Abstract
Adsorption characteristics of new coccine on chitosan 10B were examined in aqueous medium. The zero point charge (pHzpc) of chitosan 10B was evaluated to be 6.45. In order to find out an optimum condition for maximum dye elimination by chitosan 10B, the kinetic investigations were carried out as a function of contact time, solution pH, initial dye concentration, temperatures, and ionic strengths, respectively. The maximum dye removal by chitosan 10B was found in acidic medium at pH 4. The kinetics of dye adsorption data was analyzed by pseudo first-order, pseudo second-order, Elovich, film diffusion and intraparticle diffusion kinetic models, respectively. The kinetic data complied with pseudo second-order kinetic model. Adsorption isotherms were agreed perfectly with Langmuir isotherm model having maximum dye adsorption ability 90.77 µmol/g at 30°C. New coccine adsorption onto chitosan 10B was proven by the Fourier Transform Infrared (FTIR) spectroscopy. Thermodynamic parameters indicate that the new coccine dye elimination from aqueous solution by chitosan 10B is an exothermic spontaneous physisorption process. New coccine desorption from dye-loaded chitosan 10B was performed in 0.1 mol/L NaOH solution (pH 13) and the reusability of chitosan 10B was also investigated.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
Now-a-days the discharge of azo dyes into the nature is a big trouble for the pigmentation of the native waters, their toxicity, carcinogenicity, mutagenicity, and also their biotransformation products [1]. The colored wastewater imposes serious health problems for both aquatic and human lives. The dyed wastewater also interrupts aesthetics, water clarity, and gas solubility in water environments [2]. Moreover, there is a typical concern that the azo dyes diminish the permeation of light into water and there by disturbing the photosynthetic activity of aquatic floras [3]. So, it is very urgent to eliminate azo dyes from wastewater.
Various techniques such as oxidative degradation [4], photocatalytic degradation [5–7] coagulation [8], flocculation [9], membrane filtration [10], adsorption [11–14], ozonation [15], liquid-liquid extraction [16], and biological process [17] are used to remove dyes from wastewater. Each method presents some drawbacks. The major drawbacks are cost, efficiency, selectivity to toxic elements, availability etc. Among the mentioned processes, adsorption is one of the best methods due to its small expense, ease mode of operation and finally in selectivity to toxic materials. Various adsorbents such as activated carbon [18], graphene [19], clay [20], silica [21], fly ash [22], peat [23] and agro wastes [24–26] etc. have been utilized to eliminate the dyes from aqueous solution.
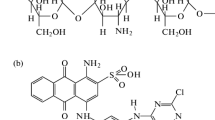
On other hand, chitosan is a linear polysaccharide made in arbitrarily scattered β-(1 → 4)-coupled D-glucosamine (deacetylated unit) and N-acetyl-D-glucosamine (acetylated unit). Chitosan is synthesized by treating chitin with sodium hydroxide. Chitin is usually obtained from garbage of the seafood handling industries, commonly shells of krill, shrimp, crabs, and prawns. Shrimp shell rubbish is a bulky garbage of seafood handling industries in Bangladesh (more than 30 000 tons per year) [12]. The large numbers of amino and hydroxyl functional groups in chitosan assemble it valuable for eliminating various dyes from aqueous systems. Recently, we have investigated the adsorption behaviors of remazol brilliant violet, reactive blue 4 (RB4), reactive yellow 145 (RY145), and reactive red 239 (RR239), and onto chitosan in aqueous media [11, 12, 27, 28]. Chitosan showed intense dye adsorption pursuit. This significant discovery inspired us to examine adsorption features of new coccine dye (Fig. 1a) onto chitosan in aqueous medium.
In this paper, chitosan 10B (100% deacetylated chitin, Fig. 1b) was utilized as an adsorbent to eliminate an azo dye new coccine from aqueous system. The influences of contact time, solution pH, dye concentration, temperatures and ionic strengths on the kinetics of new coccine adsorption onto chitosan 10B were examined in aqueous medium through batch adsorption studies. The equilibrium adsorption of new coccine onto chitosan 10B was explored at four different temperatures. Dye adsorption kinetics and mechanisms were inspected by pseudo first-, second-order, Elovich, film, and intraparticle diffusion models. Freundlich, Langmuir and Temkin isotherm models were applied to assess new coccine dye adsorption equilibrium. Thermodynamics of the sorption phenomena and reusability of recycled chitosan 10B were also investigated.
EXPERIMENTAL
Materials
New coccine (C20H11N2Na3O10S3; MW: 604.47; dye content 75%; Fig. 1a) dye was bought from Sigma-Aldrich, Germany and was used without any sanitization. Chitosan 10B (Fig. 1b) was bought from Katokichi Bio Co., Ltd., Japan and employed as received. Other chemicals employed in this research were of pure analytical grade. Water used in all experiments was distilled and deionized.
Characterization of Chitosan 10B
The mass median diameter of the chitosan 10B particles was estimated to be (228 ± 5) µm by a laser scattering particle size analyzer (LDSA-240DA, Tonichi Computer Applications, Japan) coupled with a dry dispersing apparatus (PD-IOS, Tonichi Computer Applications, Japan). The point of zero charge (pHzpc) of chitosan 10B was determined by pH drift method [29]. A solution of 0.1 mol/L KCl solution was prepared and boiled to remove dissolved CO2 and then cooled to room temperature. The initial pH of KCl solution was adjusted between 2.0 and 12.0 by using 0.5 mol/L HCl or 0.5 mol/L NaOH. Chitosan 10B (0.05 g) was added into 25 mL of the pH-adjusted 0.1 mol/L KCl in a 125 mL reagent bottle. The bottles containing chitosan 10B samples were kept closed and equilibrated for 24 h. After 24 h, the final pH of each sample was measured and plotted against the initial pH. The pH at which the curve crosses the pHinitial = pHfinal line is taken as pHpzc of chitosan 10B. Fourier transformation infrared (FTIR) spectra of chitosan 10B and dye-loaded chitosan 10B were recorded in KBr at the frequency range 400–4000 cm‒1 by using FTIR spectrometer (IRPrestige-21 FTIR Spectrophotometer, Shimadzu, Japan).
Adsorption Study
To evaluate the features of new coccine dye adsorption onto chitosan 10B, group adsorption tests were executed in stoppered bottle (125 mL ) having chitosan 10B (0.05 g) and dye solution (25 mL; 50 μmol/L) [11]. The primary pH of dye solution was corrected with 1 mol/L HCl or NaOH solution, by employing a pH meter (Adwa AD8000). Sample bottles were agitated in a thermostated shaker at room temperature (30 ± 0.2°C) with 120 rpm until equilibrium. Every bottle was plugged to prevent vaporization at higher temperature. Sample bottles were taken at definite time period for computing dye concentration in solution. The samples were centrifuged at 4000 rpm for 5 min. New coccine concentration in the supernatant was computed by the spectrophotometric method, employing a Shimadzu UV-1800 spectrophotometer (Shimadzu, Japan), at λmax: 507 nm (pH 1–10) and 438 nm (pH 11–13). The apparent molar absorptivity constants of new coccine were determined to be 18 × 103 L mol–1 cm–1 at 507 nm and 9 × 103 L mol–1 cm–1 at 438 nm. The amount of new coccine adsorbed per unit mass of chitosan 10B at time t, qt (μmol/g) was computed by Eq. (1)
where C0 (μmol/L) and Ct (μmol/L) are new coccine strengths in solution at zero and at time t, respectively; V (L) is the volume of new coccine solution and m (g) is the quantity of dry chitosan 10B utilized.
The adsorption kinetics was also examined at different solution pHs (4–10), dye concentration (100–2000 μmol/L), temperatures (30, 35, 40 and 45°C), and ionic strengths (0.01–0.05 mol/L), respectively. The ionic strength of dye solution was controlled with 1 mol/L KCl solution. Adsorption equilibrium was executed in aqueous medium (pH 4) at various temperatures (30, 35, 40 and 45°C) in absence KCl. The extent of new coccine adsorbed onto adsorbent at equilibrium time, qe (μmol/g) was computed by Eq. (2)
where Ce (μmol/L) is the new coccine strength in solution at equilibrium time; C0, V and m persist analogous as illustrated earlier.
In desorption experiment, chitosan 10B was treated with new coccine solution (50 μmol/L; 25 mL) for 120 min, separated and dye loaded-chitosan 10B was dehydrated at room temperature (30°C) for 12 h. New coccine filled-chitosan 10B particles were shaken in 0.1 mol/L NaOH solution (25 mL; pH 13) for 120 min. The extent of dye adsorption was ascertained in the similar way as mentioned previously. All data reported in this article are the average of dual measurements.
RESULTS AND DISCUSSION
Interaction Time Effect
The influence of interaction time on new coccine adsorption kinetics was observed in aqueous medium (pH 4) at 30°C. The effects are exhibited in Fig. 2. The typical time-resolved UV–visible spectra of new coccine during adsorption onto chitosan 10B are shown in Fig. 2a. It is noted that the absorbance of new coccine at 507 nm was gradually diminished with rising contact time. These effects signify that the magnitude of new coccine removal by chitosan 10B was gradually amplified with rising contact time. The influence of interaction time on the degree of new coccine adsorption (qt) onto adsorbent is exhibited in Fig. 2b. It is perceived that the degree of new coccine adsorption was very rapid for first 20 min, later the dye adsorption rate diminished slowly and accomplished to equilibrium within 60 min. At the starting, the rate of dye adsorption was fast as the dye molecules were adsorbed by the peripheral surface of chitosan 10B. When the surface adsorption attained to saturate, the dye molecules slowly penetrate into the holes of the adsorbent particles [30]. However, the extent of adsorption was measured for 120 min as an optimum equilibrium time for further studies.
(a) Typical UV–visible spectra of new coccine taken at 1—0, 2—5, 3—10, 4—20, 5—40, 6—60, 7—90, and 8—120 min during adsorption onto chitosan 10B in aqueous medium (pH = 4) at 30°C. (b) The extent of new coccine adsorption, qt (μmol/g) onto chitosan 10B with contact time, t (min). [New coccine]0: 50 μmol/L; solution volume—25 mL; adsorbent dosage—0.05 g; solution pH 4; temperature—30°C.
Effect of Solution pH
The exterior charges of adsorbent and the structure of dye molecules are greatly influenced by solution pH [31]. Hence, the solution pH plays a major role on the dye elimination process. Here, the adsorption of new coccine onto chitosan 10B was performed in aqueous media at pH 4–10 with a fixed dye concentration (50 μmol/L) and chitosan 10B (0.05 g) for 120 min. Adsorption research was not performed at very low pH due to the solubility of chitosan 10B [13]. New coccine dye adsorption kinetic sketches at numerous solution pHs are shown in Fig. 3a. Initial dye elimination rate, h ((μmol/g) min), fallen considerably with rising solution pHs (Table 1). From Fig. 3b, it is said that the equilibrium dye adsorption quantity, qe (μmol/g), also decreased with rising solution pHs. These results can be explained with the help of pHzpc of chitosan 10B. The pHzpc of chitosan 10B was found to be 6.45 (figure not shown), which is consistent with the reported pHzpc: 6.3 of chitosan [32]. At pH 4, the surface of chitosan 10B became positively charged which attracted the anionic dye molecules, hence the new coccine adsorption rate and adsorption quantity became high at this solution pH. On the contrary, with rising the solution pH > pHzpc, the surface of chitosan 10B particles converted into negative charges resulting electrostatic repulsion between chitosan 10B and new coccine dye molecules [13], which ultimately results in less adsorption of dye. The highest quantity of new coccine dye adsorption onto chitosan 10B was obtained to be 24.72 μmol/g in aqueous solution (pH 4). A comparable result was also noticed in RB4 anionic dye adsorption onto chitosan 10B [12]. Hence, all other kinetic experiments were conducted at solution pH 4.
(a) The changes in extent of new coccine adsorption, qt (μmol/g), onto chitosan 10B with contact time, t (min), at various solution pHs. [New coccine]0: 50 μmol/L, solution volume—25 mL, adsorbent dosage—0.05 g, temperature—30°C, solution pHs: 1—pH 4, 2—pH 5, 3—pH 6, 4—pH 7, 5—pH 8, 6—pH 9, 7—pH 10. All lines were numerically mimicked by utilizing Eq. (4), qe(cal) and k2 recorded in Table 1. (b) The extent of equilibrium new coccine adsorption, qe (μmol/g), onto chitosan 10B at several solution pHs.
Dye Concentration Effect
The extent of new coccine adsorption (qt) onto chitosan 10B with contact time (t) at various initial concentration of dye solutions (pH 4) and at 30°C is illustrated in Fig. 4. The initial dye elimination rate, h ((μmol/g) min), increased with rising dye concentration in solution (Table 1), which indicates that the new coccine elimination by chitosan 10B definitely depends on its concentration in solution. The values of qe were noticed to be 24.72 μmol/g for 50 μmol/L and 94.12 μmol/g for 2000 μmol/L new coccine dye solutions, respectively. The dye concentration in solution affords a prominent potency to devastate the mass transfer confrontation of all molecules between the liquid and solid phases [11]. A comparable phenomenon was also seen in the elimination of remazol brilliant violet by chitosan 10B and reactive orange (RO13) by jackfruit seed flakes in aqueous medium [11, 26].
The changes in extent of new coccine dye elimination, qt (μmol/g), by chitosan 10B with contact time (t) at various initial concentration of dye solution. Solution pH 4, solution volume—25 mL, adsorbent dosage: 0.05 g, temperature: 30°C, [New coccine]0: 1—50, 2—100, 3—250, 4—500, 5—1000, 6—1500, 7—2000 µmol/L. All lines were numerically mimicked by utilizing Eq. (4), qe(cal) and k2 (Table 1).
Ionic Strength Effect
The changes in degree of new coccine adsorption (qt) onto chitosan 10B with contact time (t) in aqueous medium (pH 4) was examined at numerous ionic strengths using known amount of KCl in dye solution (50 μmol/L) at 30°C. The effects are depicted in Fig. 5. It is found that the capacity of equilibrium dye adsorption, qe (μmol/g), onto chitosan 10B gradually decreased with rising ionic strength of dye solutions. The results could be explained by a competitive effect between new coccine dye anions and Cl‒ (from KCl) for the active sorption sites of chitosan 10B as noticed in elimination of RO13 by jackfruit seed flakes [26] and RY145 by chitosan in aqueous media [27], respectively. This also demonstrates the presence of electrostatic interactions between dye molecules and adsorbent particles.
The changes in extent of new coccine adsorption, qt (μmol/g), onto chitosan 10B with contact time, t (min), at various ionic strengths of dye solution. Solution pH 4, [New coccine]0: 50 μmol/L, solution volume—25 mL, adsorbent dosage—0.05 g, temperature—30°C, ionic strength: 1—0.00, 2—0.01, 3—0.02, 4—0.03, 5—0.05 mol/L. All lines were numerically simulated by utilizing Eq. (4), qe(cal) and k2 (Table 1).
Temperature Effect
New coccine adsorption kinetics was explored in aqueous medium (pH 4) at various temperatures (30, 35, 40 and 45°C) with dye solution (50 μmol/L; 25 mL) for 120 min. The effects are exhibited in Fig. 6. The dye elimination rate, h ((μmol/g) min), and the degree of adsorption at equilibrium, qe (μmol/g), declined with rising solution temperature from 30 to 45°C (Table 1). The interaction between new coccine dye molecules and functional groups of chitosan 10B occurred less efficiently at higher temperature due to inadequate flexibility of the dye molecules at higher temperature. It is foreseen that the high solution temperature may compress the surface area and the interior configuration of chitosan 10B as observed in adsorption of brilliant green onto kaolin [33], methylene blue and indigo carmine onto Brazil nut shells [34] in aqueous solution.
The changes in extent of new coccine dye adsorption (qt) onto chitosan 10B with contact time (t) in aqueous medium at different temperatures. Solution pH 4, [New coccine]0: 50 μmol/L, solution volume—25 mL, adsorbent dosage: 0.05 g, temperature—1—30, 2—35, 3—40, 4—45°C). All lines were numerically simulated by employing Eq. (4), qe(cal) and k2 (Table 1).
Kinetic Modeling
To describe the mechanism of new coccine dye adsorption onto chitosan 10B, the data procured from batch adsorption explorations were studied by pseudo first- [35], second-order [36], Elovich [37], film diffusion [38], and intraparticle diffusion [39] kinetic models, respectively. The pseudo first-order kinetic model is known by Eq. (3) [35]:
where k1 (min–1) is the pseudo first-order sorption rate constant determined from the slope of a plot log(qe – qt) versus t.
The nonlinear form pseudo second-order kinetic model is stated by Eq. (4) [36]:
where k2 ((g/μmol) min) is a pseudo second-order adsorption rate constant obtained from Eq. (5) [36]:
The k2 and qe values were computed from intercept and slope of a plot t/qt versus t. The initial elimination rate, h ((μmol/g) min), is calculated by Eq. (6) [36]:
Elovich kinetic model is stated by Eq. (7) [37]:
where α ((µmol/g) min) denotes a primary dye adsorption rate and β (g/μmol) is involved to an activation energy of chemisorption and an extent of surface coverage. The Elovich constants were obtained from qt versus lnt plot.
The significance and validity of each model can be verified using a normalized standard deviation (∆qe %) [40], which can be determined as follows:
where N is the quantity of data points, qe, exp (µmol/g) and qe, cal (µmol/g) are the quantity of experimental and calculated equilibrium dye adsorption.
The kinetic parameters along with correlation coefficients (R2) and normalized standard deviation (∆qe %) found in different kinetic models are shown in Table 1. The R2 values attained from pseudo first-order (≤0.997) and Elovich (≤0.969) kinetic models were negligible compare to the values computed from pseudo second-order (≥0.999) kinetic model (Table 1). And the values of ∆qe (%) attained from pseudo second-order kinetic model were small compare to the values found in pseudo first-order and Elovich kinetic models (Table 1). In addition, the qe, cal values computed from pseudo second-order kinetic model and the observed qe, exp values are analogous (Table 1) implying a good agreement of pseudo second-order adsorption kinetics as found in RY145 and reactive black 5 (RB5) adsorption on chitosan in aqueous media [27, 38].
The equation of film diffusion model proposed by McKay can be written as [38]:
where kfd (1/min) is a film diffusion rate constant, F is a partial equilibrium achievement, and qt (μmol/g) and q∞ (μmol/g) are extents of new coccine adsorption onto chitosan 10B at t time and infinite time, respectively. The film diffusion rate constant was determined from the plot of ln(1 – F) versus t. The values of kfd and R2 are shown in Table 2.
The intraparticle diffusion model is denoted by Eq. (11) [39]:
where kid ((μmol/g) min0.5) represents intraparticle diffusion rate constant and I (μmol/g) signifies the intercept. Figure 7 shows the usual plots of qt versus t0.5 of new coccine dye adsorption onto chitosan 10B at different dye concentrations in solution. There were two linear portions in each plot, which suggests that two phenomena occurred in the adsorption process. The first is prompt exterior surface diffusion of the adsorbent with a rate constant kid1 ((μmol/g) min0.5) and the second is slow interior surface diffusion of the adsorbent with a rate constant kid2 ((μmol/g) min0.5) [27]. The kid1 ((μmol/g) min0.5) and kid2 ((μmol/g) min0.5) values presented in Table 2 were determined from the slope of corresponding lines in Fig. 7. Any straight line did not pass across the origin (Fig. 7) indicating the mechanism of present dye adsorption is multifaceted. Surface adsorption and intraparticle diffusion mechanisms might be responsible to the actual sorption process as noticed in the RY145 adsorption onto chitosan in aqueous medium [27].
Characteristic plots of qt versus t0.5 for the new coccine elimination by chitosan 10B at various initial dye concentration of solutions. Solution pH 4, solution volume—25 mL, adsorbent dosage—0.05 g, temperature—30°C. [New coccine]0: 1—50, 2—100, 3—250, 4—500, 5—1000, 6—1500, 7—2000 µmol/L. The values of kid1 ((μmol/g) min0.5) and kid2 ((μmol/g) min0.5) are computed in Table 2.
Adsorption Isotherm
The extent of equilibrium new coccine elimination by chitosan 10B was examined in aqueous system at numerous temperatures. The plots of qe (μmol/g) versus Ce (μmol/L) at numerous solution temperatures are depicted in Fig. 8. The present adsorption was found to be an exothermic process as the magnitude of qe (μmol/g) decreased with rising solution temperature from 30 to 45°C. Freundlich [41], Temkin [42], and Langmuir [43] isotherm models were employed to elucidate equilibrium adsorption data acquired at various temperatures. Equations of the employed isotherm models are given below:
Adsorption isotherm of new coccine onto chitosan 10B at different temperatures. Solution pH 4, [New coccine]0: 50–3000 μmol/L, solution volume—25 mL, adsorbent dosage—0.05 g, temperature: 1—30, 2—35, 3—40, 4—45°C. All lines were mimicked by employing Langmuir isotherm Eq. (19), aL and KL at numerous temperatures recorded in Table 3.
Freundlich model:
Temkin model:
Langmuir model:
where Ce (µmol/L) denotes dye concentration at equilibrium, qe (µmol/g) represents measure of new coccine adsorbed per unit weight of chitosan 10B at equilibrium, KF ((µmol/g) (µmol/L)−1/n) and n imply Freundlich isotherm constants showing the aptitude and magnitude of the adsorption. KT (µmol/L) means Temkin isotherm constant, b (J/mol) relates to the heat of adsorption, R (8.314 (J/mol) K) is an ideal gas constant, T is absolute temperature (K), KL (L/g) denotes Langmuir constant and aL (L/μmol) is the Langmuir binding constant. The highest dye adsorption onto chitosan 10B, qm (µmol/g), was computed from the ratio of KL/aL. Table 3 illustrates the values of isotherm and thermodynamic parameters. All adsorption isotherms were matched satisfactorily with Langmuir isotherm model preferably than Temkin and Freundlich isotherm models because the highest correlation coefficients (R2 ≥ 0.991) were observed in Langmuir model (Table 3). The qm values were computed to be 90.77 µmol/g at 30°C and 86.67 µmol/g at 45°C, respectively.
Confirmation of Dye Adsorption
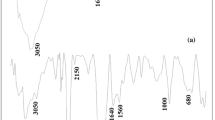
FTIR spectrum of chitosan 10B and new coccine loaded-chitosan 10B are exhibited in Fig. 9. The broad band at 3420 cm–1 for the N‒H stretching and O‒H stretching of chitosan 10B (Fig. 9(1)) was shifted to 3441 cm–1 in dye-loaded chitosan 10B (Fig. 9(2)), which indicates an interaction between the NH/OH group of chitosan 10B and dye molecule [44]. The band at 2878 cm–1 corresponding to C‒H stretching in the spectrum of fresh chitosan 10B shifted to 2881 cm–1 after adsorption. The vibration bands at 1649 cm–1 for amide-I (C=O stretching) and at 1599 cm–1 for amide-II (N‒H bending) of fresh chitosan 10B (Fig. 9(1)) shifted to 1647 and 1595 cm–1 in dye-loaded chitosan 10B (Fig. 9(2)). The peak at 1420 cm–1 associated with ‒CH2 bending in the spectrum of fresh chitosan 10B shifted to 1422 cm–1 in dye-loaded chitosan 10B (Fig. 9(2)). The typical bands of polysaccharide backbone appearing at 1153, 1080, 1024 and 897 cm–1 of fresh chitosan 10B (Fig. 9(1)) shifted to 1154, 1094, 1022 and 895 cm–1 as shown in Fig. 9(2). Hence, the adsorption of new coccine dye onto chitosan 10B can be confirmed.
Thermodynamics
Thermodynamic research on the adsorption process is essential to inspect the spontaneity of the system. The changes in Gibb’s free energy (∆G kJ/mol), enthalpy (∆H kJ/mol) and entropy (∆S (J/mol) K) were computed by using following Eq. [45]:
where (aL l/mol) denotes Langmuir binding constant, R is the universal gas constant (8.314 (J/mol) K) and T is the absolute temperature (K). The slope and y-intercept of the plot lnaL versus 1/T (R2 = 0.950) were used to calculate the values of ∆H: ‒94.54 kJ/mol and ∆S: ‒227.33 (J/mol) K, respectively (Table 3). The negative magnitude of ∆H implies that the new coccine adsorption onto chitosan 10B pursued exothermic processes. The negative ∆S confirmed the decreased of randomness due to the strong binding of new cccine dye with chitosan 10B particles [46]. The value of |∆H| is greater than that of |T∆S | at all temperatures which signifies that the adsorption process is controlled by enthalpic rather than entropic changes [46]. The Gibb’s free energy (∆G) was calculated to be ‒24.88 at 30°C and ‒21.15 kJ/mol at 45°C, respectively (Table 3). The negative values of ∆G decreased with increasing temperatures which indicates that the adsorption process was spontaneous and favored at low temperatures.
Reuse of the Chitosan 10B
The recyclable adsorbent is very important in industry to assemble an economic wastewater remedy plant [47]. Figure 10 shows the typical plot of new coccine dye adsorption-desorption-adsorption onto chitosan 10B in aqueous systems. In the adsorption step 1, dye solution (50 µmol/L; 25 mL; pH 4) was interacted with chitosan 10B (0.05 g) for 120 min at 30°C whereas the dye release from new coccine-loaded chitosan 10B was measured in 0.1 mol/L NaOH (25 mL; pH 13) for 120 min at 30°C. The value of qe was measured to be 24.72 µmol/g in the adsorption step 1. In desorption step, new coccine release rate was very fast for primary 20 min and 88% of new coccine was discharged from dye-loaded chitosan 10B in 120 min. It suggests that the anionic new coccine dye molecules was easily liberated from dye loaded-chitosan 10B in strongly basic solution (pH 13). In the adsorption step 2, recycled chitosan 10B was used to adsorb new coccine dye solution (50 µmol/L; 25 mL; pH 4) for 120 min. The quantity of dye adsorption was analogous as noticed in step 1. The value of qe was found to be 21.59 μmol/g for recycled chitosan 10B. These results indicate that the chitosan 10B can be utilized as an efficient adsorbent for removing new coccine dye from aqueous solution.
Adsorption-desorption-adsorption kinetics of new coccine onto chitosan 10B in aqueous medium at 30°C. [New coccine]0: 50 μmol/L, solution volume—25 mL, adsorbent dosage—0.05 g, 1—adsorption step 1: direct adsorption at pH 4, 2—desorption step—pH 13, 3—adsorption step 2: regenerated chitosan 10B was used at pH 4.
CONCLUSIONS
The kinetics, mechanisms, equilibrium and thermodynamics of new coccine dye adsorption onto chitosan 10B were studied in aqueous media. The pHpzc of chitosan 10B was found to be 6.45. The dye adsorption kinetics was inspected in aqueous solution as function of contact time, solution pH, initial dye concentration, ionic strengths and temperatures, respectively. Adsorption capability of the chitosan 10B was raised with increasing of interaction time, dye concentration, and decreased with rising temperatures and ionic strengths, respectively. New coccine elimination by chitosan 10B was maximized at solution pH 4. The pseudo second-order kinetic model fully approves new coccine adsorption kinetics with dynamical behavior. The qe, cal values computed from pseudo second-order kinetic model are analogous to the qe, exp values found in batch experiments. In addition, the observed adsorption kinetic traces are absolutely mimicked traces obtained from numerically analysis based on the pseudo second-order kinetic model and employing the values of k2 and qe, cal recorded in Table 1. The Langmuir isotherm model is the best-fit equilibrium isotherm preferably than the Freundlich and the Temkin models for the sorption of new coccine dye onto chitosan 10B. The highest new coccine adsorption capability of chitosan 10B was observed to be 90.77 µmol/g at 30°C. The dye adsorption onto chitosan 10B was proven by FTIR spectroscopy. The values of thermodynamic parameters (∆H: ‒94.54 kJ/mol; ∆S: ‒227.33 (J/mol) K and ∆G: ‒24.88 kJ/mol at 30°C and ‒21.15 kJ/mol at 45°C) demonstrated that the new coccine adsorption onto chitosan 10B is spontaneous exothermic physiosorption process. The dye-loaded chitosan 10B released new coccine dye in NaOH solution (0.1 mol/L; pH 13) and the recycled chitosan 10B was used in dye adsorption without significant loss its activity. This research confirms that the chitosan 10B can be employed as a productive adsorbent to eliminate new coccine from aqueous solution.
REFERENCES
Y. M. Kolekar, S. P. Pawar, K. R. Gawai, et al., Bioresour. Technol. 99, 8999 (2008). https://doi.org/10.1016/j.biortech.2008.04.073
I. M. Banat, P. Nigam, D. Singh, and R. Marchant, Bioresour. Technol. 58, 217 (1996). https://doi.org/10.1016/S0960-8524(96)00113-7
O. Anjaneya, S. S. Shrishailnath, K. Guruprasad, A. S. Nayak, S. B. Mashetty, and T. B. Karegoudar, Int. Biodeterior. Biodegrad. 79, 64 (2013). https://doi.org/10.1016/j.ibiod.2013.01.006
T. K. Saha, H. Frauendorf, M. John, S. Dechert, and F. Meyer, ChemCatChem. 5, 796 (2013). https://doi.org/10.1002/cctc.201200475
T. K. Saha, S. Karmaker, S. Debnath, and M. I. A. Biswas, Russ. J. Phys. Chem. A 94, 2723 (2020). https://doi.org/10.1134/S0036024420130233
A. A. Kashmeri, F. Nawaz, M. Yousal, A. Shameem, M. Sh. Mahr, J. Iqbal, M. Shafique, and M. A. Javed, Russ. J. Phys. Chem. B 14, 552 (2020). https://doi.org/10.1134/S1990793120030069
E. M. Bayan, T. G. Lupeiko, and L. E. Pustovaya, Russ. J. Phys. Chem. B 12, 923 (2018). https://doi.org/10.1134/S1990793118050159
F. Mcyotto, Q. Wei, D. K. Macharia, M. Huang, C. Shen, and C. W. K. Chow, Chem. Eng. J. 405, 126674 (2021). https://doi.org/10.1016/j.cej.2020.126674
K. Guo, B. Gao, R. Li, W. Wang, Q. Yue, and Y. Wang, Environ. Sci. Pollut. Res. 25, 2083 (2018). https://doi.org/10.1007/s11356-017-0835-z
C. Zhijiang, X. Ping, Z. Cong, Z. Tingting, G. Jie, and Z. Kongyin, Cellulose 25, 5123 (2018). https://doi.org/10.1007/s10570-018-1914-0
S. Karmaker, A. J. Nag, and T. K. Saha, Cellulose Chem. Technol. 53, 373 (2019). https://doi.org/10.35812/CelluloseChemTechnol.2019.53.38
S. Karmaker, A. J. Nag, and T. K. Saha, Russ. J. Phys. Chem. A 94, 2349 (2020). https://doi.org/10.1134/S0036024420110126
T. K. Saha, S. Karmaker, H. Ichikawa, and Y. Fukumori, J. Colloid Interface Sci. 286, 433 (2005). https://doi.org/10.1016/j.jcis.2005.01.037
V. B. Ivanov, A. A. Zavodchikova, I. I. Barashkova, E. V. Solina, and A. M. Wasserman, Russ. J. Phys. Chem. B 8, 233 (2014). https://doi.org/10.1134/S1990793114020067
M. Khadhraoui, H. Trabelsi, M. Ksibi, S. Bouguerra, and B. Elleuch, J. Hazard. Mater. 161, 974 (2009). https://doi.org/10.1016/j.jhazmat.2008.04.060
P. Pandit and S. Basu, J. Colloid Interface Sci. 245, 208 (2002). https://doi.org/10.1006/jcis.2001.7939
R. R. M. Khan, A. Adnan, M. Pervaiz, M. A. Raza, and M. Y. Naz, Russ. J. Phys. Chem. B 10, 517 (2016). https://doi.org/10.1134/S1990793116030234
K. N. Aboua, Y. A. Yobouet, K. B. Yao, D. L. Goné, and A. Trokourey, J. Environ. Manage. 156, 10 (2015). https://doi.org/10.1016/j.jenvman.2015.03.006
Y. Li, Q. Du, T. Liu, et al., Chem. Eng. Res. Des. 91, 361 (2013). https://doi.org/10.1016/j.cherd.2012.07.007
E. Errais, J. Duplay, M. Elhabiri, et al., Colloids Surf., A 403, 69 (2012). https://doi.org/10.1016/j.colsurfa.2012.03.057
G. Mckay, Chem. Eng. Sci. 39, 129 (1984). https://doi.org/10.1016/0009-2509(84)80138-4
J. X. Lin, S. L. Zhan, M. H. Fang, X. Q. Qian, and H. Yang, J. Environ. Manage. 87, 193 (2008). https://doi.org/10.1016/j.jenvman.2007.01.001
Y. S. Ho and G. McKay, Chem. Eng. J. 70, 115 (1998). https://doi.org/10.1016/S0923-0467(98)00076-1
X. Ren, X. Zhang, L. Zhang, and R. Han, Desalin. Water Treatm. 51, 4514 (2013). https://doi.org/10.1080/19443994.2012.741776
T. K. Saha, R. K. Bishwas, S. Karmaker, and Z. Islam, ACS Omega 5, 13358 (2020). https://doi.org/10.1021/acsomega.0c01493
S. Karmaker, M. N. Uddin, H. Ichikawa, Y. Fukumori, and T. K. Saha, J. Environ. Chem. Eng. 3, 583 (2015). https://doi.org/10.1016/j.jece.2014.09.010
S. Karmaker, T. Sen, and T. K. Saha, Polym. Bull. 72, 1879 (2015). https://doi.org/10.1007/s00289-015-1378-4
S. Karmaker, F. Sintaha, and T. K. Saha, Adv. Biol. Chem. 9, 1 (2019). https://doi.org/10.4236/abc.2019.91001
M. Kosmulski, J. Colloid Interface Sci. 337, 439 (2009). https://doi.org/10.1016/j.jcis.2009.04.072
M. M. Rahman, N. Akter, M. R. Karim, et al., J. Environ. Chem. Eng. 2, 76 (2014). https://doi.org/10.1016/j.jece.2013.11.023
L. Ai, C. Zhang, F. Liao, Y. Wang, K. Li, L. Meng, and J. Jiang, J. Hazard. Mater. 198, 282 (2011). https://doi.org/10.1016/j.jhazmat.2011.10.041
P. Udaybhaskar, L. Iyengar, and A. V. S. P. Rao, J. Appl. Polym. Sci. 39, 739 (1990). https://doi.org/10.1002/app.1990.070390322
B. K. Nandi, A. Goswami, and M. K. Purkait, J. Hazard. Mater. 161, 387 (2009). https://doi.org/10.1016/j.jhazmat.2008.03.110
S. M. O. Brito, H. M. C. Andrade, L. F. Soares, and R. P. Azevedo, J. Hazard. Mater. 174, 84 (2010). https://doi.org/10.1016/j.jhazmat.2009.09.020
S. Lagergren, K. Sven. Vetenskapsakad. Handl. 24, 1 (1898).
Y. S. Ho and G. McKay, Process Biochem. 34, 451 (1999). https://doi.org/10.1016/S0032-9592(98)00112-5
S. Y. Elovich and O. G. Larinov, Izv. Akad. Nauk SSSR, Otd. Khim. Nauk. 2, 209 (1962).
T. K. Saha, N. C. Bhoumik, S. Karmaker, M. G. Ahmed, H. Ichikawa, and Y. Fukumori, Clean-Soil, Air, Water 39, 984 (2011). https://doi.org/10.1002/clen.201000315
W. J. Weber and J. C. Morris, J. Sanit. Eng. Div. Proc. Am. Soc. Civ. Eng. 89, 31 (1963). https://doi.org/10.1061/JSEDAI.0000430
A. Abdelhay, A. A. Bsoul, A. Al-Othman, N. M. Al-Ananzeh, I. Jum’h, and A. A. Al-Taani, Adsorp. Sci. Technol. 36, 46 (2018). https://doi.org/10.1177%2F0263617416684347
H. Freundlich, Z. Phys. Chem. 57, 384 (1906). https://doi.org/10.1515/zpch-1907-5723
M. I. Temkin and V. Pyzhev, Acta Physicochim. USSR 12, 327 (1940).
I. Langmuir, J. Am. Chem. Soc. 40 1361 (1918). https://doi.org/10.1021/ja02242a004
S. Pervin, C. K. Shaha, S. Karmaker, and T. K. Saha, Polym. Bull. 78, 4527 (2021). https://doi.org/10.1007/s00289-020-03331-z
Y. Liu, Colloids Surf., A 274, 34 (2006). https://doi.org/10.1016/j.colsurfa.2005.08.029
A. A. Atia, A. M. Donia, and W. A. Al-Amrani, Chem. Eng. J. 150, 55 (2009). https://doi.org/10.1016/j.cej.2008.12.004
B. Zhao, W. Xiao, Y. Shang, H. Zhu, and R. Han, Arab. J. Chem. 10, S3595 (2017). https://doi.org/10.1016/j.arabjc.2014.03.010
ACKNOWLEDGMENTS
We are thankful to the Ministry of Science and technology, Government of the People’s Republic of Bangladesh for giving research grant to Prof. Dr. Tapan Kumar Saha to complete the present work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Islam, M., Ghosh, P., Karmaker, S. et al. Kinetics, Equilibrium and Thermodinamic Investigation of New Coccine Adsorption onto Chitosan 10B in Aqueous Solution. Russ. J. Phys. Chem. B 15 (Suppl 2), S189–S200 (2021). https://doi.org/10.1134/S1990793121100043
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1990793121100043