cm−1, respectively. In addition, the intensity of these peaks also varied more or less. These changes confirmed the chemical and hydrogen bond interreaction between Fe3O4@SA-Fe polymer gel beads and dye molecules [63], and Fe3O4 particles in Fe3O4@SA-Fe polymer also participated in the interaction with dye anions.
In order to further study the interaction between the adsorbent and dye molecules, the surface chemistry of the adsorbent before and after adsorption were analyzed by XPS as shown in Fig. 7. The XPS wide scan spectra of Fe3O4@SA-Fe polymer in Fig. 7a clearly showed the presence of characteristic peaks such as C 1 s, O 1 s, and Fe 2p, confirming that these elements are the main components of Fe3O4@SA-Fe polymer. However, after dye adsorption, the adsorbent spectrum changed slightly in the position and intensity of energy band of the main elements. It is found from the high-resolution C 1 s spectra of Fe3O4@SA-Fe before and after dye adsorption in Fig. 7b that C 1 s spectrum of Fe3O4@SA-Fe can be decomposed into three peaks with binding energy at 284.8, 286.6, and 288.8 eV, respectively, which should be attributed to the C atom in C − C, C − O, and COO− [64], respectively. After dye adsorption, the binding energy of each peak can be shifted slightly and the area ratio of each peak can be reduced. The two peaks of O1s spectrum for Fe3O4@SA-Fe (Fig. 7c) at 530.6 and 532.6 eV assigned to the O atom in iron-oxide (Fe-O) and COO− [65] were shifted to 530.3 and 532.7 eV for CR and 530.3 and 532.3 eV for DR 23 after dye adsorption, respectively, and the area ratio of each peak at 530.6 and 532.6 eV can be increased. The area ratios of peak corresponded to O spectrum of Fe-O and COO− are 1:3 and 1:2 for Fe3O4@SA-Fe and Fe3O4@SA-Fe-CR and 1:1.6 and 1:1.4 for Fe3O4@SA-Fe and Fe3O4@SA-Fe-DR 23, respectively. In the high-resolution spectrum of Fe 2p in Fig. 7d, the spectra of Fe 2p3/2 and Fe2p1/2 for Fe3O4@SA-Fe were respectively divided into two peaks at 711.1 eV and 712.9 eV, 723.8 eV and 725.8 eV [66], indicating the existence of Fe3+ and Fe2+ and the existence of Fe3O4 component in the adsorbent [67]. Among them, the peaks at 711.1 eV and 723.8 eV could be assigned to Fe2+ and the peaks at 712.9 eV and 725.8 eV could be attributed to Fe3+ [68, 69]. After dye adsorption, each peak of the Fe species for Fe3O4@SA-Fe was moved. Before and after CR adsorption, the area ratios of Fe2+ peaks for Fe3O4@SA-Fe are 1:1.5 and 1:3, and those of Fe3+ peaks are 1:1.4 and 1:3 in Fig. 7e, respectively. After DR 23 adsorption, the peak areas of Fe2+ peaks can increase by 1:1.4 and 1:1.8 times, respectively, and the areas of Fe3+ peaks for Fe3O4@SA-Fe can increase by 1:1.4 and 1:2 times as shown in Fig. 7f, respectively. These changes of XPS spectra confirmed that the carboxyl groups of SA and Fe-O groups in iron-oxide were involved in dye adsorption through hydrogen bonding between carboxyl and hydroxyl groups of Fe3O4@SA-Fe and dye molecules and surface complexation (Fe-O-dye), which agreed well with the results of UV-Vis and FTIR analysis.
According to the effect of pH on the adsorbent performance and the results of UV-Vis, FTIR and XPS characterization and adsorption study, it can be deduced that the adsorption of dye anions onto Fe3O4@SA-Fe gel beads is chiefly related to electrostatic adsorption, hydrogen bonding and surface complexation besides Van der Waals forces (Fig. 8).
Conclusion
Magnetic polymer Fe3O4@SA-Fe gel beads synthesized by facile droplet polymerization at room temperature exhibiting super-high adsorption efficacy for CR and DR 23 dyes in a wide pH range of dyeing wastewater. The optimized ratio of raw materials for polymer gel beads preparation was obtained. The adsorption of two dyes onto Fe3O4@SA-Fe gel beads can reach equilibrium very quickly, which was 30 min for CR and 60 min for DR 23, respectively. The adsorption processes at different temperatures can be completely represented by the pseudo-second-order rate model and basically controlled by the intraparticle diffusion. Based on the best goodness-of-fit of equilibrium data for the Langmuir model and the exothermic properties of adsorption systems, the maximum adsorption capacities of two dyes can reach 3333 and 1429 mg/g at 298 K and natural pH of dye solutions respectively, significantly better than those of many other reported adsorbents. Analysis of FTIR, UV-Vis and XPS and the discovery of adsorption study suggested that the interaction mechanism between Fe3O4@SA-Fe adsorbent and dye molecules involved primarily electrostatic adsorption, hydrogen bonding and chemical complexation. As a feasible and cost-effective magnetic polymer, Fe3O4@SA-Fe gel beads with wide application range of acidity, the best adsorption effect and the most convenient practical operation at room temperature and short adsorption equilibrium time, would be considered as a promising environment-friendly biosorbent, which can achieve the ultra-efficient purification for high-concentration dye effluent and fast separation and recovery from treated-water.
References
Zhuang HF, Tang HJ, Shan SD, Mao ZR (2019) Ind Water Treat 39:41
Li Q, Wang M, Yuan XJ, Li DY, Xu HM, Sun L, P F, X DS (2019) Environ Technol 1
Solís M, Solís A, Pérez HI, Manjarrez N, Flores M (2012) Process Biochem 47:1723
Deepak R, Vandana M, Radhey Shyam S (2016) Chemosphere 155:591
Niu S, Xie XK, Wang Z, Zheng LH, Gao F, Miao Y (2019) Environ Technol :1
Yin HX, T YC, H XH, X LP, X MT, H W, W T (2017) Res Environ Sci 30:1105
Sun GF, Zeng HY, Song LX, Hu Y, Zhang YQ (2011) J Leshan Normal Univ 26:20
Tahreen A, Jami MS, Ali F (2020) J Water Process Eng 37:101440
Wang YX, Jiang L, Shang HG, Li Q, Zhou WZ (2020) Environ Technol Innovation 19:1
Mishra S, Nayak JKand Maiti A (2020) Clean Technol Envir 22:651
Periyasamy S, Naushad M, Viswanathan N (2020) Environ Sci Water Res Technol 6:851
Jawad AH, Mubarak NSA, Abdulhameed AS (2020) J Polym Environ 28:624
Ren JP, Tao FR, Cui YZ (2020) J Polym Environ 8:1302
Yu C, Zhang Y, Fang Y, Tan YJ, Dai K, Liu S, Huang QY (2020) Environ Sci Pollut R 27:16745
Ferrer-Polonio E, Fernández-Navarro J, Iborra-Clar M, Alcaina-Miranda M, Mendoza-Roca JA (2020) J Environ Manag 263:110368
Yu SY, Cui JL, Wang JB, Zhong CS, Wang X, Wang N (2020) Int J Biol Macromol 149:562
Li B, Ren Z (2020) J Polym Environ 28:1811
Wang HP, Lin YC, Li Y, Anudari D, Fang H, Guo L, Huang J, Yang JX (2019) J Inorg Organomet Polym Mater 29:1874
Rocher V, Siaugue J-M, Cabuil V, Bee A (2008) Water Res 42:1290
Elwakeel KZ, El-Bindary AA, El-Sonbati AZ, Hawas AR (2017) Can J Chem 95:807
Polat G, Acikel YS (2019) J Polym Environ 27:1971
Bée A, Talbot D, Abramson S, Dupuis V (2011) J Colloid Interface 362:486
Yadav S, Asthana A, Chakraborty R, Jain B, Singh AK, Carabineiro SAC, Susan MABH (2020) Nanomaterials 10:170
Ge M, Xi Z, Zhu C, Liang G, Hu G, Jamal L, Jahangir Alam SM (2019) Polymers 11:607
Metin AU, Dogan D, Can M (2020) Mater Chem Phys 256:1
Yao WH, Yu F, Ma J (2018) Prog Chem 30:1722
Dodero A, Alloisio M, Vicini S, Castellano M (2020) Carbohydr Polym 227:115
Zhang M, Bai B, Hu N, Wang HL, So YR (2019) Chem Eng 47:42
Zhang J, Lin HQ, Ma QY, Lu BH, Jiang H, Peng BX (2019) China Pharm 30:3307
Wang QQ, Liu Y, Zhang CJ, Zhang C, Zhu P (2019) Mater Sci Eng C 99:1469
Li ZM, Yao Y, Wei GT, Zhang LY, Lu MJ, Liu L, Liang DX, Sun YQ (2018) J Guangxi Univ (Nat Sci Ed) 43:2391
Sun JZ, Wang JX, Ni MJ, Zhang XB, Chen YH, Liu SY, Li H (2019) Environ Sci Technol 42:104
Chen WP, Zhang EH, Lin YB (2010) Environ Protection Sci 36:14
Verma A, Thakur S, Mamba G, Prateek Gupta RK, Thakur P, Thakur VK (2020) Int J Biol Macromol 148:1130
Zhang MY, Yi K, Zhang XW, Han P, Liu W, Tong MP (2020) J Hazard Mater 388:121
Li ZQ, Shen JF, Ma HW, Liu X, Shi M, Li N, Ye MX (2012) Polym Bull 68:1153
Li BG, Yin HY (2020) J Polym Environ 28:2137
Kaur S, Rani S, Mahajan RK (2013) J Chem 2013:1
Reza Sohrabi M, Mansouriieh N, Khosravi M, Zolghadr M (2015) Water Sci Technol 71:1367
Ho YS, McKay G (1999) Process Biochem 34:451
McKay G, Ho YS (1999) Water Res 33:578
Anitha T, Senthil Kumar P, Senthil Kumar K (2016) J Water Process Eng 13:127
Agnihotri S, Singhal R (2019) J Polym Environ 27:372
Tharaneedhar V, Senthil Kumar P, Saravanan A, Ravikumar C, Jaikumar V (2017) Sustain Mater Technol 11:1
Foo KY, Hameed BH (2010) Chem Eng J 156:2
Kang S, Park S, Park J, Baek K (2019) J Environ Manag 234:181
Fawzy MA, Gomaa M (2020) J Environ Manag 262:110380
Zhang HL, Ma JZ, Wang FY, Chu YT, Yang L, Xia MZ (2020) Int J Biol Macromol 149:1161
Leudjo T, Fosso-Kankeu A, Pillay K, Yangkou Mbianda X (2020) J Environ Chem Eng 8:1
Goddeti SMR, Bhaumik M, Maity A, Ray SS (2020) Int J Biol Macromol 149:21
Olusegun SJ, Mohallem NDS (2020) Environ Pollut 260:114019
Shahnaz T, Mohamed Madhar Fazil S, Paadmanaban VC, Narayanasamy S (2020) Int J Biol Macromol 151:322
Hu HJ, Wageh S, Al-Ghamdi AA, Yang SB, Tian ZF, Cheng B, Ho W (2020) Appl Surf Sci 511:1
Mansha M, Waheed A, Ahmad T, Kazi IW, Ullah N (2020) Environ Res 184:109337
Hebeish A, Ramadan MA, Abdel-Halim E, Abo-Okeil A (2011) Clean Techn Environ Policy 13:713
Mahmoodi NM, Maroofi SM, Mazarji M, Nabi-Bidhendi G (2017) J Surfactant Deterg 20:1085
Abbasian M, Jaymand M, Niroomand P, Farnoudian-Habibi A, Karaj-Abad SG (2017) Int J Biol Macromolecules 95:393
Dalvand A, Mahvi AH (2019) Water Quality Res J 1
Mahmoodi NM, Mokhtari-Shourijeh Z, Ghane-Karade A (2017) Water Sci Technol 75:2475
Kasperiski FM, Lima EC, Reis GS, Da Costa JB, Dotto GL, Dias SLP (2018) Chem Eng Commun 205:1520
Almasian A, Chizari Fard G, Parvinzadeh Gashti M, Mirjalili M, Mokhtari Shourijeh Z (2015) Desalin Water Treat 57:10333
Wen RT, Tu BY, Guo XH, Hao XQ, Wu X, Tao HS (2020) Int J Biol Macromol 146:692
Mansha M, Waheed A, Ahmad T, Kazi IW, Ullah N (2020) Environ Res 184:109
Dong X, Lin YC, Ma YY, Zhao L (2020) Inorg Chim Acta 510:119748
Wu ZG, Deng WJ, Zhou W, Luo JW (2019) Carbohyd Polym 216:119
Liu Y, Chen JS, Liu ZK, Xu HY, Shi ZQ, Yang QL, Hu GH, Xiong CX (2020) J Colloid Interf Sci 576:119
Yamashita T, Hayes P (2008) Applied Surf Sci 254:2441
Lu FF, Xu CB, Meng FC, Xia T, Wang RH, Wang JP (2017) Adv Mater Interfaces 4:1700639
Lu ZY, Xu WW, Zhu W, Yang Q, Lei XD, Liu JF, Li YP, Sun XM, Duan X (2014) Chem Commun 50:6479
Acknowledgments
This study was supported by the National Natural Science Foundation of China (21167011), the Natural Science Foundation of Inner Mongolia Autonomous Region (2020LH02009), the Science Research Foundation of Inner Mongolia Normal University (112129K18ZZYF006) and Research and Innovation Foundation for Postgraduates of Inner Mongolia Normal University (CXJJS19119).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lv, T., Li, B. Preparation of Novel Magnetic Sodium Alginate-Ferric(III) Gel Beads and Their Super-Efficient Removal of Direct Dyes from Water. J Polym Environ 29, 1576–1590 (2021). https://doi.org/10.1007/s10924-020-01977-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10924-020-01977-4