Abstract
The crosslinking of chitosan with cyanoguanidine shows some advantages, such as the improved the stability in acid solutions and the decrease of adsorbent cost. In this work, cyanoguanidine-crosslinked chitosan and pure chitosan were prepared to apply in the adsorption of Food Yellow 4 (FY4) and Food Blue 2 (FB2), in single and binary systems. Effects of pH and deacetylation degree (DD) of chitosan in adsorption were evaluated. The adsorbents were characterized by Fourier transform infrared spectroscopy and scanning electron microscopy. The kinetic data were analyzed by pseudo-first order, pseudo-second order and Avrami models. The conditions of pH 3 and DD 95% were the more suitable to reach the highest adsorption capacities in all experimental assays. Under these conditions, the adsorption capacities for FY4 were approximately of 392 and 200 mg g−1 and, for FB2 were approximately of 370 and 184 mg g−1, respectively, in the single and binary systems. The Avrami model was suitable to represent the kinetic curves in all conditions, and the highest adsorption capacities were found for FY4 in binary aqueous system, being for the pure chitosan of 229 mg g−1 and crosslinked chitosan of 218 mg g−1. The Langmuir and extended Langmuir models presented a good fit to the equilibrium data in both systems. It was found that, the chitosan crosslinked with cyanoguanidine improved the chemical stability of chitosan as adsorbent.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Dyes have been widely employed in the food industry, once are important additives for several products. Therefore, the excessive use of these compounds have contributed to the environmental pollution, due to the toxic and carcinogenic properties of most dyes [1,2,3]. Even at low concentrations, the dyes can cause a decrease of photosynthetic activity in aquatic environments and, thus, becoming difficult the penetration of light and oxygen [4]. There are numerous operations for the treatment of dye-containing effluents, and the adsorption operation stands out when compared with conventional methods due to its simplicity, effectiveness and fast kinetics [5, 6].
Adsorption is an operation in which dissolved molecules or ions are captured on a suitable interface [7]. The most widely used adsorbent is activated carbon, however, presents high cost and difficult regeneration, thus, increasing the search for more suitable materials [8]. The biopolymer chitosan has attracted considerable attention for the dyes removal from effluents [3, 6, 9]. Chitosan is a polysaccharide obtained from chitin deacetylation, normally found in the exoskeletons of crustaceans, such as, crabs and shrimps [5]. Chitosan is used in a wide range of applications due to its properties, such as, hydrophilicity, biodegradability, biocompatibility, and shows high affinity for most dyes [3, 5]. However, this biopolymer has several disadvantages, including its agglomeration, the high crystallinity and the low mechanical strength [10]. Crosslinking of chitosan has been widely investigated in literature to improve its chemical stability and mechanical resistance [3, 6, 11, 12].
Many studies demonstrated the applicability of pure chitosan to removal food dyes, such as, FD&C Red No. 40, Acid Blue 9, Food Yellow 3, Acid Blue 9 and Food Yellow 3 [13,14,15]. Crosslinked chitosan is an alternative to improve the performance of chitosan as adsorbent. The adsorption capacity depends of the deacetylation degree, solvent, pH, temperature, nature of the adsorbate molecules, type of aqueous system, among others. Under acid conditions, chitosan is a cationic polysaccharide and in solution may protonate the amino groups (–NH2), being a potential adsorbent [13, 16]. The deacetylation degree is an important factor in the chitosan properties and in its adsorption capacity. Therefore, it is relevance the study of suitable conditions regarding the deacetylation degree, which provides high adsorption capacity [4, 6].
This work aimed to obtain and characterize pure chitosan and cyanoguanidine-crosslinked chitosan to apply in the food dyes adsorption. The adsorbents were prepared and characterized by Fourier transform infrared spectroscopy and scanning electron microscopy. The effects of pH and of deacetylation degree on the adsorption in single and in binary aqueous systems using crosslinked chitosan and pure chitosan were verified. The adsorption kinetic curves were obtained using the adsorbents in binary system, and the pseudo-first order, pseudo-second order and Avrami models were fitted to experimental data. The equilibrium isotherms for the both systems were fitted by Langmuir and extended Langmuir models.
Materials and Methods
Preparation and Characterization of the Adsorbents
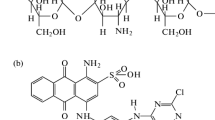
Chitosan in powder form was obtained from shrimp (Penaeus brasiliensis) wastes by the steps of demineralization, deproteinization and deodorization [17] and, afterwards, by drying operation [18]. The deacetylation reaction was carried out in according to method described by Moura et al. [19], obtaining different degrees deacetylation (75, 85 and 95%). Cyanoguanidine-crosslinked chitosan was prepared according to the method presented by Wang et al. [20], where 1.00 g of chitosan was dissolved in 100 mL of 1% (v/v) hydrochloric acid solution under magnetic agitation. After the complete dissolution, it was added 0.53 g of cyanoguanidine (99.9% Sigma-Aldrich).
The functional groups of pure chitosan and crosslinked chitosan were identified by Fourier transform infrared spectroscopy (FT–IR) (Prestige, 21210045, Japan) [21]. The textural characteristics were observed by scanning electron microscopy (SEM) (Jeol, JSM−6610LV, Japan) [22].
Food Dyes Characteristics
The food dyes of industrial grade, purity higher than 85%, were Food Yellow 4 (FY4) (azo dye, molecular weight, 534.4 g mol−1, C.I. 19,140, λmax 425 nm) and Food Blue 2 (FB2) (indigoid dye, molecular weight 466.3 g mol−1, C.I. 73,015, λmax 610 nm) and were supplied by Duas Rodas Ind., Brazil. All solutions were prepared with distilled water.
Effects of Solution pH and of Deacetylation Degree on the Food Dyes Adsorption
The assays were carried out in different pH values (from 3 to 7) at different deacetylation degrees (75, 85 and 95%). Firstly, stock-solutions of dye (1.0 g L−1) were prepared. Dyes solutions samples of 100 mL with concentration of 100 mg L−1 (50 mg L−1 of each dye) were prepared and placed in flasks. The pH was adjusted through buffer disodium phosphate/citric acid solution 0.1 mol L−1 (the buffer not presented significant interaction with the dyes). Then, 250 mg of pure chitosan (or crosslinked chitosan) were added in each dye solution, being the flasks agitated at 100 rpm using a thermostated type Wagner agitator (Fanem, 315 SE, Brazil) for 24 h at 298 K. All assays were carried out in two repetitions. The dyes concentration was determined by a spectrophotometer (Biospectro, SP–22, Brazil). For CBlue (FB2) and CYellow (FY4) in binary system, the concentrations were calculated by Eqs. 1 and 2, respectively [23]:
where, k B1 , k A1 , k B2 and k A2 are the calibration constants for FB2 and FY4, at 610 nm and 425 nm respectively, d 1 and d 2 are the optical densities.
The adsorption capacities of the dyes, q Blue and q Yellow (mg g−1), were obtained by Eqs. 3 and 4, respectively.
where, C B0 and C Y0 are, respectively, the initial concentrations of FB2 and FY4 in liquid phase (mg L−1), m is the amount of adsorbent (g) and V is the volume of solution (L).
Kinetics Analysis
In the best condition (pH and deacetylation degree) of the adsorption process previously determined, the assays were carried out to obtain the kinetics data. In the kinetics assays, the pH was adjusted to 3 and the stirring rate was of 100 rpm at 298 K. The initial concentration was of 50 mg L−1 for each dye, using pure chitosan and crosslinked chitosan. Aliquots were removed at 2, 4, 6, 8, 10, 15, 20, 25, 30, 40, 50, 60, 80, 90, 100, 120, 140, 160 and 180 min [15].
The adsorption kinetics was elucidated by the fitted of three models to the experimental data. The adsorption rate can be determined, respectively, by the models of pseudo-first (Eq. 5) and pseudo-second order (Eq. 6) [24, 25]:
where q t is the amount of dyes adsorbed at time t (mg g−1), k 1 and k 2 are the rate constants of pseudo-first and pseudo-second order models, respectively, in (min−1) and (g mg−1 min−1), q 1 and q 2 are the theoretical values of the adsorption capacity (mg g−1) and t is the time (min).
The Avrami equation is an alternative kinetic model, which has been used for adsorption systems, as presented in Eq. 7 [26].
where, k AV is the Avrami kinetic constant (min−1), q AV is the theoretical Avrami adsorption capacity (mg g−1) and n is a fractional exponent.
Adsorption Isotherms
The equilibrium isotherms were obtained for the simple system and binary system, at the samples temperature of 298 K and, all other experimental conditions were determined by preliminary tests and literature [5, 14]. The assays were performed using dye concentrated solutions to obtain the dye solutions concentrations from 50 to 400 mg g−1, for both system. The pH was corrected to 3.0 and, it was added 25 mg of crosslinked chitosan. The flasks were stirred (100 rpm) at temperatures of 298 K for 24 h. The dye concentrations in the liquid phase of the binary system were determined by Eqs. (1) and (2), The adsorption capacities for dyes qBlue and qYellow (mg g−1) were obtained by Eqs. 3 and 4, respectively. The data were adjusted by Langmuir model for the simple system and, by extended Langmuir model for the binary system [12].
Statistical Analysis
The Tukey test was used to compare the adsorption capacities obtained for the pure chitosan and the crosslinked chitosan with different deacetylation degrees, at different pH values. Significance level was of 95% confidence (p < 0.05). The kinetics parameters were determined by nonlinear regression employing a Hooke–Jeeves estimation method. The calculations were realized using Statistica 7.0 software (Statsoft, USA), and the fit quality was measured according to the coefficient of determination (R 2) and average relative error (ARE) [13, 14].
Results and Discussion
Characterization of the Adsorbents
The adsorbents were characterized according to the functional groups and surfaces features. The functional groups of the adsorbents were identified by FT–IR, and the spectra are presented in Fig. 1a (pure chitosan) and Fig. 1b (crosslinked chitosan).
The characteristic bands of pure chitosan can be observed in Fig. 1a. The broad band around 3050 cm−1 can be assigned to the typical N–H and O–H stretchings [24]. The stretching vibrations of C=O were observed at 1640 cm−1. At 1550 cm−1, the C–N stretching vibration of amide was observed. The band around 1000 cm−1 could be assigned to a C–O stretching. It can be seen at 680 cm−1, the angular deformation of H–N–H [27, 28].
Cyanoguanidine-crosslinked chitosan (Fig. 1b) presented an intense band around 2200 cm−1, which can be assigned to a bond of doped quaternary ammonium salt formed [29]. A little C≡N band of cyanoguanidine appeared at 2150 cm−1. The broad band around 3050 cm−1 decreased due to the insertion of cyanoguanidine into the amino group of chitosan. Therefore, based on these statements, it can be seen that cyanoguanidine was successfully inserted on the chitosan polymeric chains [20].
Figure 2 presents the SEM micrographs of the adsorbents. In Fig. 2a, the pure chitosan showed more smooth and homogeneous surface, and the crosslinked chitosan in Fig. 2b presented concavities and protuberances.
Effects of pH and of Deacetylation Degree on the Food Dyes Adsorption
Figures 3, 4 and 5 show the effects of pH and of deacetylation degree on the adsorption of food dyes by pure chitosan and by cyanoguanidine-crosslinked chitosan, in single and binary systems.
Effects of pH in the adsorption capacity for the food dyes adsorption at deacetylation degree 75%: a Food Yellow 4 in single system, b Food Blue 2 in single system, c Food Yellow 4 in binary system, d Food Blue 2 in binary system. Legend: (filled triangle) pure chitosan and (filled circle) cyanoguanidine-crosslinked chitosan
Effects of pH in the adsorption capacity for the food dyes adsorption at deacetylation degree 85%: a Food Yellow 4 in single system, b Food Blue 2 in single system, c Food Yellow 4 in binary system, d Food Blue 2 in binary system. Legend: (filled triangle) pure chitosan and (filled circle) cyanoguanidine-crosslinked chitosan
Effects of pH in the adsorption capacity for the food dyes adsorption at deacetylation degree 95%: a Food Yellow 4 in single system, b Food Blue 2 in single system, c Food Yellow 4 in binary system and, d Food Blue 2 in binary system. Legend: (filled triangle) pure chitosan and (filled circle) cyanoguanidine-crosslinked chitosan
In Figs. 3, 4 and 5 were found that, for both dyes and adsorbents, the pH decrease led to an increase in the adsorption capacity. This behavior can be explained because, under acid conditions, the hydrogen atoms (H+) present in solution tends to facilitate the protonation of the amino groups of chitosan, which are converted into NH3 + [5]. The attraction is facilitated due to the dyes are anionic. Dotto and Pinto [15] verified similar behavior in the adsorption of food dyes onto chitosan powder.
In relation to the DD effect, it was verified that the DD increase caused an increase in the adsorption capacity. This occurred because higher DD led to an increase in the protonation of chitosan amino groups, therefore, leading to a more number of active sites which favored the dye adsorption, and consequently, the food dyes adsorption capacity was increased. Piccin et al. [13] reported the same behavior, that in the higher DD was obtained the highest adsorption capacity of the FD&C Red No. 40 dye onto chitosan. In Figs. 3, 4 and 5 it was verified that in the crosslinked chitosan were practically maintained the adsorption capacities when compared with the pure chitosan, not presenting a significance difference (p > 0.05). Generally, the modification can reduce the adsorption capacity, due to the crosslinking agent connect with functional groups such as amino groups making them unavailable [2]. However, cyanoguanidine-crosslinked chitosan improved the chemical stability of adsorbent in acid solution and, also resulted a lower cost adsorbent, because the cyanoguanidine presents lower cost [30].
In the binary systems there were competitions between the dye adsorption sites, reducing the adsorption capacities (Figs. 3, 4, 5). This same behavior was observed by Mahmoodi et al. [31] in the adsorption of dyes in binary system using alginate as adsorbent and, by Oladipo et al. [32] in the adsorption of Erichrome Black T and Reactive Blue 2 by chitosan hydrogel. They explained your results based on the competition between the dyes for the binding sites on the adsorbent and, the molecular weight and the molecular polarity of each dye. In present work, the adsorbents presented more affinity for Food Yellow 4, which can be attributed to the dye chemistry (one carboxylic group and two sulfonated groups), to its higher molecular weight, as well as, its higher solubility [12, 13].
The more suitable conditions for the FY 4 and FB 2 adsorption onto pure chitosan and crosslinked chitosan, in both systems, were obtained in pH 3 and DD 95%. In these conditions, the adsorption capacities were around of 370 and 184 mg g−1 for FB 2 in the single and binary systems, respectively, and for FY 4 were around of 392 and 200 mg g−1 in the single and binary systems, respectively.
Kinetic Results
The kinetic study was realized using the more suitable conditions (pH 3, chitosan DD 95%), and the study was continued only in binary system, because this was the work major focus. To investigate the adsorption kinetics, the experimental data were fitted by the pseudo-first order, pseudo-second-order and Avrami models. Figure 6 shows the experimental curves of adsorption kinetic for FY 4 (Fig. 6a) and for FB 2 (Fig. 6b) onto pure chitosan and crosslinked chitosan in binary system.
Figure 6 shows that the pure chitosan presented higher adsorption capacity than the crosslinked chitosan and, the equilibrium was reached practically at 100 min for both dyes. Other works found similar results, such as Oladipo et al. [32] for binary system and Gonçalves et al. [12] for crosslinked chitosan. The kinetic constants are shown in Table 1. The Avrami model was the most suitable to represent the adsorption kinetics of both dyes, for both adsorbents, due to the highest values of coefficient of determination (R 2 > 0.99) and lowest values of average relative error (ARE < 5.0%). Table 1 shows that the higher values of the q av parameter were for FY 4, when adsorbed onto pure chitosan (229 mg g−1) and crosslinked chitosan (218 mg g−1). The highest values of the q av and k av parameters of the FY 4 were due to the dye chemistry, the higher molecular weight and the greater solubility.
Equilibrium Studies
Figure 7 shows the adsorption equilibrium isotherms of dyes onto crosslinked chitosan in both systems. It can be observed that, the equilibrium isotherms were of a type L, based on the adsorption on a uniform and simple surface distributed in a monolayer.
In Fig. 7, the adsorption capacity values for the concentration of 100 mg L−1 in the binary system were around 200 mg g−1. Thus, corroborating that the values found in the kinetics were already at equilibrium in 100 min, because they present the same values. It can also be verified that, the simple system presented double the adsorption capacity with respect to the binary system, confirming the competition between the dyes in this system.
Vakili et al. [33] studied the adsorption of chitosan beads modified with 3-aminopropyl triethoxysilane for the removal of reactive blue 4 and, their results showed that in the dye concentration of 100 mg L−1 was obtained an adsorption capacity of approximately 300 mg g−1 in a binary system. Thus, it can be highlighted that values found in our work were similar to the literature.
The experimental equilibrium isotherms were fitted by Langmuir model and by extended Langmuir model, and the results are shown in Table 2. Non-linear regression method was used to determine the parameters q m , K L(FB2) and K L(FY4) , being employed a Quasi-Newton estimation method. The fit quality was measured according to the coefficient of determination (R 2) and average relative error (ARE) [12]. It was observed in Table 2 that, the q m values for FY4 were higher than the q m values for FB2, showing the preference of crosslinked chitosan for the FY4 in relation to the FB2 in both systems.
Conclusions
In this work, the pure chitosan and the cyanoguanidine-crosslinked chitosan were used to remove food dyes from single and binary aqueous systems. The adsorbents were characterized according to its functional groups and surface features. The effects of pH and of chitosan deacetylation degree (DD) in dyes adsorption were evaluated. The insertion of cyanoguanidine on the polymeric chain of chitosan was verified by FT-IR spectra. The pH value of 3 was the more suitable for adsorption in the both aqueous systems and adsorbents. The DD increase was favorable for the adsorption, being that the samples with DD 95% were the most suitable adsorbents. The pure and crosslinked chitosans presented similar adsorption capacities for both dyes, being that the modification is justified by economic aspects. The Avrami model was the most suitable to represent the experimental kinetic curves. In binary system, the adsorption was fastest for FY 4. The experimental equilibrium data were fitted satisfactorily by the Langmuir and Langmuir extended models for the two systems. The equilibrium experiments corroborated with the values obtained in the kinetics. The values of adsorption capacities in binary aqueous system with pure chitosan and crosslinked chitosan were, respectively, for FY 4 of 229 and 218 mg g−1 and, for FB 2 of 207.0 and 204.4 mg g−1.
References
Yagub MT, Sen TK, Afroze S, Ang HM (2014) Adv Colloid Interface Sci 209:172
Guibal E (2004) Sep Purif Technol 38:43
Liu Q, Yang B, Zhang L, Huang R (2015) Int J Biol Macromol 72:1129
Crini G (2006) Bioresour Technol 97:1061
Crini G, Badot PM (2008) Prog Polym Sci 33:3996
Vakili M, Rafatullah M, Salamatinia B, Abdullah AZ, Ibrahim MH, Tan KB (2014) Carbohydr Polym 113:115
Rajeswari A, Amalraj A, Pius AJ (2016) Water Process Eng 9:123
Shen YH (2002) Water Res 36:11079
Muzzarelli RAA, Boudrant J, Meyer D, Manno N, Demarchis M, Paoletti MG (2012) Carbohydr Polym 87:995
Anirudhan TS, Rijith S (2012) J Environ Radioact 106:8
Cestari AR, Vieira EF, Tavares AM, Bruns RE (2008) J Hazard Mater 153:566
Gonçalves JO, Dotto GL, Pinto LAA (2015) J Mol Liq 211:425
Piccin JS, Vieira MLG, Gonçalves J, Dotto GL, Pinto LAA (2009) J Food Eng 95:16
Gonçalves JO, Duarte DA, Dotto GL, Pinto LAA (2013) Clean-Soil Air Water 42:1
Dotto GL, Pinto LAA (2011) J Hazard Mater 187:164
Sakkayawong N, Thiravetyan P, Nakbanpote W (2005) J Colloid Interface Sci 286:36
Weska RF, Moura JM, Batista LM, Rizzi J, Pinto LAA (2007) J Food Eng 80:749
Dotto GL, Souza VC, Moura JM, Moura CM, Pinto LAA (2011) Drying Technol 44:1786
Moura CM, Moura JM, Soares NM, Pinto LAA (2011) Chem Eng Process 50:351
Wang Y, Qi Y, Li Y, Wu J, Ma X, Yu C, Ji L (2013) J Hazard Mater 260:9
Pillai CKS, Paul W, Sharma CP (2009) Prog Polym Sci 34:641
Goldstein JI, Newbury DE, Echil P, Joy DC, Romig AD Jr, Lyman CE, Fiori C, Lifshin E (1992) Scanning electron microscopy and x-ray microanalysis. Plenum Press, New York
Choy KKH, Porter JF, Mckay G (2000) J Chem Eng Data 45:575
Qiu H, Pan LL, Zhang QJ, Zhang W, Zhang (2009) J Zhejiang Univ Sci A10:716
Ho YS, Mckay G (1998) Proc Saf Environ Protect 76:183
Avrami M (1939) J Chem Phys 7:1103
Dotto GL, Moura JM, Cadaval TRS Jr, Pinto LAA (2013) Chem Eng J 214:8
Cadaval TRS Jr, Camara AS, Dotto GL, Pinto LAA (2013) Desalin Water Treat 51:7690
Zhao X, Qiao ZZ, He JX (2012) J Eng Fabr 5:3
Ma X, Li Y, Yeb Z, Yanga L, Zhoua L, Wang L (2011) J Hazard Mater 185:1348
Mahmoodi NM (2011) J Chem Eng Data 56:2802
Oladipo AA, Gazi M, Yilmaz E (2015) Chem Eng Res Des 104:264
Vakili M, Rafatullah M, Ibrahim MH, Abdullah AZ, Salamatinia B, Gholami Z (2016) Carbohydr Polym 139:46
Acknowledgements
The authors would like to thank CAPES (Brazilian Agency for Improvement of Graduate Personnel) and CNPq (National Council of Science and Technological Development) for the financial support. The authors would like to thank CEME–SUL/FURG (Electron Microscopy Center of southern/Federal University of Rio Grande) due to the scanning electron microscopy images.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gonçalves, J.O., Silva, K.A., Dotto, G.L. et al. Adsorption Kinetics of Dyes in Single and Binary Systems Using Cyanoguanidine-Crosslinked Chitosan of Different Deacetylation Degrees. J Polym Environ 26, 2401–2409 (2018). https://doi.org/10.1007/s10924-017-1133-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10924-017-1133-z