Abstract
This study deals with 2,2,6,6-tetramethylpiperidine-1-oxyl (TEMPO)-mediated oxidation of cellulose. Softwood and hardwood pulp fibers were suspended in water and oxidized to various extents at pH 10 and 22 °C using sodium hypochlorite in the presence of TEMPO radical and sodium bromide. This reaction system is known to be the most efficient one for the introduction of both surface carboxyl and aldehyde groups. Important relationships between formation of these functional groups and the fibrillation yield, light transmittance of the water dispersions and degree of polymerization of the oxidized softwood and hardwood pulps were established in the present study. A birefringence test confirmed the presence of nanofibers which according to atomic force microscopy analyses had diameters in the 1.6–3.8 nm range.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Microfibrillated cellulose (MFC) can be obtained from different cellulosic resources. The most common ones are wood and cotton fibers. Bacterial cellulose, flax, ramie or cotton may be used for the production, yielding fibrils with different dimensions and crystallinities [1, 2]. Each fibril is composed of cellulose macromolecules lying parallel and joined together laterally by hydrogen bonds and hydrophobic interactions (Van-der-Waals forces, London forces, etc.). These primary structures, also called cellulose microfibrils, build up the wall layers and finally the whole cell wall of the fiber.
Cellulose can be oxidized by a variety of oxidation reactants. Cellulosic hydroxyls can be converted to aldehyde, ketone or carboxyl groups. 2,2,6,6-Tetramethylpiperidine-1-oxyl or TEMPO is a stable and water-soluble nitroxyl radical, which has been used in organic syntheses in aqueous conditions as an effective catalyst for selective oxidation of primary alcohol groups to aldehydes and carboxylic groups [3–6]. Secondary hydroxyls are little affected in this oxidation process.
TEMPO-oxidized cellulose nanofibers (TOCN) reveal some unique properties when compared to MFC produced from wood celluloses by conventional high-pressure homogenization without chemical pretreatment. The main difference is that the TOCN consist of highly crystalline and mostly individualized nanofibrils with 3–4 nm widths and aspect ratios of more than 100 [7] while conventional MFCs may have bundles of cellulose microfibrils and separated fibrils with diameters of 3.5–20 nm and lengths of several tens of micrometers. TEMPO-mediated oxidation of cellulosic fibers only takes place on the microfibril surfaces and causes negative charges on them [8, 9]. The reaction affects the disintegration process by loosening the hydrogen bonds and causing electrostatic repulsion between the fibrils [10] but maintaining the microfibrillar nature of the cellulose [7, 8]. Potential applications for TOCN may include oxygen barrier coatings [11], paper wet strength additives [12, 13], food additives [14] and nanocomposites [15, 16].
From an environmental point of view and compared to other oxidation systems, TEMPO-mediated oxidation is a clean process; as only small amounts of sodium hypochlorite and sodium bromide are required for obtaining a high yield of converted product. Sodium hypochlorite and sodium hydroxide are the only chemicals being consumed during reaction. Sodium bromide and TEMPO are still present in the liquor after finished oxidation. From this point of view, the spent reaction liquor may be reused effectively with only the addition of sodium hypochlorite [17].
It has been proven by numerous studies that the crystal widths and high crystallinity of the original wood pulps are preserved during the oxidative modification with sodium hypochlorite [10, 18, 19]. Also, there were no significant differences observed in the carboxylate contents and nanofiber conversion between the never-dried and the once-dried celluloses [19]. Surface carboxylate concentration of TEMPO-oxidized celluloses has an important role e.g. its increase is associated with a corresponding increase in the amounts of adsorbed surfactants on cellulose surface [20] and may facilitate fiber homogenization process [21, 22]. It is also expected that the viscosity and optical properties of the fibrillated dispersions can be controlled by the increasing oxidation levels.
The main objective of the present work was to create a rather comprehensive study on the TEMPO-mediated oxidation of hardwood and softwood celluloses and understand how the properties of the obtained nanofibers and nano-dispersions may vary at gradually increasing degrees of oxidation. This has not been previously studied and is essential knowledge for optimization of chemical consumption to reach a desired product quality of the TOCN. An oxidation system of TEMPO/NaBr/NaClO was used to prepare TOCN from two commercial pulps (one softwood and one hardwood). The reaction yield was controlled by the amount of primary oxidant (NaClO). The materials were chosen based on different chemical composition and fiber morphology as the properties of nanofibers with respect to carboxylate contents and wood pulps are supposed to be different. The characterization of the obtained nanofibers/nano-dispersions was done with respect to various parameters: carboxylate contents, fibrillation yields, morphology, sugar composition, light transmittance and birefringence.
Experimental Section
Preparation of the Cellulose Nanofibers and Nano-Dispersions
Materials
TEMPO-oxidized nanofibers were prepared using never-dried kraft pulps made of Norway spruce (Picea Abies) and eucalyptus (51 % Eucalyptus globulus, 20 % Eucalyptus grandis and 29 % Eucalyptus maidenii). Composition characteristics of the pulps as well as the physical properties of the fibers were provided by the pulp manufacturer and are listed in Table 1. Fiber characteristics (aspect ratio, coarseness) were measured using L&W FiberMaster, and carbohydrate analyses were done by liquid chromatography. TEMPO, sodium bromide, sodium hypochlorite and other chemicals were of laboratory grades (Wako Pure Chemicals, Co., Japan).
Pulp Pretreatment
The original pulps were subjected to purification pretreatment: dispersion in water and, after adjustment to pH 3, stirring for 30 min with further filtration. The procedure was repeated three times. The overall experimental scheme for this study is presented in Fig. 1.
TEMPO-Mediated Oxidation
5 g of pretreated pulp (dry weight) was placed into a reaction vessel containing water (500 mL), NaBr (0.5 g) and TEMPO (0.08 g). The desired amount of NaClO solution, corresponding to 1–10 mmol g−1 cellulose, was added to the cellulose suspension. The pH of the mixture was maintained at 10 by adding 0.5 M NaOH using a pH-stat for the reaction times from 10 min to 4 h at 22 °C. In the case of reactions exceeding 4 h, ethanol (ca. 5 mL) was added to inhibit the oxidation. The oxidized pulp was thoroughly washed with water and stored at 4 °C without drying. A summary of the reaction times and NaClO additions are shown in Table 2.
Mechanical Treatment
0.1 % dispersion of oxidized pulp in water (30 mL) was disintegrated using a Physcotron (double cylinder mechanic homogenizer) at 7,500 rpm for 2 min and treated for 2 more min with ultrasound (300 Watts of ultrasonic power).
Characterization of the TEMPO-Oxidized Celluloses
Determination of Carboxylate and Aldehyde Contents
The carboxylate contents of the oxidized pulps were measured using electric conductivity titration. Further oxidation with NaClO2 was done to convert aldehyde groups to carboxylate. The total carboxylate content after additional oxidation was used to calculate aldehyde content of the pulps [18].
Degree of Polymerization
Freeze-dried samples of pretreated (acid washed) unmodified spruce and eucalyptus and TEMPO-oxidized pulps were dissolved in 0.5 M copper ethylenediamine solution (cuen). Intrinsic viscosities were measured at 20 °C using a capillary viscometer. Viscosity average degrees of polymerization (DP v) were calculated according to the ASTM D 4243-99 method [23].
Characterization of the Nano-Dispersions
Fibrillation Yield
Disintegrated 0.1 % dispersions of oxidized pulps were centrifuged at 9,000 rpm for 10 min and 2 ml of the supernatant was placed in a Teflon cup for overnight drying at 60 °C. A very thin film was obtained. The fibrillation yield was calculated based on the film weight.
Light Microscopy (LM)
0.1 % dispersions of oxidized pulps in water were observed using a photomicroscope (Olympus BX50) equipped with a phase-contrast lens (Olympus UPlanFLN-PH).
Atomic Force Microscopy (AFM)
TOCN dispersions were diluted with water to 0.001 % concentration to observe separate nanofibers. A drop of the dispersion was placed on a flat mica surface and the excess liquid was absorbed by filter paper before drying at 22 °C. The nanofibers were observed using AFM (Nanoscope III Multimode) in the tapping mode under ambient air conditions (temperature and relative humidity). The widths were determined for 60 nanofibers from the AFM images.
UV–Vis Transmittance
Light transmittance of the 0.1 % nano-dispersions containing both unfibrillated and fibrillated pulps were measured using Shimadzu UV-1700 spectrophotometer in the wavelength range from 300 to 900 nm. The length of the light path was 1 cm.
Visual Examination
The nano-dispersions obtained by the mechanical treatment were centrifuged for 10 min at 9,000 rpm to remove the unfibrillated part and to confirm the presence of birefringence when observed between crossed polarizers. Non-centrifuged dispersions were used to examine visual appearance. Pictures were taken with a digital camera 2 min after shaking.
Results and Discussion
Both carboxylate and aldehyde groups are formed in cellulose as a result of the TEMPO/NaBr/NaClO oxidation at pH 10. The relationships between the amounts of NaClO (used as the primary oxidant) and the carboxylate/aldehyde content of both TEMPO-oxidized eucalyptus and spruce pulps are depicted in Fig. 2. In spite of 1, 3, 5 and 10 mmol/g of NaClO being added, the corresponding carboxylate contents of the oxidized pulps are in the range 0.3–1.4 mmol g−1. The cellulose content of the original pulp as well as the oxidation efficiency was noted to be higher for spruce pulp compared to eucalyptus. It was also observed that the amount of oxidized C6 hydroxyls was higher for spruce than for eucalyptus. This might be due to the high xylan level of eucalyptus (Table 1). Xylan has no C6 primary hydroxyls to be oxidized [7] and, correspondingly, eucalyptus has less C6 hydroxyls than the spruce. Significant amounts of carboxylate groups and small amounts of aldehyde groups were formed in both pulps.
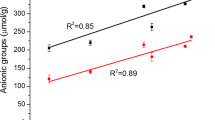
The TEMPO-mediated oxidation and especially the increase of the NaClO content inevitably lead to proportional depolymerization of the starting material. Figure 3 depicts the relationship between the carboxylate contents of the oxidized pulps and viscosity average degrees of polymerization (DP v). Some of the DP v reductions under the alkaline conditions have probably occurred as a result of β-elimination of glucose bonds, accelerated by the presence of C6 carboxylate and aldehyde groups and also C2 or C3 ketones of the water-insoluble fractions [7]. The DP v of the hardwood and softwood pulps dropped within 4 h of oxidation from 1130 to 520 and from 1,120 to 370, respectively. Cellulose molecules have longer chains than hemicellulose. Hemicelluloses have only 150–200 monomer units and low degrees of polymerization [24]. The DP v of the hardwood drops more slowly than for softwood at low carboxylate contents. This could be due to the differences between the hardwood and softwood hemicelluloses. Xylan in hardwood pulps is not oxidized by TEMPO-mediated oxidation, whereas glucommanan is. Possibly, oxidized glucommanan has lower viscosity than xylan even though they have the same degree of polymerization. As the oxidation proceeds to carboxylate contents higher than 0.8 mmol g−1, most of the hemicelluloses are removed by TEMPO-oxidation with large amounts of NaClO. This will result in similar carboxylate/DP v patterns are observed for both softwood and hardwood pulps.
Mechanical disintegration was applied for the preparation of TOCN dispersions in water. A significant increase of viscosity was observed due to increases of the nanofiber conversion and carboxylate contents. The high oxidation levels of wood pulps are important for obtaining dispersions containing mostly individualized nanofibers. Dispersions with high nanofiber contents become transparent as the nano-sized fibers do not scatter the visible light anymore. Transparent and highly viscous gels were obtained from TEMPO-oxidized hardwood and softwood with carboxylate contents of 1.2 and 1.4 mmol g−1, respectively. Partially disintegrated fiber fragments were to a certain extent still present in all of the dispersions even after introducing large amounts of carboxylate groups. All the nano-dispersions prepared from the oxidized spruce pulp were relatively stable and gave no sedimentation for at least 1 day. On the other hand, the eucalyptus sampled E1, E3 and E5 showed signs of sedimentation already after 2 min (Fig. 4).
The TEMPO-oxidized celluloses with carboxylate contents form 0.3 to 1.4 mmol g−1 were disintegrated under the same conditions, showing different degrees of fibrillation (Fig. 5). The largest conversion was registered for the softwood prepared with 10 mmol g−1 NaClO and a carboxylate content of 1.4 mmol g−1. It was also observed that the higher the fibrillation, the more stable dispersions were obtained.
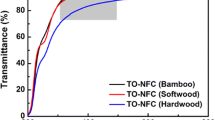
Figure 6 represents UV–Vis transmittance of the 0.1 % dispersions prepared from the TEMPO-oxidized pulps. It was impossible to evaluate the E1, E3 and E5 samples due to the too fast sedimentation, giving unreliable data. The rest of the dispersions showed transmittance variation from slightly translucent to highly transparent. The highest transmittance was registered for the S10 samples, reaching a value of 95 %. For both S10 and E10 it was observed an increase after 300 nm, quickly reaching a plateau in the visible light region. A gradual increase in the percent of transmittance following the wavelength increase in the whole region from 300 to 900 nm was registered for the S1, S3 and S5 dispersions.
All the oxidized nano-dispersions showed clear birefringence (double refraction) between crossed polarizers (Fig. 7) confirming presence of individual nanofibers [25]. There is a significant difference in the birefringence intensity between dispersions made from spruce and eucalyptus, being higher for the spruce samples. The intensity in general is increasing gradually following the increase of the carboxylate content and the amounts of nanofibers in the oxidized cellulose dispersions.
The cellulose, hemicellulose and lignin contents predominantly affect the chemical and physical properties of wood pulps. It is expected that highly oxidized pulps contain very little amounts lignin [26]. The hemicellulose content of spruce was 16 % and somewhat higher (21 %) in eucalyptus (Table 1). Softwood and hardwood differ not only in the percentages of the hemicelluloses but also in the chemical composition of the hemicelluloses. In eucalyptus, the primary hemicellulose is a partially acetylated (4-O-methylglucurono) xylan with a low fraction of glucomannan. In spruce, the main fraction of hemicellulose is partially acetylated galactoglucomannans [27]. Hemicelluloses are soluble in aqueous alkali and are partially removed by washing after a TEMPO-mediated oxidation at pH 10. After the reaction is terminated, the spent liquor is drained and the fiber mass is thoroughly washed with distilled water. Neutral sugar and lignin compositions of the original and TEMPO-oxidized spruce and eucalyptus pulps are presented in Table 3. Most of the neutral sugars and lignin were removed as water-soluble compounds from non-soluble fraction of the TEMPO-oxidized pulp. The total sugar composition is described as unknown and includes hydrolyzed uronic acids, unhydrolyzed uronic acids and unhydrolyzed neutral sugars. These compounds are possible to be present in the TEMPO-oxidized pulps due to difficulties in the hydrolysis of aglycones when uronic acid units are formed.
Figure 8 shows representative optical microscope images of unfibrillated fiber fractions after the TEMPO-mediated oxidation and disintegration in water. The majority of the fibers appear unchanged compared to those of the unmodified pulps. However, mechanical treatment and depolymerization occurring in the amorphous regions as a result of the oxidation may cause formation of the short particles. After extended reaction times of 1 and 4 h, reduction of large particles and fiber fragments were clearly observed (Fig. 8).
Atomic force micrographs (AFMs) were acquired to examine the presence of cellulose nanofibers and fiber fragments in 0.001 % nano-dispersions of the TEMPO-oxidized celluloses (Fig. 9). Mostly well individualized nanofibers are present in the images. There was visible absence of fiber fragments in eucalyptus pulp dispersions before and after centrifugation. This can be explained by almost full conversion to nanofibers already at 1.2 mmol g−1. The spruce dispersions showed presence of nanofiber bundles, for example, the object marked on Fig. 9 S10f has a width of 7.6 nm and might be comprised of 2 or 3 single nanofibers.
It can be noted from the AFM images that eucalyptus has longer nanofibers. Spruce, on the other hand, exhibits shorter fibrils as well as having more kinks and twists occurred as a result of the mechanical treatment (and/or higher carboxyl contents than for eucalyptus).
The average nanofibers widths were measured from the fibril height in the AFM images and showed the same values for both types of pulps—2.5 nm. However, the width distributions presented in Fig. 10 confirm that the eucalyptus samples were mainly comprised of 2–3 nm nanofibers while spruce had equal proportions of both wider and thinner objects.
Conclusions
TEMPO-mediated oxidation is a promising technique applicable for a variety of cellulosic materials. The reaction can be used for an effective introduction of both aldehyde and carboxylate groups, giving high reaction rate and yield, high selectivity and low degradation of the substrate. TEMPO-oxidized celluloses from both hardwood and softwood can be used for the preparation of nanofibers with different properties depending on the carboxylate contents. However, it was confirmed that TEMPO-mediated oxidation of spruce was more efficient than the oxidation of eucalyptus. Spruce gave higher carboxylate contents and nanofiber yields, higher light transmittance and stability of the nano-dispersions.
References
Ioelovich M (1991) Wood Chem 4:27
Ioelovich M (1992) Acta Polym 43:110
de Nooy A, Besemer A, van Bekkum H (1995) Carbohydr Res 269:89
Isogai A, Kato Y (1998) Cellulose 5:153
Bragd P, Besemer A, van Bekkum H (2000) Carbohydr Res 328:355
Tahiri C, Vignon M (2000) Cellulose 7:177
Isogai A, Saito T, Fukuzumi H (2011) Nanoscale 3:71
Okita Y, Saito T, Isogai A (2010) Biomacromolecules 11:1696
Hirota M, Furihata K, Saito T, Kawada T, Isogai A (2010) Angew Chem Int Ed 49:7670
Saito T, Nishiyama Y, Putaux J-L, Vignon M, Isogai A (2006) Biomacromolecules 7:1687
Fukuzumi H, Saito T, Iwata T, Kumamoto Y, Isogai A (2009) Biomaromolecules 10:162
Saito T, Isogai A (2005) Tappi J 4(3):3
Saito T, Isogai A (2007) Ind Eng Chem Res 46:773
Suh D, Lee K, Chang P, Kim K (2007) J Food Sci 72:C235
Li Z, Renneckar S, Barone J (2010) Cellulose 17:57
Johnson R, Zink-Sharp A, Renneckar S, Glasser W (2009) Cellulose 16:227
Mao L, Ma P, Law K, Daneault C, Brouillette F (2010) Ind Eng Chem Res 49:113
Saito T, Isogai A (2004) Biomacromolecules 5:1983
Saito T, Kimura S, Nishiyama Y, Isogai A (2007) Biomacromolecules 8:2485
Alila S, Aloulou F, Beneventi D, Boufi S (2007) Langmuir 23:3723
Syverud K, Chinga-Carrasco G, Toledo J, Toledo P (2011) Carbohydr Polym 84:1033
Besbes I, Alila S, Boufi S (2011) Carbohydr Polym 84:975
ASTM D4243-99 (1999) Standard test method for measurement of average viscometric degree of polymerization of new and aged electrical papers and boards, ASTM International, p 6
Timell T (1964) Adv Carbohydr Chem 19:247
de Souza MM, Borsali R (2004) Marcomol Rapid Comm 25:771
Okita Y, Saito T, Isogai A (2009) Holzforschung 63:529
Sjostrom E (1993) Wood chemistry: fundamentals and applications. Academic Press, San Diego
Acknowledgments
The authors would like to thank all the members of Prof. Isogai’s laboratory (The University of Tokyo) for valuable assistance during the experimental work, Prof. Torbjørn Helle for linguistic help and the project partners in the SustainBarrier project at PFI for the financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rodionova, G., Saito, T., Lenes, M. et al. TEMPO-Mediated Oxidation of Norway Spruce and Eucalyptus Pulps: Preparation and Characterization of Nanofibers and Nanofiber Dispersions. J Polym Environ 21, 207–214 (2013). https://doi.org/10.1007/s10924-012-0483-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10924-012-0483-9