Abstract
In this work, the effect of ethylene vinyl acetate (EVA) content on the rheology, mechanical properties, and morphology of poly (lactic acid) (PLA)/EVA blends was investigated. Differential Scanning Calorimetry (DSC) showed that the addition of EVA to PLA decreased the degree of crystallinity of PLA. Dynamic Mechanical Thermal Analysis (DMTA) results showed that the presence of EVA copolymer increased the cold crystallization temperature (Tcc) of PLA. The addition of 10% of EVA to PLA substantially increased the impact strength. Morphology analysis by Scanning Electron Microscopy (SEM) indicated the immiscibility between PLA and EVA phases and an in increase the EVA average dispersed phase domains size with the increase in EVA content. Rheological measurements under oscillatory shear flow showed that storage modulus (G′) increased with the increase in the EVA content at low frequencies and is related to the increase in the EVA dispersed phase domains size, as also observed by relaxation spectrum analysis. Cole–Cole plots of imaginary viscosity (η″) versus real viscosity (η′) and Han plots of storage modulus (G′) versus loss modulus (G″) indicated that PLA and EVA phases are immiscible, as also observed by SEM.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Most of the petroleum (nonrenewable source) based polymers are resistant to degradation, causing environmental impacts due to inappropriate disposal into the environment, mainly in the form of packaging and bottles. This concerning situation has led many researchers to seek new alternatives to the use of these petroleum-based polymers [1, 2]. Among these alternatives, is the use of bio-based polymers, which are polymers obtained from raw materials derived from renewable sources [3,4,5,6,7] such as corn, sugar cane, cellulose, chitin, etc. [7, 8]. In recent years, research with bio-based polymers such as poly (lactic acid)—PLA, which can be obtained from corn and sugar cane [9], has attracted the attention of many researchers around the world. PLA is a biodegradable, compostable, and biocompatible thermoplastic polyester [10, 11] and has advantages such as easy processability and good mechanical properties [12, 13]. However, it has some limitations, like brittleness, low crystallization rate and low thermal stability [14, 15].
In order to overcome this problem, the properties of PLA may be improved by the addition of other polymers or copolymers (biodegradable and non-biodegradable) [16,17,18,19,20,21,22,23,24,25,26], forming polymer blends. Polymer blends, a mixture of two or more polymers/copolymers, has emerged as an alternative of the synthesis of new polymers, since it is possible to combine the best properties of each of the constituent polymer/copolymer, thus achieving desirable properties. One of the copolymers that are being used in the literature to improve the properties of PLA is Ethylene–Vinyl Acetate (EVA), which has a rubbery nature, flexibility at low temperatures, and high impact strength [27,28,29]. Singla et al. [30] studied PLA blends with EVA containing a high content of vinyl acetate (VA). The authors observed that for small EVA contents there was the formation of a dispersed phase–matrix morphology. For 30% EVA, a co-continuous morphology was formed. The rheological measurements under oscillatory shear flow showed that the complex viscosity (η*) and the storage modulus (G′) of the blends increased with the frequency indicating a strong interaction between the PLA and EVA phases. By means of the Cole–Cole and Han curves, partial miscibility and incompatibility between the PLA/EVA blend phases were observed.
Ma et al. [31] studied the PLA toughness by adding EVA containing different vinyl acetate contents. The authors observed that there was an improvement in the compatibility between the phases of PLA and EVA with the increase in the vinyl acetate content. With low vinyl acetate content, high dispersed EVA phase domains and low interfacial adhesion was observed, which compromised the PLA toughness.
Sangeetha et al. [28] investigated the effect of different EVA, containing 40% of vinyl acetate (VA), content (5, 10, 15 and 20%) on the mechanical and thermal properties of PLA/EVA blends. The authors found that the impact strength of PLA/EVA blend was significantly increased with the addition of 15% of EVA. From morphology analysis, an increase in EVA particles size distribution with the increase in the EVA content was observed. The thermal stability of PLA was decreased with the addition of EVA.
As can be observed, there are only a few works in the literature that studied the effect of EVA copolymer on the rheological properties of PLA and its correlation with the morphology of PLA/EVA blends. The aim of this work is to evaluate the effect of different EVA content on the rheology, mechanical properties, and morphology of PLA/EVA blends.
Experimental
Materials
Poly (lactic acid)—PLA Ingeo™ 2003D - Nature Works, MFI = 6 g/10 min (210 °C/2.16 kg), was obtained from Cargill, Brazil. Ethylene Vinyl Acetate (EVA) copolymer, vinyl acetate (VA) content of 19 wt%, Evateno® 3019 PE, MFI = 2.5 g/10 min (190 °C/2.16 kg) was provided by Braskem.
Blends Preparation
To prepare PLA/EVA blends containing 5, 10, 15 and 20 wt% of EVA, PLA and EVA were dry blended and fed into the Coperion Werner & Pfleiderer ZSK 18 mm (L/D = 40) modular twin screw extruder with a screw speed of 250 rpm and feeding rate of 5 kg/h. The thermal profile from the hopper to die was: 180:180:180:180:180:180:180 °C. Before blending with EVA, PLA was dried under vacuum at 80 °C during 4 h. The screw profile, containing distributive and dispersive elements is shown in Fig. 1.
Samples Preparation
PLA/EVA blends samples for tensile and impact strength tests were injection molded in an ARBURG Allrounder 270C golden edition injection molding machine at a temperature of 180 °C in all zones and mold temperature of 20 °C.
X-Ray Diffraction (XRD)
X-Ray Diffraction (XRD) analysis was carried out in a Shimadzu XRD-6000 Diffractometer (Cu Kα radiation, λ = 1. 5406 Å) at scanning speed of 2°/min and 2θ angle ranging from 2.0 to 30°.
Differential Scanning Calorimetry (DSC)
DSC analysis was performed, under N2 with a flow rate of 50 mL/min, in a TA Instruments SDT Q20 Differential Scanning Calorimeter. The samples, weighing about 3 mg, were heated from room temperature to 200 °C, at a heating rate of 10 °C/min. The degree of crystallinity (Xc) of neat PLA and of PLA present in the blends was calculated according to Eq. 1.
where Xc = Degree of Crystallinity; ΔHm = Melting Enthalpy; ΔHcc = Cold Crystallization Enthalpy; ΔH ºm = Melting enthalpy of 100% crystalline PLA, which is assumed to be 93 J/g [32]; WPLA = Weight fraction of PLA.
Dynamic Mechanical Thermal Analysis (DMTA)
Dynamic Mechanical Thermal Analysis (DMTA) was performed in an Anton Paar Physica MCR 301 rheometer, equipped with solid rectangular fixtures accessory. The samples were heated from 28 to 150 °C at a heating rate of 5 °C/min, a frequency of 1 Hz and strain 0.1%, under an air atmosphere. DMTA analysis was in rectangular samples, with dimensions of 54.0 × 13.7 × 3.2 mm, cut from injection molded specimens that are used for impact tests.
Rheological Measurements Under Dynamic-Oscillatory Shear Flow
Rheological measurements under dynamic-oscillatory shear flow were carried out in an Anton Paar Physica MCR 301 rheometer, equipped with parallel plate geometry of 25 mm diameter, and a gap between the plates of 1 mm, at a temperature of 180 °C. Frequency sweeps from 628 to 0.0628 rad/s were carried out at low strains (1%) within the linear viscoelastic region. These strains were previously determined by the strain sweeps at 180 °C.
Mechanical Properties
Tensile tests were carried out in a SHIMADZU AG-IS 100 kN Universal Testing Machine, according to ASTM-D 638, in Type I samples, operating at a crosshead speed of 50 mm/min. Izod impact strength tests were performed according to ASTM-D 256 in notched samples, with dimensions of 63.5 × 12.7 × 3.2 mm, at room temperature using a CEAST Resil 5.5 apparatus. The results reported are the average of 6 samples.
Scanning Electron Microscopy (SEM)
For the morphology analysis, cryo-fractured surfaces of the PLA/EVA blends were coated with gold and analyzed using a SHIMADZU SS X550 Super Scan Scanning Electron Microscope. The voltage used in the filament was 15 kV. The SEM micrographs were analyzed using an Image J free image processing software. The number-average diameter (dn), weight-average diameter (dw) and the volume-average diameter (dv) of the particles were calculated using Eqs. 2, 3, 4 [33, 34] respectively.
The interparticle distance (ID) was determined according to Eq. 5 [33]. At least 400 particles from 4 SEM images were used to estimate the diameter of the EVA particles.
where ϕ is the volumetric fraction of the dispersed phase.
Results and Discussion
Influence of the EVA Copolymer on the Crystallinity of PLA
Figure 2 shows the X-Ray Diffraction (XRD) patterns of PLA and of PLA/EVA blends. PLA presents a broad halo over the entire 2θ range. The presence of a halo is an indication that PLA presents an amorphous structure. This may be ascribed to the rapid cooling of the sample in the mold during the injection molding process, preventing the crystallization of PLA. For the PLA/EVA blends, a diffraction peak at 2θ around 21.3° is observed. This peak is related to the polyethylene (PE) present in the EVA copolymer and becomes more intense with the increase in EVA content. The presence of EVA does not affect the structure of PLA, which remains amorphous. Since PLA is biodegradable, it is subjected to the attack of microorganisms during composting. The attack of microorganisms in polymers occurs preferentially in the amorphous regions [8].
Figure 3 shows the Differential Scanning Calorimetry (DSC) thermograms of PLA and of PLA/EVA blends containing 5–20% of EVA copolymer. PLA presents an endothermic peak at around 55 °C, related to the glass transition temperature (Tg), and an exothermic peak at around 100 °C, ascribed to the cold crystallization temperature (Tcc). It also presents an endothermic peak characterized by a double melting peak at temperatures around 151 and 155 °C, related to the melting temperature (Tm) of β and α phases, respectively [35]. PLA/EVA blends present, besides the Tg, Tcc, and Tm peaks of PLA, an endothermic peak at around 83 °C, related the melting temperature of EVA copolymer. With the addition of EVA to PLA, the Tg and Tcc of PLA are shifted to higher temperatures, indicating that EVA is making the crystallization of PLA difficult. Aghjeh et al. [36] also observed an increase in the Tcc of PLA with the addition of EVA. The authors reported that before the cold crystallization of PLA, the EVA droplets are already in the molten state and the presence of these droplets decreases the crystallization rate and the crystallinity of PLA, leading to the increase in Tcc. Different results, however, were obtained by Sangeetha et al. [28]. The authors reported a decrease in the Tcc of PLA with the increase in the EVA content and ascribed this decrease to the nucleating effect of EVA.
Table 1 summarizes the degree of crystallinity (Xc) of neat PLA and of PLA present in the PLA/EVA blends calculated according to Eq. 1. It can be observed that the degree of crystallinity of PLA decreases with the addition of EVA, confirming that the presence of EVA dispersed phase is making the crystallization of PLA difficult.
Figure 4 depicts the results of DMTA analysis of PLA and of PLA/EVA blends containing 5–20% of EVA copolymer. Figure 4a shows the plots of storage modulus (E′) as a function of temperature. PLA presents a sharp decrease in E′ at around 60 °C which is related to the glass transition temperature of PLA. An increase in E′ is observed at a temperature around 98 °C, which is related to the cold crystallization temperature of PLA. The addition of EVA copolymer to PLA leads to the decrease in E′ and hence, of the stiffness, at temperatures between 28 and 60 °C. This decrease may be ascribed to the rubbery nature of EVA copolymer and is more pronounced for the PLA/EVA blend containing 20% of EVA. The cold crystallization temperature (Tcc) of PLA is increased in the presence of EVA, indicating that EVA is affecting the cold crystallization. Figure 4b shows the loss tangent (tan δ) plots as a function of temperature. PLA shows a peak at around 74 °C. This peak is related to the glass transition temperature (Tg) of PLA. With the addition of EVA to PLA, this peak, and hence, the Tg, is shifted towards higher temperatures, being more pronounced for the PLA/EVA blend containing 20% of EVA, indicating that EVA is restricting the mobility of PLA chains, making the crystallization of PLA difficult. Singla et al. [30]. also observed a shift in the Tg (tan δ peak) of PLA to higher temperatures with the addition of EVA. The authors ascribed this increase in the Tg to the enhanced phase adhesion, due to strong polar–polar interaction, between PLA and EVA phases. It can also be observed that the tan δ peak intensity is decreased with the addition of EVA, indicating that the blends are more elastic.
Influence of EVA Content on the Mechanical Properties of PLA/EVA Blends
Table 2 presents the results of the mechanical properties of PLA and of PLA/EVA blends containing different EVA content. The addition of EVA to the PLA matrix leads to the decrease in the modulus and the tensile strength, which may be ascribed to the low modulus (flexibility) of EVA as also observed by Ma et al. [31]. The elongation at break (EB) increases with the increase in the EVA content up to 15%. Further increase in the EVA content to 20% leads to a decrease in the EB. This decrease may be related to the higher EVA dispersed phase domains size for the blend containing 20% of EVA (Table 3). The impact strength is substantially increased with the addition of EVA. The increase is more pronounced for the blends containing 10–20% of EVA. This increase may be ascribed to the elastomeric characteristic of EVA particles. For higher EVA content, from 10 to 20%, there is no significant difference in the impact strength.
Influence of the EVA Content on the Morphology of PLA/EVA Blends
Figure 5 shows the Scanning Electron Microscopy (SEM) micrographs of PLA/EVA blends containing 5–20% of EVA. All the blends present droplet-matrix morphology, where PLA is the matrix and the droplet is the EVA dispersed phase, indicating immiscibility between PLA and EVA phases. For all blends, many EVA dispersed phase particles are pulled out from the PLA matrix, indicating a poor adhesion between the PLA and EVA phases. The higher is the EVA content, higher is the EVA dispersed phase domains size. The increase in the EVA domains size with the increase in EVA content may be ascribed to the coalescence of EVA particles. A similar result was reported by Ma et al. [31], that observed an increase in the EVA domains size with the increase in the EVA content. The authors ascribed this increase to the coalescence of EVA droplets during melt blending due to the higher amount of droplets present in the blends. Sundararaj and Macosko [37] reported that for uncompatibilized blends the final particle size is increased with the dispersed phase concentration due to the increase in the coalescence.
Table 3 summarizes the values of the number-average diameter (dn), weight-average diameter (dw), volume-average diameter (dv), and the interparticle distance (ID) calculated according to Eqs. 2–5. It can be observed that the average diameter of EVA particles increases with the increase in EVA content from 5 to 20%. On the other hand, the distance between EVA particles decreases with the increase in the EVA content, which is also an indication of the coalescence of EVA dispersed phase particles.
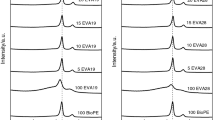
Figure 6 shows the histograms of the EVA particles size distribution. For PLA EVA blend containing 5% of EVA, the distribution of particles is narrow, becoming broader as the EVA content increases.
Figure 7 shows the complex viscosity (η*) (Fig. 7a), Storage Modulus (G′) (Fig. 7b) and damping factor (tan δ) (Fig. 7c) curves as a function of angular frequency (ω) for PLA and PLA blends containing different EVA content (5–20%). PLA exhibits a Newtonian behavior at low frequencies (< 1 rad/s) whereas EVA shows a shear thinning behavior over the entire frequency range. PLA/EVA blends with EVA content up to 15% exhibit a Newtonian behavior at frequencies below 1 rad/s whereas the PLA/EVA blend containing 20% of EVA exhibit a Newtonian behavior only at frequencies below 0.1 rad/s. There is an increase in the complex viscosity of PLA/EVA blends at low frequencies (Fig. 7a) with the increase in EVA content. This increase may be related to the higher viscosity of EVA copolymer when compared to that of PLA. To better understand the effect of EVA content on the viscosity of PLA/EVA blends at low frequencies (< 1 rad/s), the zero shear viscosity of PLA, EVA, and of PLA/EVA blends containing 5–20% of EVA was obtained by fitting the plots of η* versus ω using the Cross model shown in Eq. 6.
where η0 = Zero shear rate viscosity; \(\dot{\gamma }\) = shear rate, τ* = the critical shear stress at the transition from the Newtonian plateau, n = power law index.
The values of the zero shear viscosity (η0) are summarized in Table 4. It can be observed that the η0 of the EVA copolymer is much higher than those of PLA and of PLA/EVA blends. An increase in the values of η0 is observed with the increase in EVA content, being more pronounced for the blend containing 20% of EVA.
At higher frequencies (> 10 rad/s) PLA and the PLA/EVA blends exhibit shear thinning behavior and the values of the complex viscosity of the blends approach the values of the viscosity of the neat PLA.
Figure 7b shows de plots of G′ as a function of ω. At low frequencies (terminal zone), PLA and PLA/EVA blends exhibit a liquid-like behavior (G′ α ω2) whereas EVA exhibits a predominant solid-like behavior (G′ α ω0) as can be observed by the slopes of the plots. G′ increases with the increase in the EVA content, which may be attributed to the increase in the EVA dispersed phase domains size. It is known that the greater is the dispersed phase domains size, longer is the relaxation time, resulting in an increase of G′ at low frequencies [38,39,40]. From SEM micrographs and Table 3 it can be observed that the averaged EVA particles domains size increases with the increase in the EVA content. Figure 7c shows the tan δ plots as a function of ω. PLA shows a liquid-like behavior whereas EVA exhibits a predominant solid-like behavior at low frequencies. For PLA/EVA blends, as the EVA content increases, the slopes of the plots at low frequencies decreases. The decrease in the slope at low frequencies is an indication that the behavior is changed from liquid-like to solid-like, that is, it becomes more elastic than viscous. This behavior is more pronounced for the PLA/EVA blend containing 20% of EVA, where tan δ at low frequencies is almost independent of the angular frequency.
Cole–Cole plots are plots where the imaginary variable is plotted as a function of the real variable [41]. From Cole–Cole plots of imaginary viscosity (η”) versus real viscosity (η’), it is possible to evaluate if the components of the blends are miscible or immiscible. For immiscible blends, Cole–Cole plots of η” versus η’ result in two arcs, which are related to the occurrence of two processes with different relaxation times [42]. However, these plots alone are not sufficient to determine whether or not the phases of the blends are miscible or immiscible. Plots of G’ versus G” (loss modulus) can also be used to evaluate the miscibility between the phases of the blends. G ‘versus G” plots, known as Han plots, are commonly used to detect phase separation in polymer blends [43]. For miscible polymer blends, the Han plots are independent of the composition and temperature and present the same slopes [44].
Figure 8 shows Cole–Cole and Han plots of PLA and of PLA/EVA blends. Figure 8a shows the Cole–Cole plots of η” versus η’. For PLA/EVA blends containing 5 and 10% of EVA, only one arc is observed indicating some miscibility between PLA and EVA phase. For the blends containing 15 and 20% of EVA, the presence of two distinct arcs is observed indicating immiscibility of PLA and EVA phases. Figure 8b shows the Han plots of G’ versus G”. It can be seen that PLA/EVA blends have different slopes at low frequencies. The slope decreases with the increase in the EVA content from 5 to 20%. This is an indication of the immiscibility between PLA and EVA phases, corroborated by the results obtained by SEM.
The weighted relaxation spectrum is sensitive to the structural changes in immiscible polymer blends [45]. Figure 9 shows the weighted relaxation spectra of PLA and of PLA-EVA blends containing 5–20% of EVA copolymer. In the relaxation spectra of the PLA/EVA blends two peaks are observed: the first one is related to the relaxation time of PLA and the second one, is related to the relaxation time of the EVA dispersed phase droplets, as also observed by Lacroix et al. [46] when studying PP/EVA blends. The relaxation time (λ) of the EVA droplets increases from 0.30 to 2.40 s with the increase in the EVA content. According to Calvão et al. [39], the shape relaxation time is directly proportional to the size of the dispersed phase. From SEM analysis (Figs. 5 and 6) it is observed that the EVA dispersed phase particles average size increases with the increase in the EVA content.
Conclusions
The effect of EVA content on the rheology and morphology of PLA/EVA blends was investigated Differential Scanning Calorimetry (DSC) showed that the addition of EVA to PLA decreased the degree of crystallinity of PLA. Dynamic Mechanical Thermal Analysis (DMTA) results showed that The addition of EVA copolymer to PLA decreased the storage modulus (E′) at temperatures between 28 and 60 °C, which may be ascribed to the rubbery nature of EVA, and increased the cold crystallization temperature (Tcc) of PLA. The addition of 10% of EVA to PLA substantially increased the impact strength. Further addition of 15 and 20% of EVA did not affect the impact strength of the blend. From morphology analysis the immiscibility between PLA and EVA phases was observed and that the increase in the EVA content increased the EVA average dispersed phase domains size, which may be ascribed to the coalescence between EVA particles. This result was further confirmed with the particles size distribution analysis. From the rheological measurements under oscillatory shear flow, it was observed that zero shear viscosity (η0) of EVA was much higher than that of PLA and increased with the increase the EVA content in the PLA/EVA blends. An increase in the storage modulus (G′) of PLA/EVA blend at low frequencies with the increase in EVA content was also observed and may be related to the increase in the EVA dispersed phase domains size. These results were corroborated by the relaxation spectrum analysis. Cole–Cole plots of η″ versus η′ and Han plots of G′ versus G″ indicated immiscibility between PLA and EVA phases, corroborated by the morphology analysis.
References
Foruzanmehr M, Vuillaume PY, Elkoun S, Robert M (2016) Mater Des 106:295
Lee JY, Kwon SH, Chin I-J, Choi HJ (2019) Polym Bull
Sangeetha VH, Deka H, Varghese TO, Nayak SK (2018) Polym Compos 39:81
Meng X, Bocharova V, Tekinalp H, Cheng S, Kisliuk A, Sokolov AP, Kunc V, Peter WH, Ozcan S (2018) Mater Des 139:188
Iwata T (2015) Angew Chem Int Ed Engl 54:3210
Nakajima H, Dijkstra P, Loos K (2017) Polymers 9:1
Imre B, Pukánszky B (2013) Eur Polym J 49:1215
Webb H, Arnott J, Crawford R, Ivanova E (2012) Polymers 5:1
Satti SM, Shah AA, Marsh TL, Auras R (2018) J Polym Environ 26:3848
Jalali A, Huneault MA, Elkoun S (2016) J Mater Sci 51:7768
Lim LT, Auras R, Rubino M (2008) Prog Polym Sci 33:820
Chow WS, Teoh EL, Karger-Kocsis J (2018) Express Polym Lett 12:396
Jing M, Sui G, Zhao J, Zhang Q, Fu Q (2019) Compos Part A 117:219
Kakroodi AR, Kazemi Y, Nofar M, Park CB (2017) Chem Eng J 308:772
Chuayjuljit S, Wongwaiwattanakul C, Chaiwutthinan P, Prasassarakich P (2017) Polym Compos 38:2841
Brito GF, Agrawal P, Araújo EM, Mélo TJA (2012) Polímeros 22:427
Gug J, Sobkowicz MJ (2016) J Appl Polym Sci 133:43350
Zhao H, Zhao G (2016) J Mech Behav Biomed Mater 53:59
Wang S, Pang S, Xu N, Pan L, Lin Q (2016) J Appl Polym Sci 133:43424
de Araújo JP, Silva RC, Lima JCC, Agrawal P, de Mélo TJA (2016) Macromol Symp 367:82
Zhang N, Wang Q, Ren J, Wang L (2008) J Mater Sci 44:250
D’Amico DA, Iglesias Montes ML, Manfredi LB, Cyras VP (2016) Polym Test 49:22
Gerard T, Budtova T (2012) Eur Polym J 48:1110
Fortelný I, Ostafińska A, Michálková D, Jůza J, Mikešová J, Šlouf M (2015) Polym Bull 72:2931
Luyt AS, Gasmi S (2016) J Mater Sci 51:4670
Liu Y, Cao K, Karpova S, Olkhov A, Filatova A, Zhulkina A, Burkov A, Fomin SV, Rosa DS, Iordanskii AL (2018) Macromol Symp 381:1800130
Aghjeh MR, Nazari M, Khonakdar HA, Jafari SH, Wagenknecht U, Heinrich G (2015) Mater Des 88:1277
Sangeetha VH, Valapa RB, Nayak SK, Varghese TO (2018) J Polym Environ 26:1
Moura I, Botelho G, Machado AV (2013) J Polym Environ 22:148
Singla RK, Zafar MT, Maiti SN, Ghosh AK (2017) Polym Test 63:398
Ma P, Hristova-Bogaerds DG, Goossens JGP, Spoelstra AB, Zhang Y, Lemstra PJ (2012) Eur Polym J 48:146
Srithep Y, Nealey P, Turng L-S (2013) Polym Eng Sci 53:580
Faker M, Razavi Aghjeh MK, Ghaffari M, Seyyedi SA (2008) Eur Polym J 44:1834
John B, Varughese KT, Oommen Z, Thomas S (2010) Polym Eng Sci 50:665
Mohapatra AK, Mohanty S, Nayak SK (2012) Polym Compos 33:2095
Aghjeh MR, Asadi V, Mehdijabbar P, Khonakdar HA, Jafari SH (2016) Compos Part B 86:273
Sundararaj U, Macosko CW (1995) Macromolecules 28:2647
Yee M, Calvão PS, Demarquette NR (2007) Rheol Acta 46:653
Calvão PS, Yee M, Demarquette NR (2005) Polymer 46:2610
Wu D, Zhang Y, Zhang M, Zhou W (2008) Eur Polym J 44:2171
Dealy JM, Wang J (2013) Melt rheology and its applications in the plastics industry engineering materials and processes, 2nd edn. Springer, Netherlands
Wu D, Zhang Y, Yuan L, Zhang M, Zhou W (2010) J Polym Sci Part B 48:756
Chen Y, Zou H, Liang M, Liu P (2013) J Appl Polym Sci 129:945
Shen G, Shen H, Xie B, Yang W, Yang M (2013) J Appl Polym Sci 129:2103
Kwon MK, Cho KS (2016) Korea-Aust Rheol J 28:23
Lacroix C, Aressy M, Carreau PJ (1997) Rheol Acta 36:416
Acknowledgements
The authors thank CAPES and CNPq for financial support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Agrawal, P., Araújo, A.P.M., Lima, J.C.C. et al. Rheology, Mechanical Properties and Morphology of Poly(lactic acid)/Ethylene Vinyl Acetate Blends. J Polym Environ 27, 1439–1448 (2019). https://doi.org/10.1007/s10924-019-01445-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10924-019-01445-8