Abstract
In order to improve the crosslinking reaction rate of two-component waterborne polyurethanes (2K-WPUs) in the absence of ungreen external catalysts, waterborne polyols containing different tertiary amines in molecular structure were synthesized from a turpentine-based epoxy resin (TME) and secondary amines (N-benzylethanolamine and diethanolamine). Then self-catalytic 2K-WPUs were prepared with the waterborne amino polyols and a hydrophilically modified hexamethylene diisocyanate tripolymer. The structure and micromorphology of TME based polyols were identified using nuclear magnetic resonance (1H NMR, 13C NMR), Fourier transform infrared spectroscopy (FT-IR) and transmission electron microscopy techniques. The crosslinking reaction of 2K-WPUs was monitored using differential scanning calorimetry (DSC) and FT-IR methods. Kinetics analysis from DSC showed the activation energy of the crosslinking reaction of 2K-WPUs reduced obviously by using amino polyols, which indicated the tertiary amines chemically bonded in the polyols could self-catalyze the crosslinking reaction of 2K-WPUs without adding small molecular toxic catalysts. Small steric hindrance of the tertiary amines led to high catalytic activity. The properties of the crosslinked products of the waterborne TME based polyols were investigated by DSC, thermogravmetric analysis and dynamic mechanical analysis (DMA). The results showed high hydroxyl value of the polyol and aromatic ring in the polyol structure resulted in high storage modulus (G′) and glass transition temperature (Tg) of the crosslinked products. More C–N bond content of the crosslinked products of the waterborne amino polyols decreased the thermal stability in the first degradation stage of the products.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Waterborne polyurethanes (WPUs) have the merits of environmental friendly, non-toxic, odorless and safe to use, and therefore are now substituting for traditional solvent-based resins [1,2,3,4]. However, most WPUs are linear thermoplastic polymers with hydrophilic ionic groups in the main chain, so the mechanical properties and water resistance of WPUs are lower than those of solvent-based counterparts [5,6,7,8]. Two-component waterborne polyurethanes (2K-WPUs) provide an external crosslinking way to offer attractive potential in WPUs enhancement by increasing crosslinking density among water-dispersed particles, and have almost the same high performance as solvent-based resins. Due to the high performance, such as good abrasion resistance, hardness and flexibility, and good chemical and solvent resistance, 2K-WPUs have grown commercially application in coating and adhesive fields over the last decade [9,10,11]. 2K-WPUs typically consist of hydroxyl-functional resin dispersion and hydrophilically modified polyisocyanate. However, compared with homogeneous crosslinking process of solvent-based two-component polyurethane systems, the crosslinking process of 2K-WPUs is a more complicated heterogeneous phase process including water volatilizing, particle merging and chemical crosslinking [12, 13]. Therefore, the crosslinking process of 2K-WPUs is slower and more inefficient than that of solvent-based two-component polyurethane systems.
Adding catalysts can accelerate the crosslinking process of 2K-WPUs. Metal organic compounds and tertiary amines have been reported to be good catalytic activity for the crosslinking reaction of isocyanate groups (–NCO) and hydroxyl groups (–OH), and are widely used as catalysts for the crosslinking reaction of polyurethane system [14, 15]. At present, tin, bismuth, zinc, zirconium and other metal contained organometallic compounds have been used as catalysts for the crosslinking reaction of waterborne polyurethane [16]. Cakic et al. compared the effects of organo-zirconium and organo-manganese (III) on the crosslinking reaction of 2K-WPUs [17].The results showed organo-manganese obtained higher catalytic activity than organo-zirconium, and both catalysts enabled accomplishing more optimal physical-mechanical properties of 2K-WPUs coatings. Werner Blank et al. studied the catalytic effect of organotin and organo-zirconium on the crosslinking reaction of 2K-WPUs [18,19,20].The results showed the organic zirconium catalyst was more selective for the reaction of –NCO with –OH in 2K-WPUs system than dibutyltin dilaurate. However, with the increasing requirements of environmental protection and human health, organometallic compounds catalysts have been restricted in many application fields of coatings, especially in food packaging and children's toys. Triethylamine, triethanolamine, N,N-dimethylethanolamine and other small molecular tertiary amines are another kind of widely used catalysts for the crosslinking reaction of polyurethane. Van Maris systematically studied the catalytic activity of tertiary amines for the reaction of –NCO with –OH with kinetic models, and clarified the relationship between the structure and catalyzing property of small tertiary amines [21]. The results showed the catalytic activity of tertiary amines was mainly determined by the alkaline strength of tertiary amines and the steric effects of the substitution groups bonded to nitrogen atom. Generally, strong alkalinity and small steric hindrance of the tertiary amine lead to the high catalytic activity [22]. However, small molecular tertiary amines only play a catalyst role in polyurethane crosslinking reaction and do not participate in the crosslinking reaction. They exist in the cross-linked polymer in a free state and are easy to volatilize, which not only produces unpleasant odor, but also increases the emission of toxic volatile organic compounds (VOCs). Recent studies have shown workers exposed to a certain concentration of tertiary amines in the air for above 30 min can cause blurred vision, and long-term exposure can cause occupational diseases such as glaucoma [23]. The National Institute of Occupational Safety and Health of the United States has studied the volatility of small molecular tertiary amines used in polyurethane industry and their hazards. A method for determining the concentration of tertiary amines in the air has been developed for enterprises to monitor the concentration of tertiary amines in operation area to ensure workers' health [24].
In view of the potential embryotoxicity and teratogenicity of bisphenol A epoxy resin, synthesis of epoxy resin from renewable feedstock has been growing concerned [25, 26]. Resent years, we have prepared a biomass based epoxy resin from turpentine [27, 28]. In order to improve the crosslinking reaction rate of 2K-WPUs in the absence of external catalysts, in this paper a new type of waterborne polyols containing different tertiary amines in molecular structure was designed and synthesized from the turpentine based epoxy resin and secondary amines. The tertiary amine groups chemically bonded in polyols can self-catalyze the crosslinking reaction between polyols and polyisocyanate to formulate 2K-WPUs in the absence of small molecular tertiary amines, which could solve the key problem of toxic emission of VOCs. Waterborne turpentine-diethanolamine based polyol (WTDP) contained tertiary amines with two hydroxyethyl groups, and waterborne turpentine-benzylethanolamine based polyol (WTBP) contained tertiary amines with a hydroxyethyl group and a benzyl group (Scheme 1). Different structures of tertiary amines with different steric hindrance effects led to different self-catalytic activities. The structure and micromorphology of the polyols were confirmed by Fourier transform infrared (FT-IR) spectrum, nuclear magnetic resonanc (NMR) spectrum and transmission electron microscopy (TEM). The effects of tertiary amine groups on self-catalytic activity of the crosslinking reaction of 2K-WPUs were determined by differential scanning calorimetry (DSC) by comparing with a similar structure polyol, waterborne turpentine-neopentylglycol based polyol (WTNP) without tertiary amine groups. The relationship between the structure of the polyols and the properties of the crosslinked products of the polyols was investigated by DSC, thermogravmetric analysis (TGA) and dynamic mechanical analysis (DMA).
Experimental Details
Materials
The turpentine based epoxy resin with endocyclic structure (Scheme 1) and epoxy value of 3.5 mmol g−1 was synthesized from turpentine, maleic anhydride and epichlorohydrin, named as terpene-maleic ester type epoxy resin (TME) [27]. N-Benzylethanolamine (BEA), hexamethylene diisocyanate (HDI) and 2,2-bis (hydroxymethyl) propionic acid (DMPA) were purchased from Aladdin Industrial Co., Shanghai, China. Neopentyl glycol (NPG), Diethanolamine (DEA), and Sodium hydroxide (NaOH) were supplied by Shanghai Lingfeng Chemical Reagent, Co., Ltd., China. Acetone and alcohol were obtained from Nanjing Chemical Reagent, Co., Ltd., China. The hydrophilically modified hexamethylene diisocyanate (HDI) tripolymer with isocyanate group (–NCO) content of 13% and solid content of 80 wt% was supplied by Wuhan Shiquanxing Polyurethane Technology Co., Ltd., China.
Preparation of TME Based Polyols
Turpentine-neopentylglycol based polyol (TNP) was synthesized as follow (Scheme 1): A 500 mL four-necked flask equipped with stirrer, dropping funnel, thermometer, condenser, and heating mantle was charged with 100.0 g TME, 36.4 g NPG and 5.0 g acetone. The reactants were heated to 90 °C and mixed for 20 min with constant stirring. Then 1.0 g boron fluoride ethyl ether was added drop by drop in about 1 min to catalyze the reaction of epoxy groups and hydroxyl groups. The reaction was continued for 1 h at 100–110 °C. A yellow transparent solid product TNP was obtained, yield > 98%.
Turpentine-diethanolamine based polyol (TDP) and turpentine-benzylethanolamine based polyol (TBP) were synthesized as follow (Scheme 1): A 500 mL three-necked flask equipped with stirrer, thermometer, condenser, and heating mantle was charged with 100.0 g TME, 36.8 g DEA (or 52.9 g BEA) and 16.0 g alcohol. The reactants were heated to 80 °C and reacted for 1 h with constant stirring. Finally, remove ethanol with vacuum distillation, and a yellow transparent solid product TDP (or TBP) was obtained, yield > 98%.
TNP, 1H NMR (500 MHz, CDCl3, δ): 5.98–6.25 (–CH=CH– in endocyclic structure); 4.75–5.25 (–CH–O–); 3.10–4.40 (–CH2–O–); 2.00–2.40 (–CH–C=O); 1.60–1.90 (–CH–C–); 1.11–1.55 (–CH2–C–); 0.61–1.05 (CH3–C). 13C NMR (500 MHz, CDCl3, δ): 170.9–173.5 (–CO–O–); 134.3–136.0 (–CH=CH– in endocyclic structure); 60.9–72.7 (–O–CH2–, –CH–OH, –CH2–OH); 42.8 (–CH–C=O); 33.2–37.0 (quaternary carbons); 28.5–31.5 (tertiary carbons); 16.1–24.2 ( –CH2–CH2– and –CH3). FT-IR (cm−1): 3100–3700 (O–H); 1738 (C =O in ester); 2954, 2876, 1458, 1376 (–CH3, –CH2–); 1170,1110 (–C–O–C–); 1040 (–CH2–OH).
TDP, 1H NMR (500 MHz, CDCl3, δ): 5.98–6.25 (–CH=CH– in endocyclic structure); 4.75–5.25 (–CH–O–); 3.10–4.40 (–CH2–O–); 2.35–3.05 (–CH2–N–); 2.00–2.40 (–CH–C=O); 1.60–1.90 (–CH–C–); 1.11–1.55 (–CH2–C–); 0.61–1.05 (CH3–C–C). 13C NMR (500 MHz, CDCl3, δ): 170.9–173.5 (–CO–O–); 134.3–136.0 (–CH=CH– in endocyclic structure); 60.2–69.9 (–O–CH2–, –CH–OH, –CH2–OH); 58.0 (–CH2–N–); 42.8 (–CH–C=O); 33.2–37.0 (quaternary carbons); 28.5–31.5 (tertiary carbons); 16.1–24.2 ( –CH2–CH2– and –CH3). FT-IR (cm−1): 3000–3700 (O–H, N–H); 1738 (C=O in ester); 2954, 2876, 1458, 1376 (–CH3, –CH2–); 1170 (–C–O–C–); 1068 (–C–N–); 1040 (–CH2–OH).
TBP, 1H NMR (500 MHz, CDCl3, δ): 6.88–7.90 (–CH=CH– in aromatic ring); 5.98–6.25 (–CH=CH– in endocyclic structure); 4.75–5.25 (–CH–O–); 3.10–4.40 (–CH2–O–); 2.35–3.05 (–CH2–N–); 2.00–2.40 (–CH–C=O); 1.60–1.90 (–CH–C–); 1.11–1.55 (–CH2–C–); 0.61–1.05 (CH3–C). 13C NMR (500 MHz, CDCl3, δ): 170.9–173.5 (–CO–O–); 137.4–139.0, 126.5–130.6 (–CH=CH– in aromatic ring); 134.3–136.0 (–CH=CH– in endocyclic structure); 60.9–79.7 (–O–CH2–, –CH–OH, –CH2–OH); 58.0 (–CH2–N–); 42.8 (–CH–C=O); 33.2–37.0 (quaternary carbons); 28.5–31.5 (tertiary carbons); 16.1–24.2 ( –CH2–CH2– and –CH3). FT-IR (cm−1): 3000–3700 (O–H, N–H); 1738 (C=O in ester); 2954, 2876, 1458, 1376 (–CH3, –CH2–); 1600, 1500 (aromatic ring);1170 (–C–O–C–); 1068 (–C–N–); 1040 (–CH2–OH); 737, 698 (one-substituted aromatic ring).
Preparation of Waterborne TME Based Polyols
Waterborne TME based polyol dispersions were prepared as follow (Scheme 2): 6.0 g DMPA, 15.2 g HDI and 21.0 g acetone were placed in a 500 mL four-necked flask equipped with stirrer, dropping funnel, thermometer, condenser, and heating mantle. The mixture was heated to 56 °C (the reflux temperature of acetone) and reacted for 4 h with constant stirring. Then, 52.3 g TNP (or 52.4 g TDP or 58.8 g TBP) was added into the four-necked flask. The reaction was continued for 2–5 h at about 60 °C. 1.8 g NaOH dissolved in 10 g water was added drop by drop in about 1 min and continued stirring for 30 min. The reaction product was dispersed with water directly at stirring rate of 400 rpm at 60 °C, and the water was added drop by drop in about 30 min. Finally, anionic TME based polyol dispersions with solid content of 35 wt% were obtained after removal of acetone by a rotary evaporator under reduced pressure. The waterborne polyol prepared with TNP, TDP or TBP, was denoted as WTNP, WTDP or WTBP, respectively. The technical parameters of WTNP, WTDP, WTBP resins and dispersions were summarized in Table 1.
WTNP, 1H NMR (500 MHz, CDCl3, δ): 8.20–8.50 (–O–CO–NH–); 5.98–6.25 (–CH=CH– in endocyclic structure); 4.75–5.25 (–CH–O–); 4.01–4.60 (–CH2–O–CO–NH–); 3.30–4.40 (–CH2–O–); 2.60–3.20 (–CO–NH–CH2–); 2.00–2.40 (–CH–C=O); 1.60–1.90 ( CH–C–); 1.11–1.55 (–CH2–C–); 0.61–1.05 (CH3–C). 13C NMR (500 MHz, CDCl3, δ): 170.9–173.5 (–CO–O–); 157.0 (–O–CO–NH–); 134.3–136.0 (–CH=CH in endocyclic structure); 60.1–71.0 (–O–CH2–, –CH–OH, –CH2–OH); 56.2 (–CH2–O–CO–NH–); 42.8 (–CH–C=O); 33.2–37.0 (quaternary carbons); 28.5–31.5 (tertiary carbons); 16.1–24.2 (–CH2–CH2– and –CH3). FT-IR (cm−1): 3100–3700 (O–H, N–H); 1738 (C=O in ester); 1700 (C=O in urethane); 1540 (–CO–NH–); 2954, 2876, 1458, 1376 (–CH3, –CH2–); 1250 (–C–O–C– in urethane); 1130 (–C–O–C–); 1040 (–CH2–OH).
WTDP, 1H NMR (500 MHz, CDCl3, δ): 8.20–8.50 (–O–CO–NH–); 5.98–6.25 (–CH=CH– in endocyclic structure); 4.75–5.25 (––CH–O–); 3.90–4.60 (–CH2–O–CO–NH–); 3.30–4.40 (–CH2–O–); 2.30–3.10 (–CO–NH–CH2–); 2.00–2.30 (–CH–C=O); 1.60–1.90 (–CH–C–); 1.11–1.55 (–CH2–C–); 0.61–1.05 (CH3–C). 13C NMR (500 MHz, CDCl3, δ): 170.9–173.5 (–CO–O–); 157.0 (–O–CO–NH–); 134.3–136.0 (–CH=CH– in endocyclic structure); 60.1–70.0 (–O–CH2–, –CH–OH, –CH2–OH); 58.0 (–CH2–N–); 57.4 (–CH2–O–CO–NH–); 42.8 (–CH–C=O); 33.2–37.0 (quaternary carbons); 28.5–31.5 (tertiary carbons); 16.1–24.2 ( –CH2–CH2– and –CH3). FT-IR (cm−1): 3000–3700 (O–H, N–H); 1738 (C=O in ester); 1700 (C=O in urethane); 1540 (–CO–NH–); 2954, 2876, 1458, 1376 (–CH3, –CH2–); 1170,1130 (–C–O–C–); 1068 (–C–N–); 1040 (–CH2–OH).
WTBP, 1H NMR (500 MHz, CDCl3, δ): 8.20–8.50 (–O–CO–NH–); 6.88–7.90 (–CH=CH– in aromatic ring); 5.98–6.25 (–CH=CH– in endocyclic structure); 4.85–5.25 (––CH–O–); 4.01–4.80 (–CH2–O–CO–NH–); 3.30–4.35 (–CH2–O–); 2.40–3.30 (–CO–NH–CH2–); 2.00–2.40 (–CH–C=O); 1.60–1.90 (–CH–C–); 1.05–1.55 (–CH2–C–); 0.61–1.05 (CH3–C). 13C NMR (500 MHz, CDCl3, δ): 170.9–173.5 (–CO–O–); 157.0 (–O–CO–NH–); 137.4–139.0, 126.5–130.6 (–CH=CH– in aromatic ring); 134.3–136.0 (–CH=CH– in endocyclic structure); 60.9–71.7 (–O–CH2–, –CH–OH, –CH2–OH); 59.0 (–CH2–N–); 56.2 (–CH2–O–CO–NH–); 42.8 (–CH–C=O); 33.2–37.0 (quaternary carbons); 28.5–31.5 (tertiary carbons); 16.1–24.2 (–CH2–CH2– and –CH3). FT-IR (cm−1): 3000–3700 (O–H, N–H); 1738 (C=O in ester); 1700 (C=O in urethane); 1540 (–CO–NH–); 2954, 2876, 1458, 1376 (–CH3, –CH2–); 1170 (–C–O–C–); 1068 (–C–N–); 1040 (–CH2–OH); 737, 698 (one-substituted aromatic ring).
Preparation of 2K-WPUs from Waterborne TME Based Polyols
Three kinds of 2K-WPUs were prepared by blending waterborne TME based polyols (WTNP, WTDP or WTBP) with the hydrophilically modified HDI tripolymer respectively in molar ratio of hydroxyl group to isocyanate group 1:1.2. The well-mixed blend dispersions were degassed under vacuum at ambient temperature. Subsequently, the resulting dispersions were cast on clean Teflon plates and dried for 7 days at room temperature. The crosslinked films with a thickness of approximately 100 µm were obtained.
Kinetics Model for the Crosslinking Reaction of 2K-WPUs from Waterborne TME Based Polyols
DSC is a convenient way to study the crosslinking reactions of thermosetting polymers. The heat release of the crosslinking reactions can be precisely measured with DSC. The effects of tertiary amine containing polyols on self-catalytic activity of the crosslinking reaction of 2K-WPUs was determined with DSC. DSC kinetic data are always analyzed with the following general expression:
where α is the conversion, dα/dt is the crosslinking rate, f(α) is some function of α [29]. The apparent rate constant k is usually assumed to be in the Arrhenius form:
where A is the pre-exponential factor, Ea is the activation energy, R is the gas constant (8.314 J/mol K), and T is the absolute temperature.
Then, the reaction rate equation can be obtained as follows:
According to the Arrhenius form, the apparent rate constant k of the reaction is exponentially dependent on the the activation energy (Ea). The crosslinking reaction with low Ea has larger rate constant and high reaction rate. So Ea values can indicate the catalytic activity of the crosslinking reaction of 2K-WPUs. We used the Friedman and Flynn–Wall–Ozawa methods which are two the most frequently cited model-free methods to calculate Ea of the crosslinking reaction of the waterborne polyols containing different structures [30, 31].
Sample tubes filled with about 1 g sample of each 2K-WPUs were freeze-dried in a vacuum freeze-drying machine with the sampling temperature below − 30 °C for 24 h to remove water. After dried place about 5 mg 2K-WPUs samples in hermetically sealed high-volume stainless-steel pans, weighed, and then scanned with a Perkin–Elmer Diamond DSC from 30 to 200 °C at three different heating rates: 5, 10 and 20 K/min. Each DSC test used a fresh specimen. High-volume stainless-steel pans capable of withstanding vapor pressure up to 3.8 MPa were used to prevent the test samples from evaporating at high temperatures. The DSC tests on every condition were duplicated twice.
Characterizations
FT-IR spectra of the polyol samples were measured on an ALPHA-II Fourier transform infrared spectrophotometer (Bruker, Switzerland) in an attenuated total reflection (ATR) mode. Each sample was scanned ranging from 4000 to 500 cm−1 with an average of 32 scans.
NMR spectra were recorded on an AVANCE AV-500 nuclear magnetic resonance spectrometer (Bruker, Switzerland). The polyol samples were solved in deuterated chloroform. Chemical shifts were calculated relative to TMS for NMR control.
Gel permeation chromatography (GPC) was used to calculate the average molecular weight (Mw) of the polyols. The GPC analysis was performed on a Waters 1550 GPC system (Waters, USA) consisting of two styragel columns connected in series, a Waters 2487 ultraviolet detector and a Waters 2414 refractive index detector. The polyol samples were solved in tetrahydrofuran at a concentration of 10 mg mL−1 and filtered through a 0.45 µm hydrophobic fluoropore polytetrafluoretyhylene filter before injection into the GPC system.
Particle size test was carried out on a Nano-ZS ZEN3600 Zeta-sizer (Malvern Instrument Co., UK). The polyol dispersions were diluted to a solids content of 0.2%–0.3% with deionized water, and 1 mL of the dispersion was placed in a cuvette for performing particle size analysis.
The particle morphology of polyol dispersions were observed with a Tecnai G2 20 S-TWIN transmission electron microscopy (FEI, USA). The waterborne TME based polyols were diluted in water to 0.5 wt% and dispersed by sonication for 10 min. A drop of diluted polyol dispersion was dripped on a C-coated Cu grid, and dried for 2 h for TEM measurement.
The glass transition temperature (Tg) of the crosslinked products of 2K-WPUs was measured with DSC. DSC measurements were carried out on a differential scanning calorimeter (PerkinElmer Diamond, USA) at heating rate of 20 K min−1 and nitrogen gas flow of 20 mL min−1. The specimen was crumple-sealed in aluminum crucible and scanned from − 50 to 180 °C.
The thermal stability of the crosslinked products of 2K-WPUs was measured with TGA. TGA) was performed with STA 409 PC/PG thermogravimetric analyzer (Netzsch Co., Germany) at a heating rate of 10 K min−1 under a nitrogen atmosphere from 25 to 800 °C.
DMA was carried out on a Q800 dynamic mechanical analyzer (TA instrument, USA) at frequency of 1 Hz, temperature range of −100–180 °C, and heating rate of 3 K min−1. The size of the specimens cut from the crosslinked products of 2K-WPUs was 40 mm × 6.5 mm × 0.1 mm.
Drying time was measured according to standard test method GB/T 1728-1979. Gloss was measured according to standard test method GB/T 9754-2007. Mechanical properties of the films were evaluated according to standard test methods (impact strength GB/T 1732-1993, adhesion GB/T 1720-1989, flexibility GB/T 1731-1993, pencil hardness GB/T 6739-1996). Water resistance, antifouling and blocking resistance properties were measured according to standard test method GB/T 23999-2009.
Results and Discussion
Micromorphologies of Waterborne TME Based Polyols
Figure 1 shows the TEM images of waterborne TME based polyols dispersions. The particle sizes of WTNP and WTBP dispersions distributed in nano scale with particle sizes from 80 to 500 nm. The storage stability of WTNP and WTBP dispersions was longer than one year. However the particle size of WTDP dispersion distributed in a wide range, and some micron-sized particles existed in the range of 1–3 µm. The particle size uniformity and the storage stability of WTDP dispersion were poor. Because TDP contained four primary hydroxyl groups, while TBP and TNP contained only two primary hydroxyl groups. When TDP was extended by hydrophilic chain segments (Scheme 2), it was easier for TDP to carry out intramolecular chain extension reaction, which resulted in the other TDP grafting less hydrophilic chain segments. As a result, the particle size uniformity of WTDP dispersion was poor.
Kinetics of the Crosslinking Reaction of 2K-WPUs from Waterborne TME Based Polyols
Figure 2 shows the DSC curves of the crosslinking reaction of 2K-WPUs from waterborne TME based polyols. By integrating the area under the exotherm peak of DSC curves, α versus time (t) curves of the crosslinking reaction were obtained (Fig. 3). As seen the consuming time at the same α of the three polyols in Fig. 3, amino polyols (WTDP and WTBP) got fast crosslinking reaction rate, while the similar structure polyol (WTNP) without tertiary amine group got the slowest crosslinking reaction rate among the three polyols. The catalytic mechanism of tertiary amine was that –NCO groups were attacked by tertiary amine to form intermediates and reacted with –OH groups (Scheme 3) [22]. Compared with WTBP, WTDP contained tertiary amines with smaller steric hindrance effect, resulting in higher self-catalytic activity and faster crosslinking reaction rate. In order to investigate the kinetics of the crosslinking reaction of the three polyols, Ea of the crosslinking reactions were calculated with the Friedman and Flynn–Wall–Ozawa methods.
Friedman gave a straightforward method to determine Ea as a function of the extent of α, which is the most common differential isoconversional method for kinetic analysis [32]. According to the Friedman method, Ea can be calculated by using the following logarithmic form of the reaction rate equation (Eq. 3):
The reaction rate dα/dt can be obtained by differentiating the curves of α versus t. Assuming dα/dt to be only a function of α and T, ln(dα/dt) is described as Eq. (4). With Eq. (4), a plot of ln(dα/dt) versus 1/T at the same α from DSC curves with various heating rates yielded a straight line with slope—Ea/R. The relationship between Ea and α was obtained by repeating this procedure. Table 2 listed the values of Ea calculated at different conversions using the Friedman method. Ea tended to fluctuate in a conversion interval ranging from 0.2 to 0.8. The Ea values of the crosslinking reaction of the polyols containing tertiary amine groups were obviously lower than that of the crosslinking reaction of WTNP. Low Ea favors fast reaction rate, which indicates the tertiary amine groups contained in the polyol structure can self-catalyze the crosslinking reaction of 2K-WPUs. Compared with WTBP, WTDP with smaller steric hindrance effect of the tertiary amine groups got lower Ea values of the crosslinking reaction. These results agreed well with the results from Fig. 3.
The Friedman method is an isoconversional differential method. Flynn, Wall, and Ozawa have provided an isoconversional integral method which does not require any assumptions concerning the form of the kinetic equation other than the Arrhenius-type temperature dependence for the calculation of activation energy values [33,34,35]. This Flynn–Wall–Ozawa method is also a model-free method which contains measuring the temperatures corresponding to fixed values of α from DSC with various heating rates. According the Flynn–Wall–Ozawa method, dα/dt in Eq. (3) was integrated and then Doyle Eq. (5) was obtained by applying a Doyle approximation [36].
In Eq. (5) g(α) was the integral form of the kinetic model. A plot of ln β versus 1/T was obtained from thermal curves recorded at several heating rates. For constant α, this plot should be a straight line whose slope could allow evaluation of Ea. Ea values calculated at different conversions by the Flyn–Wall–Ozawa method are shown in Table 3. The calculated result from the Flyn–Wall–Ozawa method was similar with that from the Friedman method. WTNP without tertiary amine group got the highest Ea of the crosslinking reaction, and WTDP with small steric hindrance tertiary amine groups got the lowest Ea of the crosslinking reaction. These results indicated the tertiary amine groups in the polyol structure could improve the crosslinking rate of the 2K-WPUs, and tertiary amine groups with smaller steric hindrance exhibited higher catalytic activity.
FT-IR Analysis of the Crosslinking Reaction at Room Temperature
Most applications of 2K-WPUs require the crosslinking reaction to take place at room temperature. FT-IR was employed to investigate the crosslinking properties of the three kinds of 2K-WPUs prepared from WTBP, WTDP and WTNP polyols respectively at room temperature (25 °C and 60% humidity). The change of vibration absorption peak of –NCO group in FT-IR spectra was used to track the crosslinking reaction of the 2K-WPUs (Fig. 4). WTDP prepared with diethanolamine contained high hydroxyl content and small steric hindrance of tertiary amine group. The crosslinking reaction between WTDP and polyisocyanate was fast. And –NCO group disappeared completely in FT-IR spectra after curing for 3 days at room temperature, which indicated that the crosslinking reaction had been completed. However the application period of 2K-WPUs from WTDP was short, less than 1 h, as a result of the high self-catalytic activity of WTDP. WTNP prepared with neopentyl glycol did not contain tertiary amine group. The crosslinking reaction between WTNP and polyisocyanate was quite slow, and –NCO group still could be found in FT-IT spectra after curing for 6 days at room temperature. WTBP prepared with benzylaminoethanol contained tertiary amine groups with big steric hindrance effect. The crosslinking reaction rate of WTBP was slower than that of WTDP and faster than that of WTNP. –NCO groups of 2K-WPUs from WTBP disappeared after curing for 4 days at room temperature. The application period of the 2K-WPUs from WTBP was about 2 h.
FT-IR results of the crosslinking reaction at room temperature were in agreement with the kinetics analysis of the crosslinking reaction of 2K-WPUs from DSC. Tertiary amine groups in the polyol structure could improve the crosslinking rate of the 2K-WPUs, and tertiary amine groups with smaller steric hindrance exhibited higher catalytic activity.
Structures Characterization of the Crosslinked Products of 2K-WPUs from Waterborne TME Based Polyols
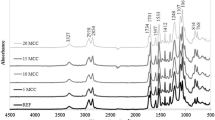
Figure 5 shows the FT-IR spectra of the crosslinked products of WTBP, WTDP and WTNP. The characteristic absorption peak of - NCO group near 2270 cm−1 all disappeared in the spectra of the three crosslinked products, indicating that the products had been crosslinked completely. Typical characteristic absorption peaks of amide bond band I (1680 cm−1, C=O) and band III (1535 cm−1, C–N) appeared in FT-IR spectra, corresponding to the molecular structure characteristics of polyurethane [37]. Comparing the spectra of the three crosslinked products, the products of WTNP had strong characteristic absorption peaks of ether-type C–O–C at 1109 cm−1, while the products of WTDP and WTBP had characteristic absorption peaks of C–N–C at 1104 cm−1, which were consistent with the structural characteristics of ether polyol (WTNP) and amino polyols(WTDP and WTBP). WTBP was prepared with benzylaminoethanol, so the characteristic absorption peaks of aromatic ring appeared at 737 cm−1 and 698 cm−1 in the FT-IR spectra of the product of WTBP.
Thermal Properties of the Crosslinked Products of 2K-WPUs from Waterborne TME Based Polyols
DSC curves of the crosslinked products of WTBP, WTDP and WTNP are shown in Fig. 6. 2K-WPUs belongs to thermosetting resin, and all the three crosslinked products got glass transition temperature (Tg) higher than room temperature. Compared with WTNP, WTDP contained higher hydroxyl value resulting to higher crosslinked density, so the Tg of its crosslinked product was higher than that of WTNP. WTBP contained lots of aromatic rings which are hard segments in molecular structure, resulting to the highest Tg among the three crosslinked products.
Figure 7 shows the thermogravimetric curves of the crosslinked products of WTDP, WTBP and WTNP. The thermal degradation of polyurethane usually involves a two-stage degradation [38, 39]. The first stage is the breaking of C–N (305 kJ/mol) bond with smaller bond energy, and the second stage is the breaking of C–C (346 kJ/mol) and C–O (358 kJ/mol) bonds with larger bond energy. The thermogravimetric curves of the crosslinked products of the three polyols showed similar trends, and there were two distinct degradation peaks in the DTG curves. Comparing the three crosslinked products, the thermal stability of the first degradation stage differed greatly due to the different C–N bond content of the three crosslinked products. Because WTNP was not an amino polyol, its crosslinked product contained the least C–N bond and exhibited the best thermal stability in the first stage among the three crosslinked products. While WTDP contained higher hydroxyl content and more C–N bonds, its crosslinked product exhibited lower thermal stability in the first stage. In the second degradation stage, the thermal stability of crosslinked product of WTBP was the best because of its aromatic ring structures.
Dynamic Mechanical Properties of the Crosslinked Products of 2K-WPUs from Waterborne TME Based Polyols
The storage modulus (G′) and loss factor (tan delta) of the crosslinked products of WTBP, WTDP and WTNP are shown in Fig. 8. The peak temperature of tan delta corresponds to the temperature at which α relaxation associated with the glass transition of the polymers occurs, i.e. glass transition temperature (Tg). The results from loss factor curves were consistent with those from DSC analysis. The Tg of the crosslinked products of WTBP was the highest, followed by that of WTDP, and that of WTNP was the lowest. The reasons had been given in DSC analysis. The storage modulus (G′) of the crosslinked products of the three polyols showed a similar trend with temperature. G′ decreased gradually with an increase of temperature. When the temperature was close to Tg, α relaxation of the crosslinked products occurred, resulting to a sharp decrease of G′. Among the three crosslinked products, the crosslinked product of WTBP got the highest G′ at the same temperature, because WTBP contained aromatic rings. G′ of the crosslinked products of WTDP was higher than that of WTNP at the same temperature, because WTDP contained higher hydroxyl value and got higher crosslinked density.
Application of the 2K-WPUs as Waterborne Wood Coatings
2K-WPUs from waterborne TME based polyols were applied as waterborne wood coatings, and a commercial 2K-WPU from Wanhua was used as a control. The properties of the films were shown in Table 4. The impact strength, adhesion, flexibility, water resistance and antifouling resistance properties of the 2K-WPUs from waterborne TME based polyols were just similar to those of the commercial product. The drying time of the 2K-WPUs with amino polyols (WTDP and WTBP) was faster than that of the commercial product as a result of the self-catalytic property of the tertiary amine groups in WTDP and WTBP. The film from WTBP had higher gloss and pencil hardness, because WTBP contained aromatic rings. The film from WTDP had faster drying time, because WTDP contained high hydroxyl value and tertiary amine group with small steric hindrance.
Conclusions
Three kinds of waterborne polyols were prepared by modifying a biomass based epoxy resin (TME) with N-benzylethanolamine, diethanolamine and neopentyl glycol, respectively. The polyols containing tertiary amines self-catalyzed the crosslinking reaction between polyols and polyisocyanate in the absence of external catalysts, which resulted in high efficiency of the film formation of 2K-WPUs. Steric hindrance effect of the tertiary amine groups of the polyols affected the catalytic activity of the reaction. Smaller steric hindrance led to faster crosslinking reaction rate. The different structures of the tertiary amines in the polyols also affected the properties of the crosslinked products. Storage modulus (G′) and glass transition temperature (Tg) of the crosslinked products were increased when the polyols contained high hydroxyl value and aromatic ring. The thermal stability of the crosslinked products of amino polyols was a little worse than that of the crosslimked products of the polyol without tertiary amines, because the amino polyols contained more C–N bond which had smaller bond energy than those of C–C and C–O bonds. Compared with the commercial 2 k-WPU, using waterborne amino polyols to formulate 2K-WPUs can improve the film formation efficiency without adding small molecular catalysts, which could reduce the toxic emission of VOCs.
References
Kang SY, Ji ZX, Tseng LF, Turner SA, Villanueva DA, Johnson R, Albano A, Langer R (2018) Design and synthesis of waterborne polyurethanes. Adv Mater 30:1706237
Wang J, Zhang HM, Miao YY, Qiao LJ, Wang XH (2017) A whole-procedure solvent-free route to CO2-based waterborne polyurethane by an elevated-temperature dispersing strategy. Green Chem 19:2194–2200
Zafar F, Ghosal A, Sharmin E, Chaturvedi R, Nishat N (2019) A review on cleaner production of polymeric and nanocomposite coatings based on waterborne polyurethane dispersions from seed oils. Prog Org Coat 131:259–275
Etxaniz I, Llorente O, Aizpurua J, Martin L, Gonzalez A, Irusta L (2019) Dispersion characteristics and curing behaviour of waterborne UV crosslinkable polyurethanes based on renewable dimer fatty acid polyesters. J Polym Environ 27:189–197
Zhou WS, Liu DY, Liu T, Ni LJ, Quan H, Sun QC (2019) Emulsion stability and water tolerance of cationic waterborne polyurethane with different soft segment ratios between trifunctional polyether and bifunctional polyester. Mater Res Express 6:065303
Kim HA, Kim BK (2019) Synthesis and properties of waterborne polyurethane/hydroxyapatite chemical hybrids. Prog Org Coat 128:69–74
Ding JH, Rahman OU, Peng WJ, Dou HM, Yu HB (2018) A novel hydroxyl epoxy phosphate monomer enhancing the anticorrosive performance of waterborne graphene/epoxy coatings. Appl Surf Sci 427:981–991
Mondragon G, Santamaria-Echart A, Hormaiztegui MEV, Arbelaiz A, Pena-Rodriguez C, Mucci V, Corcuera M, Aranguren MI, Eceiza A (2018) Nanocomposites of waterborne polyurethane reinforced with cellulose nanocrystals from sisal fibres. J Polym Environ 26:1869–1880
Wang L, Xu F, Li HX, Liu YY, Liu YL (2017) Preparation and stability of aqueous acrylic polyol dispersions for two-component waterborne polyurethane. J Coat Technol Res 14:215–223
Wu GM, Liu D, Chen J, Liu GF, Kong ZW (2019) Preparation and properties of super hydrophobic films from siloxane-modified two-component waterborne polyurethane and hydrophobic nano SiO2. Prog Org Coat 127:80–87
Wicks Z, Wicks D, Rosthauser J (2002) Two package waterborne urethane systems. Prog Org Coat 44:161–183
Wu GM, Chen J, Huo SP, Liu GF, Kong ZW (2014) Thermoset nanocomposites from two-component waterborne polyurethanes and cellulose whiskers. Carbohydr Polym 105:207–213
Winnik MA (2002) Interdiffusion and crosslinking in thermoset latex films. J Coat Technol 74:49–63
Villegas-Villalobos S, Diaz LE, Vilarino-Feltrer G, Valles-Lluch A, Gomez-Tejedor J, Valero A MF (2018) Effect of an organotin catalyst on the physicochemical properties and biocompatibility of castor oil-based polyurethane/cellulose composites. J Mater Res 33:2598–2611
Gogol R, Niyogi UK, Alam MS, Mehra DS (2012) Effect of organometallic and tertiary amine catalyst on the properties of polyurethane prepolymer. J Polym Mater 29:451–462
Sardon H, Irusta L, Fernandez-Berridi MJ (2009) Synthesis of isophorone diisocyanate (IPDI) based waterborne polyurethanes: Comparison between zirconium and tin catalysts in the polymerization process. Prog Org Coat 66:291–295
Cakic S, Lacnjevac C, Stamenkovic J, Ristic N, Takic L, Barac M, Gligoric M (2007) Effects of the acrylic polyol structure and the selectivity of the employed catalyst on the performance of two-component aqueous polyurethane coatings. Sensors 7:308–318
He ZA, Werner JB, Picci ME (2002) A selective catalyst for two-component waterborne polyurethane coatings. J Coat Technol 74:31–36
Werner JB, He ZA, Hessell ET (1999) Catalysis of the isocyanate-hydroxyl reaction by non-tin catalysts. Prog Org Coat 35:19–29
Werner JB (2002) Advances in catalysis for organic coatings. Chim Int J Chem 56:191–196
Van Maris R (2005) Polyurethane catalysis by tertiary amines. J Cell Plast 41:305–322
Silva AL, Bordado JC (2004) Recent developments in polyurethane catalysis: catalytic mechanisms review. Catal Rev 46:31–51
Jang JK (2016) Amines as occupational hazards for visual disturbance. Ind Health 54:101–115
Foley GD, Tucker SP, Cooper CV (1991) Analysis of air for tertiary amine catalysts used in the polyurethane foam industry. Am Ind Hyg Assoc J 52:664–665
Huang K, Zhang P, Zhang JW, Li SH, Li M, Xia JL, Zhou YH (2013) Preparation of biobased epoxies using tung oil fatty acid-derived C21 diacid and C22 triacid and study of epoxy properties. Green Chem 15:2466–2475
Mantzaridis C, Brocas A, Llevot L, Cendejas A, Auvergne G, Caillol R, Carlotti S, Cramail S H (2013) Rosin acid oligomers as precursors of DGEBA-free epoxy resins. Green Chem 15:3091–3098
Wu GM, Kong ZW, Huang H, Chen J, Chu FX (2007) Synthesis of epoxy resin from hydrogenated terpinene- maleic anhydride. Chem Ind Forest Prod 27:57–62
Wu GM, Liu D, Liu GF, Chen J, Huo SP, Kong ZW (2015) Thermoset nanocomposites from waterborne bio-based epoxy resinand cellulose nanowhiskers. Carbohydr Polym 127:229–235
He Y (2001) DSC and DEA studies of underfill curing kinetics. Thermochim Acta 367:101–106
Yousefi A, Lafleur PG, Gauvin R (1997) Kinetic studies of thermoset cure reactions: a review. Polym Compos 18:157–168
Sbirrazzuoli N, Vyazovkin S (2002) Learning about epoxy cure mechanisms from isoconversional analysis of DSC data. Thermochim Acta 388:289–298
Friedman HL (1965) Kinetics of thermal degradation of charforming plastics from thermo-gravimetry. Application to a phenolic plastic. J Polym Sci C 50:183–195
Ozawa T (1965) A new method analyzing thermogravimetric data. Bull Chem Soc Jpn 38:1881–1886
Flynn JH, Wall LA (1966) General treatment of the thermogravimetry of polymers. J Res Natl Bur Stand Sect A 70:487–523
Ozawa T (2000) Thermal analysis – review and prospect. Thermochim Acta 355:35–42
Doyle C (1962) Estimating isothermal life from thermogravimetric data. J Appl Polym Sci 6:639–642
Nagle DJ, Celina M, Rintoul L, Fredericks PM (2007) Infrared microspectroscopic study of the thermo-oxidative degradation of hydroxy-terminated polybutadiene/isophorone diisocyanate polyurethane rubber. Polym Degrad Stab 92:1446–1454
Levchik SV, Weil ED (2004) Thermal decomposition, combustion and fire-retardancy of polyurethanes—a review of the recent literature. Polym Int 53:1585–1610
Allan D, Daly J, Liggat JJ (2017) Structural and thermal degradation properties of novel metallocenepolyurethanes. Polym Degrad Stab 136:39–47
Acknowledgements
The authors gratefully acknowledge the financial support from the Fundamental Research Funds for the Central Non-profit Research Institution of Chinese Academy of Forestry (CAFYBB2017ZC005) and the Natural Science Foundation of Jiangsu Province (BK20191134).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wu, G., Bian, J., Liu, G. et al. Self-Catalytic Two-Component Waterborne Polyurethanes with Amino Polyols from Biomass Based Epoxy Resin. J Polym Environ 28, 713–724 (2020). https://doi.org/10.1007/s10924-019-01638-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10924-019-01638-1