Abstract
Ambulatory blood pressure monitoring (ABPM) has become an essential tool in the diagnosis and management of hypertension. Current standard ABPM devices use an oscillometric cuff-based method which can cause physical discomfort to the patients with repeated inflations and deflations, especially during nighttime leading to sleep disturbance. The ability to measure ambulatory BP accurately and comfortably without a cuff would be attractive. This study validated the accuracy of a cuff-less approach for ABPM using pulse arrival time (PAT) measurements on both healthy and hypertensive subjects for potential use in hypertensive management, which is the first of its kind. The wearable cuff-less device was evaluated against a standard cuff-based device on 24 subjects of which 15 have known hypertension. BP measurements were taken from each subject over a 24-h period by the cuff-less and cuff-based devices every 15 to 30 minutes during daily activities. Mean BP of each subject during daytime, nighttime and over 24-h were calculated. Agreement between mean nighttime systolic BP (SBP) and diastolic (DBP) measured by the two devices evaluated using Bland-Altman plot were −1.4 ± 6.6 and 0.4 ± 6.7 mmHg, respectively. Receiver operator characteristics (ROC) statistics was used to assess the diagnostic accuracy of the cuff-less approach in the detection of BP above the hypertension threshold during nighttime (>120/70 mmHg). The area under ROC curves were 0.975/0.79 for nighttime. The results suggest that PAT-based approach is accurate and promising for ABPM without the issue of sleep disturbances associated with cuff-based devices.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Increasing number of studies have validated the clinical significance of ambulatory blood pressure (ABP) monitoring and suggested ABP to be an adjunct to office BP to provide complementary information in clinical practice for the management of hypertension [1]. In addition, ABP monitoring has been shown predictive of cardiovascular events as well as target-organ deterioration in hypertensive patients after the adjustments of office BP and other covariates [2–4], especially night-time ABP [2]. Furthermore, ABP monitoring can provide more effective evaluation of antihypertensive treatments on target-organ damage [5, 6]. With the emerging field of mobile health in recent years [7, 8], smart health monitoring has already begun to play unique and important roles in improving clinical practices and outcomes [9, 10]. It is anticipated that ABP monitoring will also become increasingly popular and important in assisting clinical diagnosis and treatment in the near future.
Although an amount of evidence have shown the superiority of ambulatory to office BP in the clinical management of hypertensive patients, ABP monitoring has its drawbacks for routine use. Adequate number of expected measurements are required to guarantee sufficient accuracy and reproducibility of ABP monitoring. Due to the frequent cuff inflation and long recording period in ABP measurements, it would pose great influences to the subject’s daily activities as well as cause disturbances during sleeping, thus mitigating the clinical value of ABP monitoring [11]. It is therefore of great importance to develop a new device for ABP monitoring without interrupting the subject’s daily life.
Photoplethysmography is an optical technique to measure blood volume changes in microvascular beds at peripheral body sites using a light source and a photo detector. This technique has been applied in various applications of cardiovascular monitoring [12–15]. Pulse arrival time (PAT) which is the time for a pressure pulse to travel from the heart to a peripheral site has been suggested to be a potential surrogate of BP [16–18]. The main advantage of the PAT-based method for BP measurement is that it can be simply measured from electrocardiogram (ECG) and photoplethysmogram (PPG) cuff-lessly by wearable devices [19–22], or even contactlessly using a high speed video camera [23]. This cuff-less solution overcomes the disturbance issue of the traditional cuff-based method and is thus very promising for ABP monitoring. Many previous studies have been conducted to investigate the potential of PAT as a surrogate of BP under different protocols [16–18, 24]. One of our previous studies have validated the PAT-based method against two cuff-based BP devices under static situation using the standard protocol required by the Association for the Advancement of Medical Instrumentation (AAMI) [16]. However, these studies were conducted in a relatively short period (minutes to hours) under well-controlled laboratory or hospital settings. One of our recent studies showed that PAT is also potential for estimating overnight BP in healthy subjects in ambulatory settings, especially for an averaged BP over a long period [25]. It is unknown whether this method still works on cardiovascular patients. The aim of this study is to validate a wearable and cuff-less PTT-based BP monitor against a standard cuff-based ABP monitor on measuring averaged BP under ambulatory settings in both healthy and hypertensive subjects, and to explore the potential of the wearable device for hypertension diagnosis.
Subjects and methods
Subjects
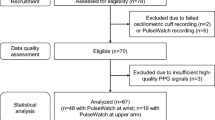
The inclusion criteria for patients include those: 1) who have diagnosed with essential hypertension or have been put on antihypertensive drugs; and 2) without evidence of cardiovascular end organ damage. Patients with any of the following conditions were excluded: 1) secondary hypertension with underlying causes or hypertensive emergency; 2) patients with atrial fibrillation or diabetes mellitus or coronary heart disease or history of congestive heart failure (systolic/diastolic) or other known structural heart disease; 3) patients receiving pacemaker therapy; 4) patients with active systemic illness or end-organ disease. Twenty-four subjects (15 elderly hypertensive patients and 9 young healthy subjects) participated in this study. Among the 15 hypertensive patients, eight have hyperlipidaemia, and a few of them have one or several of the following diseases: stroke, liver disease, obesity, gout, osteoarthritis, renal disease and endocrine disease. The basic information of all subjects are summarized in Table 1.
Two ambulatory BP devices
An in-house-developed PAT-based armband wearable device was adopted for PAT-based cuff-less BP measurement [25]. As shown in Fig. 1, it contains one PPG sensor (a near-infrared LED and a photodiode), two pieces of e-textile ECG patches sewed on the inner side of the armband and an Ag/AgCl electrode placed near the chest and connected to the armband main circuit via a wire. The amplified and filtered ECG and PPG signals were digitalized at the frequency of 500 Hz and resolution of 8 bits. Data can be stored in device memory or transmitted wirelessly to a smart phone via Bluetooth in real-time. A standard 24-h ambulatory blood pressure monitor (Oscar2, SunTech Medical) was adopted as the reference device to evaluate the accuracy of the armband. Oscar2 has been clinically validated to all three internationally recognized standards (BHS, ESH and AAMI SP10) and has been recommended in a number of previous clinical studies on ambulatory BP measurements.
Data acquisition
Upon arrival, each subject was asked to rest for about 10 min. Office BP was measured by an experienced nurse with an upper arm type of oscillometric BP device (Omron, HEM7300, Japan). According to guidelines on office BP measurements [26, 27], two consecutive BP measurements were taken at 1-min interval and the average was used in Table 1.
The two ambulatory devices were worn on the right and left upper arms respectively. Preliminary studies found that the PPG signal quality was generally better when the sensor was located around the left triceps brachia and left brachialis [25]. The cuff-based ambulatory BP device was set to automatically record at every 30 min over 24 h [1]. Meanwhile, 1-min of ECG and PPG were automatically recorded at an interval of 30 min and 15 min by the PAT-based wearable device in the healthy subjects and patients, respectively. The reason for the different sampling rates in healthy and hypertensive subjects is that our previous study in healthy subjects found the issue of the misalignment between BP and PAT on their recording time [25]. To acquire PAT samples as close as possible to BP samples, we doubled the sampling rate of PAT on the patients. BP and PAT recordings from the two devices were used for further analysis only when at least 80 % expected readings were available after excluding those erroneous ones. The subjects were required to keep still at each measurement during daytime to avoid motion artefacts, and also to record their daily activities hourly in dairies together with their sleep quality, time of sleeping and waking-up. Daytime and night-time are defined according to the sleeping and waking-up time as reported by the subject.
Data analysis
Calculation of PAT
The acquired ECG and PPG were low-pass filtered at cut-off frequency of 30 and 16 Hz, respectively. ECG and PPG segments which were distorted by motion artifacts were identified through visual observation, and were excluded from further analysis (PAT calculation and BP estimation). PAT was calculated as the time difference between R-peak of ECG and the maximal slope of PPG within the same cardiac cycle (see Fig. 2). PAT was averaged over each 1-min recording period.
Synchronize BP and PAT
As mentioned in our previous study [25], the measurements of the two BP devices were not synchronized exactly because the reference BP device did not exactly follow the predefined sampling pattern. Therefore, resampling is needed to synchronize the measurements of the two BP devices. The PAT processing method proposed in [25] were adopted in this study. Briefly, the raw PAT were firstly evenly resampled at 0.00056 Hz (i.e., every 30 min or 1/30/60 Hz) and then smoothed by a low-pass filter 0.00019 Hz (1/30/60/3 Hz). Transients in PAT samples, defined as the samples deviated from the low-pass filtered PAT trend line by 9 ms or more, were removed from the raw PAT to avoid inauthentic resampling. After removing these transient samples, the new PAT were then low-pass filtered at 0.00019 Hz and linearly interpolated at the time when BP were recorded. The rationale of the proposed synchronization method can refer to the data analysis method in [25]. In general, this method provides a fairer evaluation on the accuracy of the PAT-based method against the oscillometric-based measuring method. With the synchronized BP and PAT, PAT-based BP estimation can be performed based on the BP-PAT model as described below.
Different PAT sample sets (ALLset, NEAset, FARset)
Since the sampling rate of PAT (one sample every 15 min or 0.0011 Hz) is around two times of BP (one sample every 30 min or 0.00056 Hz) in the patients, different PAT sample sets were evaluated in terms of their accuracy on BP estimation: 1) all PAT samples (ALLset); 2) PAT samples closest to BP samples (NEAset); 3) the complementary set of NEAset (FARset). The synchronization processing method mentioned above was applied to the three sample sets for further estimation of BP. We draw a figure to better illustrate the three PAT samples sets as shown in Fig. 3. It shows the time series of SBP and the three different PAT samples sets of one patient (dark dots). With the aligning lines (dotted grey lines) that marked the recording time of SBP, we can clearly see that NEAset are subset of PAT that are closer to the SBP recording time, while FARset are subset of PAT that are relatively far away from the SBP recording time. With the three different PAT sample sets, we obtained three different PAT trend lines after low pass filtering (red lines) and three different sets of PAT resamples (red triangles) aligned with SBP. The three sets of PAT resamples were used for BP estimation in the following process.
PAT-based BP estimation
Since ABP averages during 24-h, daytime and night-time are commonly used for the diagnosis and management of hypertension [1], PAT-based estimations of these BP averages will be evaluated. The difference between the PAT-based estimation and the cuff-based measurements will be evaluated by calculating the mean and standard deviation of differences as well as the mean absolute differences between the wearable PAT device and reference cuff-based device. A previous nonlinear PAT-based BP model was adopted in this study for BP estimation [16],
One-point individual calibration was applied before BP estimation for daytime and nighttime. Specifically, SBP 0 and DBP 0 are the first measurements of the cuff-based BP device for each subject during daytime or nighttime, and PAT 0 is the first measurement of the wearable PAT device for each subject during daytime or nighttime. SBP 0 , DBP 0 and PAT 0 were used as the calibration points for individual calibration of the PAT-based BP model described in equations (1–2). Through this calibration, individualized BP-PAT model can be obtained for each subject. To validate the accuracy of the PAT-based BP estimation model, the remaining samples of PAT for each subject (i.e. all PAT measurements excluding the first sample used in the calibration) were used for BP estimation. The estimation results will then be evaluated against the BP measurements. γ is a parameter related to the arterial elastic properties which may be altered with age and the development of cardiovascular diseases [28]. The method to determine γ was described in [25]. For the healthy, it was set as 0.031 mmHg−1. Based on the data from some previous studies [29] and considering the age of the hypertensive patients in our study (63 ± 4 years old), γ was set as 0.09 mmHg−1 for all patients using the same method described in [25]. The estimated BP over daytime, night-time and 24-h periods were then averaged and compared with the cuff-based calculations.
Statistical analysis
Considering that the statistical distribution of device-device measurement difference is t-distribution [30], one-tailed student’s t-test was performed to test whether the group means from the PAT-based estimation is significantly less than that from BP0-based estimation [31]. The significance level is determined as p < 0.05. All data processing and analysis were performed using MATLAB 8.01, MathWorks.
Results
BP estimation by different PAT sample sets (ALLset, NEAset, FARset)
Using the three different sets of PAT resamples, we got three sets of estimation results as shown in Table 2. As can be seen in Table 2, NEAset outperformed ALLset and FARset in both SBP and DBP estimation at all time periods. Therefore, the following results for the hypertensive patients were based on the NEAset estimation. It is also observed that the night-time estimation is within the requirement of AAMI standard (i.e., the difference should be within 5 ± 8 mmHg), while the results for daytime and 24-h are not up to the standard. In addition, it is found that BP estimation by NEAset and FARset are quite different. For all patients, the NEA_FAR differences (Diff_NEA_FAR) were 3.4 ± 6.4 and −1.1 ± 3.9 mmHg for SBP and DBP respectively. From Fig. 4, it can be seen that the individual NEA_FAR differences ranged from −7 to 15 mmHg for SBP and from −9 to 4 mmHg for DBP.
PAT-based BP estimation
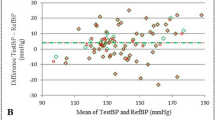
Table 3 demonstrates the estimation accuracy (mean and SD of the differences as well as mean absolute differences between the reference and the estimated BP) at different time periods for SBP and DBP. It can be seen that night-time BP showed smallest differences (−1.4 ± 6.6 and 0.4 ± 6.7 mmHg for SBP and DBP) among three time periods. Considering the PAT-based BP model was firstly calibrated using the first measurements of BP and PAT (i.e., SBP0, DBP0 and PAT0), it would be interesting to demonstrate whether PAT-based estimation (Diff_PAT_cuff) is better than BP0-based estimation (Diff_BP 0 _cuff). BP0-based estimation can be treated as the model that uses one BP measurement to represent the mean BP over a period of time. If BP does not vary too much during the measurement period, the BP0 model would work well. Therefore, only if the PAT-based estimation is better than the BP0-based estimation, we can say PAT can provide additional information for BP estimation. Otherwise, the good estimation results may simply because the PAT model was calibrated at the beginning using BP0 which is already representative of BP over the whole measuring period. Therefore, we compared PAT-based estimation in Figs. 5 (b, d) with the BP0-based estimation in (a, c) during night-time for SBP and DBP, respectively. We performed one-tail statistical test on the absolute differences between the reference BP and PAT-based estimations and that between the reference and BP0-based estimations. It showed that PAT significantly improved night-time SBP estimation compared to SBP0 (−1.4 ± 6.6 vs 2.3 ± 10.2 mmHg, p = 0.001), while DBP estimation was marginally but insignificantly improved by PAT compared to DBP0 (0.4 ± 6.7 vs 0.1 ± 8.0 mmHg, p = 0.08).
We further compared the estimation accuracy between the hypertensive and normotensive subjects for overnight average SBP and DBP in Fig. 6. The result showed that the estimation of overnight average SBP was significantly better in the healthy (1.9 ± 3.1 mmHg) than the hypertensive (3.4 ± 7.4 mmHg), while DBP was slightly worse for the healthy than the hypertensive.
Comparison between PAT-based estimation and cuff-based BP measurements for hypertension diagnosis
In addition to evaluating the accuracy of BP estimation, it is also worthwhile to evaluate the consistency of the PAT-based BP estimation with cuff-based measurements in the detection of BP above the hypertension threshold for hypertension diagnosis. The cut-off values for hypertension diagnosis using these ABP averages in clinical practice are known to be 130/80 mmHg for 24-h SBP/DBP, 120/70 mmHg for night SBP/DBP and 10–20 % day-to-night dipping [1]. Since our results showed that PAT could only estimate night-time BP accurately, we adopted a hybrid scheme (i.e., cuff-based measurements for daytime and PAT-based measurements for night-time) in the evaluation of 24-h BP. The hybrid approach implies that the subject can use the cuff-based BP monitor alone during daytime while wear the cuff-less BP monitor during nighttime. Although this is not the ideal case that a single device can provide accurate 24-h ambulatory BP measurements without discomfort, the hybrid approach indeed can reduce the discomfort and sleep disturbances during nighttime.
The diagnostic accuracy of mean night-time and 24-h SBP/DBP obtained from the PAT-based method and the hybrid method respectively in detecting BP above the hypertension threshold were evaluated against the cuff-based one by the receiver operating characteristic (ROC) as shown in Fig. 7. The area under the ROC curves (AUC) are 0.975/0.794 and 0.996/1.0 for night and 24-h SBP/DBP, respectively.
Discussion
This is the first study to evaluate the accuracy of PAT-based method for the measurement of mean BP over 24 h at out-of-hospital settings on both hypertensive and healthy subjects. The results showed that compared to the standard cuff-based measurements, PAT-based method can estimate mean night-time SBP and DBP accurately with the difference of −1.4 ± 6.6 and 0.4 ± 6.7 mmHg, respectively, but is not accurate enough for daytime BP estimation according to the requirement of AAMI standard on BP measurement [16]. The results of this study suggest that PAT-based cuff-less device has large potential to be used in mean nighttime ABP monitoring, which would bring great benefits for ABP monitoring in clinical settings such as relieving sleeping disturbances.
Different PAT sample sets for BP estimation
As shown in Table 2, the estimation by different PAT sample sets are quite different, especially for NEAset and FARset (3.4 ± 6.4 mmHg for SBP and −1.1 ± 3.9 for DBP), and the differences can be up to 15 mmHg for some individuals as seen from Fig. 4. Due to the very low sampling rate of PAT, the selection of PAT samples would greatly influence the trend lines of PAT after low-pass filtering, thus resulting in different PAT resamples. The closer PAT to BP samples on the recording time, more accurately that PAT can estimate BP. It is interesting to observe that NEAset estimation is even better than ALLset estimation considering ALLset encompasses NEAset. One reason for this is that the sampling rate of ALLset PAT is around two times of BP, so some high frequency changes which do not exist in BP may be included in PAT. Due to the frequency-dependent relationship between BP and PAT [32], NEAset performed better than ALLset in tracking BP.
Another interesting insight obtained from the different estimation results with different PAT sample sets is that the current BP sampling rate (i.e., one sample per 30 min) is inadequate to reflect the real BP level. Specifically, BP varies from time to time, so mean BP over a period are likely to be very different when they were sampled at different time. In this regard, although we showed that BP can be accurately estimated by PAT close to it (NEAset), it is difficult to be estimated by PAT far away (FARset). Previous studies also investigated the effect of sampling rate of 24-h ABP on the reproducibility of mean BP [33]. They compared mean BP measured from ABP recordings sampled every 1 h over 48-h period against that sampled at interval of 20–30 min over 48-h period. The differences of mean night-time BP calculated in their study were not as large as ours (0.1 ± 1.6 mmHg for SBP and 0.1 ± 1.4 mmHg for DBP). This may be due to the long duration of their study (48 h) which increased the number of samples to ensure the reproducibility of mean BP. It is unknown whether the sampling rate would matter when the duration is shortened to 24-h or less.
Accuracy of the PAT-based method
Nighttime vs Daytime. From Table 3, the PAT-based estimation during daytime was not as good as night-time. From some previous studies on BP-PAT relationship [34–37], it is considered that two confounding factors, i.e., vascular tone and pre ejection period (PEP), may be responsible for the larger estimation errors during daytime. Vascular tone reflects the activation level of the vessels relative to its maximal dilated state. PEP is the isovolumetric contraction period of ventricle. Vascular tone and PEP can be changed under different physiological status induced by daytime activities like posture changes, having dinner, walking and working, thus changing the BP-PAT relationship. Due to the difficulties on measuring PEP and vascular tone in ambulatory settings, the BP-PAT model in this study did not take into account the influence of PEP and vascular tone changes.
Healthy vs Hypertensive. It is also found that the PAT estimates overnight average SBP with error of 1.9 ± 3.1 mmHg in young healthy subjects, and 3.4 ± 7.4 mmHg in the elderly hypertensive subjects. Due to the different SBP ranges of the two groups (about 90–130 mmHg for the healthy and 100–150 mmHg for the hypertensive), we cannot directly conclude that PAT estimate SBP better in the healthy subjects than hypertensive subjects. One possible reason for this result is the influences of PEP and vascular tone. A number of previous studies have found that both hypertension and aging are accompanied with elevated sympathetic nerve activity which can regulate both PEP and vascular tone [38, 39]. This would cause larger variations on both PEP and vascular tone in the elderly hypertensive patients compared to the young healthy subjects, thus resulting in worse estimation results. Nevertheless, future studies are still needed to further investigate the differences on the accuracy when comparing hypertensive and normotensive subjects.
PAT vs BP 0 -based estimation. In addition, it is found that PAT significantly improved SBP estimation compared to SBP0-based estimation (from 2.3 ± 10.2 to −1.4 ± 6.6 mmHg, p < 0.05), which indicates the additional predictive value of PAT in mean night-time SBP estimation on the basis of SBP0. This result suggests that although BP during nighttime may not change too much in time, SBP0 alone was still insufficient to estimate the mean nighttime SBP. By adding PAT, the estimation significantly improved. On the other hand, the improvement in DBP estimation (from 0.1 ± 8.0 to 0.4 ± 6.7 mmHg) is not as significant as in SBP. This may because the better correlation between SBP and PAT than DBP and PAT, which has been found in many previous studies [25] [34].
There are some limitations of this study. Considering this is the first study to test if the PAT-based method has potential for cuff-less BP estimation in daily-life settings on both hypertensive patients and healthy subjects, only 24 subjects were included. Future studies with larger sample size (> = 85 subjects) should be conducted to further validate the proposed method in ambulatory settings. In addition, the hypertensive patients recruited in this study were mostly with very high BP (office SBP > 140 mmHg). Since ABPM is often recommended under situations either with very high (>140 mmHg) or very low office SBP (<90 mmHg) which are quite common in patients with uncontrolled BP, more patients with very low BP should also be included for validation.
Conclusion
This study is the first to evaluate the cuff-less PAT-based method on estimating mean night-time BP against standard cuff-based ABP measurements for potential clinical use in hypertension management. Twenty-four subjects were tested and the cuff-less method can estimate mean night-time SBP and DBP with error within −1.4 ± 6.6 and 0.4 ± 6.7 mmHg, respectively. By using the PAT-based night-time estimation and the cuff-based daytime measurements, the cuff-less method can accurately detect BP beyond the recommended hypertension cut-off values in contrast to the cuff-based ABP monitoring, showing its potential use in the clinical diagnosis of hypertension. Therefore, PAT-based method provides a cuff-less solution for night-time ABP monitoring to help alleviate the issue of sleep disturbances of traditional cuff-based ABP monitors and may greatly advance the use of ABP monitoring in clinical practices.
References
Mancia, G., Fagard, R., Narkiewicz, K., Redon, J., Zanchetti, A., et al., 2013 ESH/ESC guidelines for the management of arterial hypertension: the task force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur. Heart J. 34:2159–2219, 2013. doi:10.1093/eurheartj/eht151.
Fagard, R.H., Celis, H., Thijs, L., Staessen, J.A., Clement, D.L., De Buyzere, M.L., and De Bacquer, D.A., Daytime and nighttime blood pressure as predictors of death and cause-specific cardiovascular events in hypertension. Hypertension. 51:55–61, 2008. doi:10.1161/hypertensionaha.107.100727.
Niiranen, T.J., Maki, J., Puukka, P., Karanko, H., and Jula, A.M., Office, home, and ambulatory blood pressures as predictors of cardiovascular risk. Hypertension. 64:281–286, 2014. doi:10.1161/hypertensionaha.114.03292.
Yi, J.E., Shin, J., Ihm, S.H., Kim, J.H., Park, S., et al., Not nondipping but nocturnal blood pressure predicts left ventricular hypertrophy in the essential hypertensive patients: the Korean ambulatory blood pressure multicenter observational study. J. Hypertens. 32:1999–2004, 2014. doi:10.1097/hjh.0000000000000272.
Mancia, G., Zanchetti, A., Agebiti-Rosei, E., Benemio, G., De Cesaris, R., et al., Ambulatory blood pressure is superior to clinic blood pressure in predicting treatment-induced regression of left ventricular hypertrophy. Circulation. 95:1464–1470, 1997. doi:10.1161/01.cir.95.6.1464.
Karpettas, N., Destounis, A., Kollias, A., Nasothimiou, E., Moyssakis, I., and Stergiou, G.S., Prediction of treatment-induced changes in target-organ damage using changes in clinic, home and ambulatory blood pressure. Hypertens. Res. 37:543–547, 2014. doi:10.1038/hr.2014.24.
Baig, M.M., and Gholamhosseini, H., Smart Health Monitoring Systems: An Overview of Design and Modeling. J. Med. Syst. 37, 2013. doi:10.1007/s10916-012-9898-z.
Steinhubl, S.R., Muse, E.D., and Topol, E.J., The emerging field of mobile health. Sci. Transl. Med. 7:1–6, 2015.
Or C, Tao D (2016) A 3-Month Randomized Controlled Pilot Trial of a Patient-Centered, Computer-Based Self-Monitoring System for the Care of Type 2 Diabetes Mellitus and Hypertension. J Med Syst 40. doi:10.1007/s10916–016–0437-1
Rudner J, McDougall C, Sailam V, Smith M, Sacchetti A Interrogation of Patient Smartphone Activity Tracker to Assist Arrhythmia Management. Annals of Emergency Medicine. doi:10.1016/j.annemergmed.2016.02.039 2016.
Agarwal, R., and Light, R.P., The effect of measuring ambulatory blood pressure on nighttime sleep and daytime activity—implications for dipping. Clin. J. Am. Soc. Nephrol. 5:281–285, 2010.
Zhilin, Z., Photoplethysmography-based heart rate monitoring in physical activities via joint sparse Spectrum reconstruction. Biomed. Eng. IEEE Trans. on. 62:1902–1910, 2015. doi:10.1109/TBME.2015.2406332.
Feissel, M., Aho, L.S., Georgiev, S., Tapponnier, R., Badie, J., Bruyere, R., and Quenot, J.P., Pulse Wave Transit Time Measurements of Cardiac Output in Septic Shock Patients: A Comparison of the Estimated Continuous Cardiac Output System with Transthoracic Echocardiography. Plos One:10, 2015. doi:10.1371/journal.pone.0130489.
Ruiz-Rodríguez, J., Ruiz-Sanmartín, A., Ribas, V., Caballero, J., García-Roche, A., et al., Innovative continuous non-invasive cuffless blood pressure monitoring based on photoplethysmography technology. Intensive Care Med. 39:1618–1625, 2013. doi:10.1007/s00134-013-2964-2.
Mukkamala, R., Hahn, J.O., Inan, O.T., Mestha, L.K., Kim, C.S., Toreyin, H., and Kyal, S., Toward ubiquitous blood pressure monitoring via pulse transit time: theory and practice. IEEE Trans. Biomed. Eng. 62:1879–1901, 2015. doi:10.1109/TBME.2015.2441951.
Poon CCY, Zhang YT Cuff-less and noninvasive measurements of arterial blood pressure by pulse transit time. In: Proc. 27th Annu. Int. Conf. IEEE-EMBC, Shanghai, pp 5877–5880 2005
Gesche, H., Grosskurth, D., Küchler, G., and Patzak, A., Continuous blood pressure measurement by using the pulse transit time: comparison to a cuff-based method. Eur. J. Appl. Physiol. 112:1–7, 2011. doi:10.1007/s00421-011-1983-3.
Kim, S.H., Song, J.G., Park, J.H., Kim, J.W., Park, Y.S., and Hwang, G.S., Beat-to-beat tracking of systolic blood pressure using noninvasive pulse transit time during anesthesia induction in hypertensive patients. Anesth. Analg. 116:94–100, 2013. doi:10.1213/ANE.0b013e318270a6d9.
Zheng YL, Leung B, Sy S, Zhang YT, Poon CCY A clip-free eyeglasses-based wearable monitoring device for measuring photoplethysmograhic signals. In: Proceedings of the 34th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, San Diego. pp 5022–5025 2012
Winokur ES, He DD, Sodini, CG A wearable vital signs monitor at the ear for continuous heart rate and pulse transit time measurements. In: Proc. 34th Annu. Int. Conf. IEEE-EMBC, San Diego, CA, Aug. pp 2724–2727. doi:10.1109/embc.2012.6346527 2012.
Lin, H., Xu, W., Guan, N., Ji, D., Wei, Y., and Yi, W., Noninvasive and continuous blood pressure monitoring using wearable body sensor networks. IEEE Intell. Syst. 30:38–48, 2015. doi:10.1109/MIS.2015.72.
Dilpreet, B., Jean-Michel, R., and Mehmet Rasit, Y., A survey on signals and systems in ambulatory blood pressure monitoring using pulse transit time. Physiol. Meas. 36:R1, 2015.
Jeong, I.C., and Finkelstein, J., Introducing contactless blood pressure assessment using a high speed video camera. J. Med. Syst. 40:1–10, 2016. doi:10.1007/s10916-016-0439-z.
McCarthy, B., Vaughan, C., O'Flynn, B., Mathewson, A., and Mathúna, C.Ó., An examination of calibration intervals required for accurately tracking blood pressure using pulse transit time algorithms. J. Hum. Hypertens. 27:744–750, 2013.
Zheng, Y.L., Yan, B.P., Zhang, Y.T., and Poon, C.C.Y., An armband wearable device for overnight and cuff-less blood pressure measurement. IEEE. Trans. Biomed. Eng. 61:2179–2186, 2014. doi:10.1109/tbme.2014.2318779.
Pickering, T.G., Hall, J.E., Appel, L.J., Falkner, B.E., Graves, J., et al., Recommendations for blood pressure measurement in humans and experimental animals: part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on high blood pressure research. Hypertension. 45:142–161, 2005. doi:10.1161/01.HYP.0000150859.47929.8e.
Eguchi, K., Kuruvilla, S., Ogedegbe, G., Gerin, W., Schwartz, J.E., and Pickering, T.G., What is the optimal interval between successive home blood pressure readings using an automated oscillometric device? J. Hypertens. 27:1172, 2009.
Hughes, D.J., Babbs, C.F., Geddes, L.A., and Bourland, J.D., Measurements of Young's modulus of elasticity of the canine aorta with ultrasound. Ultrason. Imaging. 1:356–367, 1979. doi:10.1177/016173467900100406.
Bussy, C., Boutouyrie, P., Lacolley, P., Challande, P., and Laurent, S., Intrinsic stiffness of the carotid arterial wall material in essential hypertensives. Hypertension. 35:1049–1054, 2000.
ANSI/AAMI SP10: Manual, electronic, or automated sphygmomanometers Association for the Advancement of Medical Instrumentation, United States of America 2003.
Yan, I.R., Poon, C.C.Y., and Zhang, Y.T., Evaluation scale to assess the accuracy of cuff-less blood pressure measuring devices. Blood. Press. Monit. 14:257, 2009.
Liu, Q., Poon, C.C.Y., and Zhang, Y.T., Time-frequency analysis of variabilities of heart rate, systolic blood pressure and pulse transit time before and after exercise using the recursive autoregressive model. Biomed. Signal. Process. Control. 6:364–369, 2011.
Hermida, R.C., Ayala, D.E., Fontao, M.J., Mojon, A., and Fernandez, J.R., Ambulatory blood pressure monitoring: importance of sampling rate and duration-48 versus 24 hours-on the accurate assessment of cardiovascular risk. Chronobiol. Int. 30:55–67, 2013. doi:10.3109/07420528.2012.701457.
Payne, R., Symeonides, C., Webb, D., and Maxwell, S., Pulse transit time measured from the ECG: an unreliable marker of beat-to-beat blood pressure. J. Appl. Physiol. 100:136–141, 2006.
Wong, M.Y.M., Pickwell-MacPherson, E., Zhang, Y.T., and Cheng, J.C.Y., The effects of pre-ejection period on post-exercise systolic blood pressure estimation using the pulse arrival time technique. Eur. J. Appl. Physiol. 111:135–144, 2011. doi:10.1007/s00421-010-1626-0.
Zhang, G., Gao, M., Xu, D., Olivier, N.B., and Mukkamala, R., Pulse arrival time is not an adequate surrogate for pulse transit time as a marker of blood pressure. J. Appl. Physiol. 111:1681–1686, 2011. doi:10.1152/japplphysiol.00980.2011.
Liu, Q., Yan, B.P., Yu, C., Zhang, Y., and Poon, C.Y.C., Attenuation of systolic blood pressure and pulse transit time hysteresis during exercise and recovery in cardiovascular patients. Biomed Eng. IEEE Trans.on. 61:346–352, 2013. doi:10.1109/tbme.2013.2286998.
Mancia, G., Grassi, G., Giannattasio, C., and Seravalle, G., Sympathetic activation in the pathogenesis of hypertension and progression of organ damage. Hypertension. 34:724–728, 1999. doi:10.1161/01.hyp.34.4.724.
Esler, M., Hastings, J., Lambert, G., Kaye, D., Jennings, G., and Seals, D.R., The influence of aging on the human sympathetic nervous system and brain norepinephrine turnover. Am. J. Physiol. Regul. Integr. Comp. Physiol. 282:R909–R916, 2002.
Acknowledgment
The authors are thankful to Mr. Billy Leung and Mr. Ruikai Zhang for their developments of the wearable system, Mr. Ruikai Zhang and Ms. Ruoxi Yu for their contribution on developing the mobile application, and Ms. Cecilia Chan for her assistance in collecting the data from the patients. This work was supported by the Hong Kong Innovation and Technology Commission (ITS/159/11).
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
All procedures performed in the study involving human participants were in accordance with the ethical standards of the Joint Chinese University of Hong Kong – New Territories East Cluster Clinical Research Ethics Committee, and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from each participant in the study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Yali Zheng and Carmen C. Y. Poon contributed equally to this work.
This article is part of the Topical Collection on Mobile Systems
Rights and permissions
About this article
Cite this article
Zheng, Y., Poon, C.C.Y., Yan, B.P. et al. Pulse Arrival Time Based Cuff-Less and 24-H Wearable Blood Pressure Monitoring and its Diagnostic Value in Hypertension. J Med Syst 40, 195 (2016). https://doi.org/10.1007/s10916-016-0558-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10916-016-0558-6