Abstract
Bats are atypical small mammals. Size is crucial for bats because it affects most aerodynamic variables and several key echolocation parameters. In turn, scaling relationships of both flight and echolocation have been suggested to constrain bat body size evolution. Previous studies have found a large phylogenetic effect and the inclusion of early Eocene fossil bats contributed to recover idiosyncratic body size change patterns in bats. Here, we test these previous hypotheses of bat body size evolution using a large, comprehensive supermatrix phylogeny (+800 taxa) to optimize body size and examine changes reconstructed along branches. Our analysis provides evidence of rapid stem phyletic nanism, an ancestral value stabilized at 12 g for crown-clade Chiroptera followed by backbone stasis, low-magnitude changes inside established families, and massive body size increase at accelerated rate in pteropodid subclades. Total variation amount explained by pteropodid subclades was 86.3%, with most changes reconstructed as phyletic increases but also apomorphic decreases. We evaluate these macroevolutionary patterns in light of the constraints hypothesis, and in terms of both neutral and adaptive evolutionary models. The reconstructed macroevolution of bat body size led us to propose that echolocation and flight work as successive, nested constraints limiting bat evolution along the body size scale.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Body mass is the single most important variable affecting the biology of animals, especially mammals (see McNab 2007 and citations therein). Mass is even more important for one group of mammals, the bats (Chiroptera), for two major reasons: flight and echolocation. Bats are the only mammals capable of powered flight. Flight is presumed to have had an immediate impact on the great evolutionary success of bats: by the early Eocene, shortly after their origination, bats were the first mammalian group to reach a nearly cosmopolitan distribution, a fact documented in the fossil record of all continents except Antarctica (Gunnell and Simmons 2005; Eiting and Gunnell 2009; Smith et al. 2012). A spectacular diversification ensued: with > > 1200 bat species in 21 currently recognized families, chiropteran diversity is only second to rodents among extant mammals (Simmons 2005). Bats are undisputedly monophyletic, as indicated by all recent phylogenies (van den Bussche and Hoofer 2004; Eick et al. 2005; Teeling et al. 2005; Meredith et al. 2011; O'Leary et al. 2013; Amador et al. 2018). Key Eocene fossils appeared as successive sister taxa to a crown group that comprised all extant families (Simmons et al. 2008), which in turn split into two major clades, the Yinpterochiroptera, or Pteropodiformes (including fruitbats and five families of echolocating bats), and the Yangochiroptera, or Vespertilioniformes (including the remainder of echolocating bat families; see also Teeling et al. 2005). “Microbats” or echolocating bats are thus paraphyletic, so echolocation either was lost in fruitbats, or convergently evolved twice, once in each “microbat” lineage (Teeling et al. 2012). Early Eocene fossils either show clear morphological signatures of echolocation (e.g., Novacek 1987), or lack them completely, as in Onychonycteris finneyi, the most functionally primitive bat known (Simmons et al. 2008, 2010; cf. Veselka et al. 2010). Although not universally accepted (cf. Fenton and Ratcliffe 2004; Carter and Adams 2015), the lack of echolocation in Onychonycteris and the nested position of pteropodids among echolocating bats imply that flight evolved first, and echolocation soon after in more derived bats, to be secondarily lost in fruitbats (Simmons et al. 2008, 2010; cf. Veselka et al. 2010). New developmental (Wang et al. 2017) and sensory organ (Thiagavel et al. 2018) evidence supports this latter view.
Powered flight is the most strenuous mode of locomotion (Rayner 1986; Norberg and Rayner 1987; Biewener 2011). Carrying the least weight up in the air is thus naturally favored, so all flying animals, including bats, are relatively small (Norberg 1990). All bats figure in the lowest quartile of the mammalian body mass distribution (Safi et al. 2013). The distribution of body mass in bats themselves (Fig. 1) shows that half of bat species weigh 14 g or less (median value), and most (some 70% of species), weigh <30 g (see Giannini et al. 2012). A minority of bat species, most of them in the megabat family Pteropodidae, exceeds 100 g, and a couple of species exceptionally reach 1600 g: the flying foxes Pteropus vampyrus and Acerodon jubatus in the Pteropodidae (Kunz and Pierson 1994). The largest flying foxes have gone a long way in the path of size evolution: not only are they many times larger than a hypothetical median bat, they also may double the size of similarly frugivorous flying animals (e.g., the largest toucans; Aves: Ramphastidae; Norberg and Norberg 2012). Some key aerodynamic variables are typically affected by body mass (Norberg 1994) and may constitute a strong constraint to body size evolution in all vertebrates, including bats (see Smith et al. 2013).
Distribution of body mass in bats based on records for 751 extant species from several specific sources (see Methods Section and SI.1). Mode and median values are indicated
The second major factor influencing body size in bats is echolocation―a sophisticated sonar system that enables bats to navigate without visual input (e.g., in complete darkness; Kalko and Schnitzler 1998 and citations therein). Bats emit ultrasonic calls and receive air pressure waves―echoes―from objects impacted by sound; then bats process elapsed time and several information parameters contained in the returning echoes to compose instantaneous acoustic images of the surrounding space as they rapidly fly through it, or as they receive echoes from a stationary position (Kalko and Schnitzler 1998). Several key parameters of the echolocation system are expected to scale either positively or inversely with body size (see review in Jones 1999), and these scaling relationships have been suggested to constrain body size in bats (Barclay and Brigham 1991; Jones 1999).
In addition, flight and echolocation interact in ways that are relevant for the evolution of size in bats, and vice versa. For instance, synchronization of echolocation call emission and wingbeat (specifically, the downstroke) enables bats to echolocate at search-phase frequency with no additional cost while flying (e.g., Speakman and Racey 1991; Jones 1999; Parsons et al. 2010). This coupling likely represents an important selective pressure on size to keep both flight and echolocation costs as low as possible (Speakman and Racey 1991).
Clearly, body mass has the potential to affect key evolutionary variation in the bat lineage via various functional and scaling effects. Safi et al. (2013) demonstrated that most interspecific body mass variation is phylogenetically structured, although little evidence exists that body mass per se exerted any influence in the rapid diversification of bats (Isaac et al. 2005). Hutcheon and Garland (2004) estimated ancestral body mass of the crown group of extant bats between 20 and 23 g, but with exceedingly large confidence intervals (between 9 and 51 g); similarly, Safi et al. (2005) obtained a close point estimate at 19 g. More recently, Giannini et al. (2012), using optimization of continuous characters, reported a narrower and lower-valued interval (10–14 g) that included their observed median for extant bats (at ca. 14 g), and Thiagavel et al. (2018) reported 20 g. Despite methodological differences, these studies have all converged on a small value for the ancestral node of extant bat groups, with point estimates bracketed between 10 and 23 g. Examination of changes in all the family-level nodes of the bat phylogeny in analyses including key early Eocene fossil bats (Giannini et al. 2012) revealed potentially highly relevant patterns of body size change, including: initial rapid phyletic nanism, i.e., continued size reduction along successive branches of a phylogeny, sensu Gould and MacFadden (2004); stasis around the global median value along the backbone of the crown chiropteran group; evolutionary change (increases or decreases) inside major clades (established families); more net size increases than decreases; and phyletic giantism sensu Gould and MacFadden (2004), i.e., continued size increase along successive branches of a phylogeny, in several families, particularly in Old World fruit bats (Pteropodidae).
These patterns were consistent with the hypothesis of an initial evolutionary adjustment lowering the range of body mass values to first refine flight (nanism phase), next a stabilization of body mass change to establish functional echolocation parameters in all “modern” bat groups of aerial hawking bats (backbone stasis phase), and escape from stasis whenever echolocation constraints were released (e.g., giantism in Old World fruit bats; Giannini et al. 2012). This particular scenario depended on a critically small sample of just 44 extant terminals (tree from Teeling et al. 2005 and Simmons et al. 2008). In addition, release from constraint, whatever its nature, was not supported as a general explanatory mechanism driving body size evolution in mammals as a group (Baker et al. 2015), and so such finding would make of Chiroptera a notable exception among mammals.
Here, we test the hypotheses of body size evolution in bats advanced by Giannini et al. (2012) using our large-scale supermatrix phylogeny (Amador et al. 2018). This phylogeny resulted from unconstrained searches of a nine-genes supermatrix, with ca. 800 bat terminals at the species level with curated and updated taxonomy, no chimeras, and a varied placental outgroup set (see details in Methods). We intensely searched body size data from the literature and optimized the data as a continuous character on our phylogeny (see Methods). Next, we examined changes reconstructed along branches of the large bat tree, quantifying changes as net increases or decreases, phyletic (along successive branches) or apomorphic (at single branches) following Gould and MacFadden (2004), in order to characterize the nature of body size changes in the entire bat lineage. We fit models of phenotypic evolution and detected shifts in the rate of body size change in Chiroptera and in functionally significant bat subgroups. In light of these results, we evaluate hypotheses of body size evolution in bats that may result from the combined constraints of flight and echolocation (Giannini et al. 2012). In addition, we evaluated in bats hypotheses of body size trends discovered in mammals, both in terms of neutral (e.g., Bokma et al. 2016) and adaptive (e.g., Baker et al. 2015) models of character change. Our detailed analysis questions current explanations of body size evolution in mammals as extended to bats, and singles out bats as a group experiencing unique evolutionary pressures. We propose a new hypothesis of bat body size evolution based on nested constraints from echolocation and flight.
Materials and Methods
Body Mass Data Compilation
We obtained body size data, in grams, for 751 of 799 bat species plus a few distinct subspecies (making up 804 bat terminals) represented in our phylogenetic hypothesis, from specific sources (Schober and Grimmberger 1997; Bonaccorso 1998; Churchill 1998; Smith et al. 2003; Gannon et al. 2005; Goodman et al. 2006; Aguirre 2007; Decher and Fahr 2007; dos Reis et al. 2007; Garbutt 2007; Pacheco et al. 2007; Zhang et al. 2007; Magalhaēs and Costa 2009; Lim et al. 2010; Harvey et al. 2011; Mendes 2011; Threlfall et al. 2011; Hasan et al. 2012; Patterson and Webala 2012; Thong et al. 2012; Kruskop 2013; Monadjem et al. 2013; Dammhahn and Goodman 2014; Huang et al. 2014; Kawai et al. 2014; Lu et al. 2014; Sedlock et al. 2014; Table SI.1). For each species, we averaged the values from all available sources, excluding some data judged unreliable (e.g., extreme values, juveniles, etc.). In addition, for species lacking data from literature sources we searched in one museum database (National Museum of Natural History, NMNH) and, when available, averaged weight values of ten specimens (males and females; Table SI.2). For extinct bat species, we used body mass values provided by Giannini et al. (2012) from reverse estimate of mass from humerus least mid-shaft diameter using ordinary least squares regression fit with extant species.
Phylogenetic Framework
We used a pruned version of the phylogenetic hypothesis proposed by Amador et al. (2018) with, 751 out of 799 species, as a cladistic framework to search for body size evolutionary patterns (see below). This phylogeny was based on a comprehensive data matrix with updated taxonomy and no chimeric terminals, 11,489 aligned bp from nine nuclear and mitochondrial genes, and intense, unconstrained analyses including parsimony, maximum likelihood, and Bayesian inference for dating the phylogeny; the last analysis used 44 calibration points from undisputed fossil bats. The 21 currently recognized bat families were represented in the matrix, with ca. 90 and 64.4% of extant diversity at the genus and species level, respectively; i.e., 198 genera and 799 currently recognized species. The point estimate of age for crown clade Chiroptera in this phylogeny was 62.6 mya.
Also, we composed a tree adding five key Eocene bat species: Onychonycteris finneyi (Onychonycteridae), Icaronycteris index (Icaronycteridae), Archaeonycteris trigonodon (Archaeonycteridae), Hassianycteris messelensis (Hassianycteridae), and Paleochiropteryx tupaiodon (Palaeochiropterygidae), following the topology proposed by Simmons et al. (2008), with O. finneyi as the most basal taxon, and the other Eocene terminals as successive stem branches sister of the crown clade of extant terminals. This wide taxonomic sampling allowed us to test different aspects of the hypothesis of physical constraints proposed by Giannini et al. (2012) for explaining the evolution of body mass in Chiroptera (see above).
Evolution of Body Mass
We approached the macroevolutionary analysis of body mass in Chiroptera using two contrasting methodologies. First, we examined body size data applying a local, node-by-node descriptive approach that allowed an appropriate identification of evolutionary changes in the framework proposed by Gould and MacFadden (2004). Body size data were arranged in TNT data matrix format and mapped onto the phylogeny of Amador et al. (2018) as a continuous character (see Goloboff et al. 2006) with a single value (the species average) per terminal, as implemented in the phylogenetic program TNT (Goloboff et al. 2008), freely available at www.lillo.org.ar/phylogeny/tnt/. Following Giannini et al. (2012) and Amador and Giannini (2016), we chose to interpret nodal location, sign (increase or decrease), and magnitude of change, with the last being the net change or the amount of body mass increase or decrease common to all reconstructions for a given branch. We quantified frequency and magnitude of net increases and decreases along the complete phylogeny, and within the most specious families. We looked for macroevolutionary patterns in body size variation, in terms of phyletic or apomorphic changes, giantism or nanism, and their corresponding combinations (see Gould and MacFadden 2004).
To specifically test, by means of a comprehensive taxonomic sampling, the validity of patterns of body mass evolution in Chiroptera recovered by Giannini et al. (2012), we compared our results in the following aspects (Table 1): 1. value of reconstructed body mass for the ancestral node of extant bats; 2. presence of a pattern of rapid phyletic nanism in the early evolution of Chiroptera; 3. presence of stasis along the backbone of extant chiropterans; 4. amount of evolutionary net change (increases or decreases) occurring inside established families, as compared with total change; 5. relative frequency and magnitude of body mass increases and decreases; and 6. occurrence of phyletic giantism in the group that loss echolocation, the Old World fruitbats (Pteropodidae).
We assessed the global phylogenetic effect on body mass in the group, by means of estimating the fraction of total variance in body size explained by phylogeny. Previous estimates indicated ca. 39% variation explained by phylogeny (Safi et al. 2013) based on a supertree topology (from Bininda-Emonds et al. 2007). To re-evaluate this assessment, we choose a topological method, Canonical Phylogenetic Ordination (hereafter CPO; Giannini 2003). CPO considers the comparative data as dependent variable(s), here the vector of body mass data for each chiropteran species (univariate version of CPO). Tree partitions represent the independent variables in the type of analysis applied, Redundancy Analysis (Rao 1964; Ter Braak 1995), which is appropriate for continuously-varying data as body size (see Giannini 2003). CPO treats the tree as a network whose partitions are equivalent to clades of a rooted tree. Tree partitions are binary coded to represent membership of terminals (0 applied to terminals belonging to one side of the tree partition, 1 applied to the other side), and each constitutes an explanatory variable that is tested by means of an F-value using unrestricted Monte Carlo permutations (here 4999 permutations were used). These tests of individual tree partitions are followed by a forward stepwise selection procedure that seeks to produce a maximally explanatory, but non-redundant model that is a linear combination of selected partitions (clades). As a result, CPO allows the identification of major tree partitions that best explain the main patterns in the data, and provides a non-redundant estimate of total variation explained by those tree partitions. We kept groups in the model using two criteria. First, we included all the groups that individually explained 5% or more of total variation. This is a conservative approach, so we also fit the model successively adding groups that remained significant at a predefined alfa = 0.01 (see Giannini 2003). These analyses were executed using the program CANOCO version 4.0 (Ter Braak and Smilauer 1998).
We tested predictions from mammalian neutral models of body size evolution on the bat lineage. According to Bokma et al. (2016), mammalian lineages are expected to increase size on average by ca. 4.1% every 5 my with respect to the size of a given ancestor. We applied this prediction to Chiroptera and compared it to our observed values. We calculated the expected mean of bats as a group, pteropodids only, echolocating bats only, and the two clades of echolocating bats separately, i.e., Yinochiroptera (composed of families Rhinopomatidae, Megadermatidae, Craseonycteridae, Rhinolophidae, Hipposideridae and Rhinonycteridae) and Yangochiroptera (composed of Emballonuridae, Nycteridae, Myzopodidae, Mystacinidae, Thyropteridae, Furipteridae, Noctilionidae, Mormoopidae, Phyllostomidae, Natalidae, Molossidae, Miniopteridae, Cistugidae, and Vespertilionidae) sensu Amador et al. (2018). We used point estimates of crown age of each of these clades from our supermatrix phylogeny (Amador et al. 2018) and size of each ancestor from this study as departing point to apply the expected increase predicted in Bokma et al. (2016).
We used the package OUwie for R (Beaulieu et al. 2012) to determine the model of phenotypic evolution that best describes the evolution of weight in pteropodid and non-pteropodid bats. OUwie implements the Hansen model, in which a quantitative character evolves according to an Orstein-Uhlenbeck (OU) process. The model describes the change of a character through time, depending on the character evolutionary optimum (parameter θ), the strenght of selection towards this optimum (parameter α), and a variable that denotes the rate of stochastic evolution (parameter σ2). In the absence of selection (α = 0), the model resembles the Brownian motion. OUwie allows the fit of different models to a phylogeny with two predetermined regimes (or set of clades). These models differ on how the parameter values (α, θ, and σ2) vary between those two regimes. We then applied a method that identifies shifts in the evolution of a trait without the need of predetermined regimes (e.g. echolocating, non-echolocating bats). The methods implemented in the package bayou for R (Uyeda et al. 2014) identify possible shifts in phenotypic (here weight) optimum along a phylogeny using Bayesian methods (Uyeda and Harmon 2014). A MCMC chain using default, relaxed priors with 6 million generations was conducted. Parameters were sampled every 100 generations and results were summarized after discarding the first 24,000 samples (40%). A second run with the same characteristics was done to check for convergence, which was done using the Gelman and Rubin’s R statistics and plots of branch posterior probabilities.
Results and Discussion
Our analysis, based on optimization of body size as a continuous character on our large supermatrix phylogeny (from Amador et al. 2018), and models of phenotypic evolution, provides strong evidence of rapid stem phyletic nanism, followed by stasis along the backbone of the crown chiropteran subtree, considerable amount of low-magnitude change in many bat groups, and massive body size increase at accelerated pace in a few, but chiefly in one, group of bats, the Pteropodidae (see below). These results differ with respect to predictions of body size evolution in mammals as a group, either based on neutral or adaptive models, and they agree with aspects of previous hypotheses of bat body size evolution. These aspects can be linked directly with functional demands of flight, echolocation, and their interaction. In the following we dissect these results to discuss in depth bat body size evolution.
Magnitude, Location and Pattern of Bat Body Size Evolution
Optimization of body size as a continuous character data in our phylogeny of Chiroptera yielded 9.314 kg of total net changes, of which almost three quarters (72.6%) were increases (Fig. 2). In terms of frequency, stasis (no change) was dominant with 41% of nodes, while net increases and net decreases had each 16% share of total nodes; ambiguity (nodes with assignments that depend on particular reconstructions) represented 27% (Fig. 2). Distribution of changes as mapped on our tree is shown in Fig. 3 for the backbone topology of Chiroptera. The root state was assigned the interval of 30–40 g and in subsequent branches was assigned 30 g. Stem bats included in our study (five genera in five families, all extinct) varied greatly in size, from average 12 to 90 g, as estimated from humerus least shaft diameter (Giannini et al. 2012). However, as optimized in the phylogeny, a pattern of phyletic nanism (see below) was apparent along stem branches, given that size decreased from ca. 40 to 12 g (Fig. 3). Two strongly diverging changes were optimized in branches involving stem bats (Eocene fossils): the branch leading to the large Hassianycteris messelensis (+60 g), and its sister branch leading to Palaeochiropteryx tupaiodon + all extant bats (−18 g). This steep descent established a root body size (ancestral value) of 12 g for crown-clade Chiroptera (see below). This value is only 30% of the value estimated in the oldest fossils considered here (specimens of Onychonycteris finneyi, sister to all bats), and it remained stable along the entire chiropteran backbone were no net changes were reconstructed (bold branches in Fig. 3). Remarkably, these changes, and the changes toward the stem terminals, occurred from around the K–Pg boundary, through the Paleocene, to the early Eocene, given that the fossils were dated 52–49 my (see Gunnell and Simmons 2005, and citations therein) and the stem and crown age of Chiroptera were ca. 67 and 62 mya, respectively (Amador et al. 2018).
Results for body mass optimization of 751 bat species in the phylogeny of Amador et al. (2018). Relative values (%) for frequency of changes and total amount of net increases and decreases for the magnitude of change
Optimization of body mass in the backbone of bats phylogenetic tree of Amador et al. (2018), including basal extinct taxa reconstructed following Simmons et al. (2008). Black arrows denote increases and gray ones, decreases. Body mass values (in grams) indicated in brackets refers to the estimated body mass value for fossils and for the ancestral of each extant family. Ancestral reconstructed values are indicated below branches. Bold line denotes backbone stasis (i.e., no net changes)
The reconstructed size of crown-clade Chiroptera and its median value, estimated from >750 terminals, were the same at 12 g (cf. Figs. 1 and 3). This suggests highly conserved body size evolution, which is reflected in a pattern of virtual backbone stasis that accumulated <2% of total size changes in Chiroptera (see below; Fig. 3), and in the very close optimum size fitted for echolocating bats from an Ornstein-Uhlenbeck model with varying parameters (see below). Then, net changes were reconstructed independently in nodes representing the base of eight families or superfamilies, most notably in Pteropodidae, which doubled the size of the reconstructed backbone value, and continued evolving size to accumulate most of the >9 kg total change reconstructed (see below). These results match, both in pattern and magnitude, previous estimates by Giannini et al. (2012), who used the same fossil estimates, but with an extant sample limited to only 44 terminals (versus 751 terminals from Amador et al. (2018) in the present study).
Patterns of evolutionary change in extant bats can be reliably estimated with a representatively large sample of species-level terminals. Here we focus our interpretation on the effect of our large sample on the major patterns of body size evolution in bats as a group (specific patterns inside families will be dealt with elsewhere). Shifts in the interval range were mapped to the Neotropical noctilionoid branch, with intervals successively changing from 12 g (backbone) to 11–12 g and 3–12 g in noctilionoids. Net changes included three increases in the nodes corresponding to the families Pteropodidae (+16 g), Megadermatidae (+13 g), and Noctilionidae (+18 g). Five net decreases were reconstructed in the nodes corresponding to Craseonycteridae (−10 g), rhinolophoids sensu stricto (Rhynonycteridae + Hipposideridae + Rhinolophidae, −2 g), Myzopodidae (−2 g), Natalidae (−7 g), and vespertilionids sensu lato (Cistugidae + Vespertilionidae, −4 g). Total change in the backbone chiropteran tree (as seen in Fig. 3) was only 150 g. This low level of body size change is especially relevant because it is along this backbone that most of the bat divergence occurred, as all recognized bat families originated from these branches. Therefore, the majority of changes were reconstructed inside families, as summarized in Table 2. In fact, 73.4% of total magnitude of net change, or ca. 6.8 kg, was concentrated in one family, the Pteropodidae. Remarkably, only a comparatively modest increase of +16 g was reconstructed in the root of the pteropodid subtree; still, basal pteropodids were at least twice as big as the backbone ancestral bat species (28–50 g vs. 12 g; Fig. 3 and Fig. 7 from supplementary information). The greatest fraction of change occurred in more derived branches of pteropodids, principally in those leading to large or very large flying foxes (see below). Giannini et al. (2012) reconstructed phyletic increases in the pteropodid clade. In our study, pteropodids accumulated ca. 4.8 kg of increases, but also decreases were important with ca. 2.1 kg or ca. 30% of all net changes in the family (Table 2); this latter aspect is missing in Giannini et al. (2012) given the small sample of extant pteropodids included. Other aspects also reveal the peculiar evolution of size in pteropodids; the ratio of the magnitude of change to the frequency of change (which measures the average amount of change allocated to one evolutionary event), and to the number of nodes in the clade (expected amount of change per node), is one or two orders of magnitude larger in pteropodid bats than in any other bat family, both considering increases and decreases, albeit more so in the case of increases (Table 2). The “microbat” family Phyllostomidae occupied a distant second place with 708 g of total magnitude of net change (Table 2). Analysis of the nine more speciose bat families indicates patterns of increases, decreases, stasis, and ambiguity that are generally similar to that of Chiroptera as a group, with few exceptions like Miniopteridae, in which the frequency of decreases outnumbered increases, and Phyllostomidae (Table 2).
The dominant patterns of change in Chiroptera as a group were phyletic increases and apomorphic decreases (Table 3). Average phyletic vs. apomorphic total changes across families were nearly balanced (51% of phyletic changes; Table 3). This lineage-level pattern is replicated inside the five more speciose families (Vespertilionidae, Pteropodidae, Phyllostomidae, Hipposideridae, and Rhinolophidae). In Phyllostomidae there were three times more phyletic increases than decreases, and two families (vespertilionids and rhinolophids; Table 3) had 1.6–1.7 times as many increases as decreases. In Pteropodidae the pattern of apomorphic nanism dominated, in spite of the sheer magnitude of phyletic increases detected in the family (see above). In the next four diverse families (Molossidae, Miniopteridae, Emballonuridae, and Mormoopidae), all four types of change (the combination of phyletic and apomorphic increases and decreases) were nearly balanced (Table 3).
Predictions from Giannini et al. (2012) are shown in the comparative Table 1, in the context of a wider comparison between that study and this one. Methodologically, the studies included the same five fossil taxa, and differed in the number of non-bat outgroup terminals (5 vs. 20) and more crucially in the number of ingroup (bat) terminals (38 vs. 804, 751 terminals used here; Table 1). In terms of results, our study confirmed the basic trends reconstructed in Giannini et al. (2012) of initial phyletic nanism, backbone stasis, considerable amount of low-scale size evolution inside families, and phyletic giantism chiefly in pteropodids (Table 3). As expected, the total amount of size change was many times greater in our study due to the greater taxonomic sample, but this translated into a different average change, estimated as net change per terminal; in our study, this proportion was less than half the estimate in Giannini et al. (2012), indicating a dilution of perceived change in a larger sample. Put in another way, small taxonomic samples may distort this value, so comprehensive samples of the known diversity, such as the one included in the phylogeny of Amador et al. (2018), are expected to approximate this estimate of change in a more reliable fashion. Raw decrease-to-increase ratio is also different between studies, with balanced (ratio ≈ 1) estimate in our study as compared with a predominance of increases (and hence, a < 1 ratio) in Giannini et al. (2012).
In Pteropodidae in particular, the fact that the most common pattern of body size change was apomorphic decrease contrasts sharply with previous results in which only phyletic giantism was reconstructed in the family (see Giannini et al. 2012). This pattern indicates that evolution of size was very active in the group, with sharp increases in magnitude toward the largest pteropodids, but in fact most commonly changing toward decreasing relative large size in other pteropodids, likely in response to local demands or as a result of opportunistic evolutionary change (e.g., Baker et al. 2015).
Phylogenetic Effects and the Case of Pteropodidae
Results from Canonical Phylogenetic Ordination (CPO) are summarized in Table 4. Forward stepwise selection retained 20 tree partitions, all but one belonging in the single family Pteropodidae. Amount of total variation explained by these tree partitions was 86%. Deletion of pteropodid terminals did not improve fit of the remaining “microbat” groups (not shown). If we allow inclusion of partitions that explain no less than 5% of total variation, the analysis would retain only the first two groups, “pteropines” (i.e., Pteropus + Acerodon) and pteropodids except cynopterines and rousettines (i.e., the group inclusive of Macroglossinae, Harpyionycterinae, Eidolinae, Nyctimeninae, and Pterodinae). This most conservative approach demonstrates that most variation (now 67%) still is within a few major pteropodid groups.
Safi et al. (2013) determined that bats, like most mammals, have a high degree of phylogenetic signal in body size, and suggested that body size evolution is mainly phylogenetically constrained. We concur in that a strong phylogenetic signal is indeed present, but in quantitative terms, the bulk of phylogenetically structured size variation is concentrated inside just one group, the pteropodids. This perceived pattern is the result of applying CPO (but see also Thiagavel et al. 2018), which unlike other methods, dissects evolutionary change across tree partitions (i.e., monophyletic groups in rooted trees; Giannini 2003). As a consequence, patterns that corresponded with clades (e.g., clade members on average larger or smaller than the pool of all other terminals) may explain a fraction of total variation in a linear model, provided that simulations reject randomness in the data (Giannini 2003). This allows finding those clades, and calculating the amount of variation explained by such partition; because tree partitions are considered natural structures of a given lineage, it is relevant to detect variation associated with them (Giannini 2003). Here almost all significant variation was associated with subclades of megabats (Table 4). Hutcheon and Garland (2004) posed the relevant question, are megabats big? The bimodal distribution of mass in bats (Fig. 1) is explained by some species of fruitbats being generally much larger than most “microbats” (or echolocating bats). Hutcheon and Garland (2004) concluded that once phylogeny was considered, in consort with certain branch length values and neutral evolutionary models, the “signal” disappeared and Old World fruit bats were no longer distinct with respect to “microbats” (Hutcheon and Garland 2004). CPO (but see below) suggested that not “megabats” as a group, but instead particular groups inside “megabats,” explained the most conspicuous portion of chiropteran variation in size.
Predictions from Neutral Models of Size Evolution in Mammals
Predictions of body mass evolution according to a general neutral trend toward body size increase across mammalian lineages (Depéret’s, or Cope’s, rule, modeled in Bokma et al. 2016) are shown in Table 5. Based on the general average rate in Bokma et al. (2016) of 4.1% increase in size per million year with respect to a given ancestor, bats as a group are predicted to achieve a mean body size of 60.9 g, which is 35% larger than the actual estimate of extant bats at ca. 45 g (Table 5). A finer analysis per bat group, which was mandatory given the functional differences that distinguish these groups, revealed far greater discrepancies between predicted and observed values. Observed mean in pteropodid bats is ca. 213 g; predicted values range (depending on ancestral body size reconstruction) are far below, between 60 and 123 g (Table 5). Echolocating bats show the reverse pattern. Body size average of all echolocating bats (a paraphyletic array) is 15.6 g; predicted value is as for all Chiroptera (same age and ancestral size) at 60.9 g. Splitting echolocating bats into its two natural constituent groups to avoid paraphyly yielded similar results: expected vs. observed values for Yinochiroptera and Yangochiroptera were 42.4 vs. 16.8 g and 49.3 vs. 14.9 g, respectively (Table 5). In other words, the mean body size of bats as a group is close (just 15 g above) from the predicted mean using the general rate of change for mammals, but decomposing bats into meaningful functional groups (non-echolocating vs. echolocating bats, and the major groups therein) yielded mean size estimates that were too far from the expected means calculated using the general model (90–150 g below in megabats, 25–45 g above in “microbats”).
Another model from Venditti et al. (2011) predicts a mean instantaneous increase of 1.02 g per million year, with considerable (up to 52-fold) variation in rate across mammalian groups. In this model, the two more speciose mammalian orders, rodents and bats, exhibited no history of sustained body size increase. However, dinomyid rodents are known to have reached 700–1000 kg in body size (Sánchez-Villagra et al. 2003; Rinderknecht and Blanco 2008), and we estimate that the largest megabats are evolving size at a (linear) rate of 101 g per million year (notice that 101 g is greater than the expected final size of bats evolving at the average 1.02 g per million rate from a starting point at 12 g, but during ca. 62 my!). As with the previous model (from Bokma et al. 2016) there seems to be a critical lack of fit to the variation we reconstruct in bats.
Adaptive Models and Constraints to Body Size Evolution in Bats
Neutral models appear to fit poorly the reconstructed evolution of bat body size (see above). Baker et al. (2015) have suggested an adaptive basis for mammalian body size evolution. These authors found that bats were no exception to a general trend to increase size over time detected across mammals as a group, as well as within groups. Interestingly, they also detect “rapid evolutionary change toward body size decrease in the branch leading to extant bats”, which corresponds nicely with the phase of phyletic nanism that we reconstruct with early Eocene fossils, “although subsequent evolution within this group returned to a general pattern of body size increase” (Baker et al. 2015:5096), which is not obvious in our study (see above and Table 3). Baker et al. (2015) proposed adaptive explanations to these trends, which confirmed Depéret’s (= Cope’s) rule in mammals and major subclades. However, they considered unlikely that either the overall mammalian trend, or the group-specific trends that they detected, were due to the relaxation of functional unspecified constraints that allowed size to increase with respect to the ancestral condition, and a phylogenetic test failed to recognize any general pattern of evolutionary body size release across mammals. If this is generally true for mammals, then bats represent a macroevolutionary exception. We show next that bat body size evolved around conserved optima and that the “unspecified constraints” can be materialized in the abundant functional information that is available for bats.
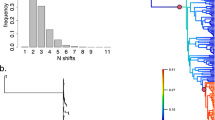
The OU model with different optima, drift, and selection parameters for each regime (OUMVA) had a significantly better fit to the evolution of weight across the bat phylogeny as compared with other fitted models (Table 6). The estimated parameters of the OUMVA model are shown in Table 7. All parameters had higher estimates for pteropodids as compared to those of the other bats. Both higher θ and σ2 favor phenotypic disparity. Remarkably, the parameter σ2 that describes the rate of stochastic evolution of the trait is 100 times higher in Pteropodidae, reflecting the much higher variance of weight in this family (Fig. 4). The Bayesian runs reached convergence (supplementary information) and ESS >100 for all parameters except for root (ESS = 70). The method identified 16 branches with a posterior probability >0.5 of having suffered shifts in the weight optima, most of which are the same as those detected by CPO (see above). All of them were located in the Pteropodidae lineage (Fig. 5 and Fig. 8 from supplementary information).
Detail of the “simmap” figure from the bayou analysis showing, in gray circles, the shift in parameters related to weight evolution according the OU process. All shifts are concentrated in the pteropodids branch (for details see Fig. 8 of supplementary information)
Besides the possible phylogenetic constraint to size variation discovered by previous authors (particularly Safi et al. 2013), bats face potentially severe physical limitations on size evolution from flight, echolocation, and their combination. Considering flight first, it is regarded as the most physiologically strenuous mode of locomotion in vertebrates, so small size should be strongly favored (Rayner 1986; Norberg and Rayner 1987; Biewener 2011). In addition, several flight parameters have a direct dependence on body size. Flight speed (ms−1) scales positively with body mass (Norberg 1994), which in turn depends on wing loading (Pa), so large bats need to fly faster to produce enough lift (Bullen and McKenzie 2002) and tend to have large home ranges and to commute long distances (Norberg 1990). By contrast, wingbeat frequency (Hz) scales inversely with body mass (Bullen and McKenzie 2002), as expected for frequencies in general (see Calder 1996). This relationship determines several aspects of bat flight; for instance, only small bats can afford the high wingbeat frequency required for hovering flight, which is required for certain life styles such as nectarivory (Rayner 1986; Norberg 1994).
Echolocation also imposes hard limitations on size derived from scaling relationships, especially frequencies. Call frequencies such as peak frequency (kHz), frequency of various harmonics (kHz), and pulse repetition rate (Hz) are expected to scale inversely with body mass (see above), which has been corroborated in some studies (e.g., Jones 1999). One significant consequence of this inverse scaling is that call parameters may constraint size evolution in echolocating bats: as bats evolve larger, their call frequencies drop (Jones 1999). Thus, for scaling reasons, larger bats fly faster at lower wingbeat frequencies while emitting low frequency calls at low repetition rates, a combination that compromises their ability to detect and track small and fast-moving, airborne prey (insects). In addition, in order to avoid call-echo overlap, most bats should hear the upcoming echo before emitting their next call, which further limits pulse repetition rate (Fenton 1990). Finally, pulse duration (ms) is a key echolocation parameter, one that is expected to scale positively with body mass (Jones 1999), but such relationship has been found only in certain bats that use quasi-constant-frequency calls (Jones 1999).
Most of the previous arguments apply chiefly to echolocating, aerial hawking bats―not to all bats. However, from an evolutionary perspective, this reasoning is especially appropriate given the history of echolocation. Simmons et al. (2008, 2010) proposed that in the bat lineage, flight evolved first and echolocation next (cf. Veselka et al. 2010). The most primitive bat known Onychonycteris finneyi, which we placed as sister of all other bats following Simmons et al. (2008), likely was an accomplished flier but lacked the morphological signature of echolocation (Simmons et al. 2008; cf. Veselka et al. 2010); by contrast, all other Eocene fossils were echolocators, as are all non-pteropodid extant bats. Available phylogenetic evidence suggests that megabats are nested inside echolocating bats (e.g., Simmons et al. 2008), so echolocation must have been lost in this group. Recent ontogenetic (late-fetal arrested cochlear development in megabats; Wang et al. 2017) and sensory organ (Thiagavel et al. 2018) evidence supports echolocation loss in megabats. So for most of the bat lineage history, echolocation has been present and presumably acting as strong constraint on size evolution (optimum from OU model at ca. 17 g) through key inverse scaling relationships associated to frequencies and timings that limit detection of airborne insects in large bats (Barclay and Brigham 1991; Jones 1996, 1999).
Only after echolocation loss, pteropodids evolved size in different directions with great increases occurring in some subclades. This was likely associated with a loss of echolocation capabilities that forced them to change diet; or more likely, a dietary shift from insectivory to frugivory made pteropodids independent of echolocation and its constraints (see also Thiagavel et al. 2018). In other words, megabats may have evolved size in all directions (increases and decreases) in the context of diet-mediated ecological release (Giannini et al. 2012). This hypothesis matches our phylogenetic results, which showed that the bulk of size variation in bats was associated with megabat subclades, particularly those of flying foxes and other large pteropodids (Table 4). The impressive evolutionary explosion of size reconstructed in flying foxes can be appreciated in the abrupt widening of the pteropodid size change envelop (shaded area in Fig. 6) and the steep slope of size change in largest megabats (see Figs. 4 and 6). For instance, the reconstructed change from ancestral Acerodon to its largest descendant A. jubatus represented at least a three-fold increase in some 7 my (the 101 g my−1 rate mentioned above).
Body mass evolution in Pteropodidae. Reconstructed values of body mass for ancestral nodes and for the extreme extant species (the lightest Megaloglosssus azagnyi and heaviest Acerodon juvatus). Values of body mass expected mean (according to neutral model of Bokma et al. 2016) and observed mean, as the theorical upper limit (Norberg and Norberg 2012), are indicated
A New Hypothesis of Nested Constraints
Flight costs limit body size expression in vertebrates in general (Pennycuick 1975; Rayner 1986; Norberg 1994). In addition, in aerial hawking bats, which represent the vast majority of Chiroptera, echolocation has been proposed to impose severe costs from the inverse scaling of frequency parameters (see Jones 1999); these combine with the direct scaling of increasing flight speed (through the wing-loading dependence on body size) required to produce enough lift in larger bats, and the coupling of wingbeat frequency and call emission for energy savings during search-phase echolocation (Jones 1999). As a result, size increase may be severely penalized in most bats (Barclay and Brigham 1994; Jones 1999). Only certain bats are expected to gain any ecological advantage from flight when increasing size: specifically, commuting bats (see above), among which large frugivores figure prominently (Norberg 1994). Echolocating bats rarely exceed 100 g and the largest aerial hawking bat, Cheiromeles torquatus (Molossidae; up to 200 g), exhibits a mixture of unusual echolocation features (including calls at two different frequencies, both higher than predicted by its mass; Heller 1995) and usual ones (including a close-to-expected call repetition rate; see Jones 1999 contra Heller 1995).
From scaling relationships, physiological constraints, and aerodynamics, Norberg and Norberg (2012) modeled the theoretical maximum size in bats using mass-specific muscle power, proportion of body mass allocated to flight muscles, and maximum attainable wing beat frequency, which together configure the maximum power output during flight in any bat. Norberg and Norberg (2012) estimated that the body mass of the largest potential bat would be 1.4–2.3 kg; the authors also give the range of 1.1–2.0 kg based on more conservative assumptions, and so we take this latter range as reference to evaluate bat body size evolution in the light of these theoretical predictions. With individual bats recorded at a maximum of 1.6 kg, it is clear that the largest bats fall within the model’s range of maximum size (Norberg and Norberg 2012), but also that the lower boundary of that range is a sub-estimation of maximum size. If we then take 2 kg as the maximum size attainable for a bat, as in Norberg and Norberg’s (2012) most conservative model, how long does it going to take for a hypothetical bat to actually start experiencing the flight constraints on maximum body size? Applying our (linear) rate of change reconstructed in large pteropodines (101 g my−1), and extending size increase in these bats with the assumption that everything else remains as in Norberg and Norberg’s (2012) model (i.e., no further evolution modifies the physiological conditions used in the model), flying foxes will experience the physical limitations of the upper boundary of body size in about 8 my from now, when these forms would reach the theoretical upper limit of 2 kg. That is, body size evolution in non-echolocating bats presently is not as strongly penalized from flight physiology as to prevent further increases, and thus these bats are expected to continue evolving size for a few million years.
Considering bats altogether, it is clear that body size evolution depends on different aspects of flight, echolocation, and their combination, as factors acting at different levels of the size scale. We propose here that these factors work as nested constraints. Echolocation would impose the strongest physical limitations, keeping most aerial hawking bats small (Barclay and Brigham 1994; Jones 1999). This first level of constraint might have been acting since the very early Eocene when bats first started using echolocation to pursue airborne prey and it might explain part of the size evolution pattern in bats: 1. initial nanism (detected in Giannini et al. 2012; Baker et al. 2015; and in this study), as a phase of adjustment to the evolving echolocating system; 2. the remarkably little variation in size in echolocating bats that is manifested in the backbone stasis; and 3. the little change, over some 60 my, from the reconstructed ancestral size of crown clade Chiroptera (at 12 g) to the average size of living echolocating bats (at 15 g; Table 1; Fig. 1). The last aspect corresponds with the finding of Cooper and Purvis (2010) that the best model of size change in bats is the OU (Ornstein-Uhlenbeck) model; the OU model is selected over other Brownian-like models whenever evolutionary character variation shows a tendency of returning to some optimum―theoretical or empirically determined (Cooper et al. 2016). We found the same result here with the optimum parameter from the OU model at ca. 17 g and we propose that this optimal value of size is determined by echolocation constraints. The optimal value is thus somewhere between the ancestral value (at 12 g) and the estimated optimum (at ca. 17 g), which comprise the mean of extant echolocating bats (at 15 g). As echolocating bats become larger, the strength of the echolocation constraint may increase and eventually limit the aerial hawking behavior to exceptions that depend on at least some energy-expensive especial traits (e.g., in Cheiromeles; see Heller 1995). Loss of echolocation may have opened up the size landscape to pteropodids so these bats accumulated most of the sampled size variation in bats; evidence presented so far indicates that these bats are not favored by size increases unless they can profit from the obligate faster flight that accompanies size increase, as in the case of large commuting frugivores (see Norberg 1994). Thus, factors limiting size evolution in bats can be described as nested constraints from echolocation and flight acting at different levels of the size scale: echolocation at ca. 200 g and flight at ca. 2000 g. For non-echolocating bats, the ultimate constraint in size comes exclusively from flight and meeting the theoretical upper limit of body size appears to be a few million years away at the estimated (linear) rates of evolution in the largest extant species.
References
Almeida FC, Giannini NP, Simmons NB, Helgen KM (2014) Each flying fox on its own branch: A phylogenetic tree for Pteropus and related genera (Chiroptera: Pteropodidae). Molecular Phylogenetics and Evolution 77:83-95
Aguirre LF (2007) Historia Natural, Distribución y Conservación de los Murciélagos de Bolivia. Centro de Ecología y Difusión Simón I. Patiño. Santa Cruz, Bolivia
Amador LI, Giannini NP (2018) Phylogeny and evolution of body mass in didelphid marsupials (Marsupialia: Didelphimorphia: Didelphidae). Org Divers Evol 16(3): 641–657
Amador LI, Moyers Arévalo RL, Almeida FC, Catalano SA, Giannini NP (2018) Bat systematics in the light of unconstrained analyses of a comprehensive molecular supermatrix. J Mammal Evol 25(1): 37–70
Baker J, Meade A, Pagel M, Venditti C (2015) Adaptive evolution toward larger size in mammals. Proc Natl Acad Sci USA 112(16): 5093–5098
Barclay RM, Brigham RM (1991) Prey detection, dietary niche breadth, and body size in bats: why are aerial insectivorous bats so small? Am Nat 137(5): 693–703
Barclay RM, Brigham RM (1994) Constraints on optimal foraging: a field test of prey discrimination by echolocating insectivorous hats. Anim Behav 48(5): 1013–1021
Beaulieu JM, Jhwueng DC, Boettiger C, O’Meara BC (2012) Modeling stabilizing selection: expanding the Ornstein–Uhlenbeck model of adaptive evolution. Evolution 66(8): 2369–2383
Biewener AA (2011) Muscle function in avian flight: achieving power and control. Philos Trans R Soc B: 366(1570): 1496–1506
Bininda-Emonds ORP, Cardillo M, Jones KE, MacPhee RDE, Beck RMB, Grenyer R, Price SA, Vos RA, Gittleman JL, Purvis A (2007) The delayed rise of present-day mammals. Nature 446(7135): 507–512
Bokma F, Godinot M, Maridet O, Ladevèze S, Costeur L, Solé F, Gheerbrant E, Peigné S, Jacques F, Laurin M (2016) Testing for Depéret’s Rule (body size increase) in mammals using combined extinct and extant data. Syst Biol 65: 98–108
Bonaccorso FJ (1998) Bats of Papua New Guinea. Conservation International, Washington, D.C.
Bullen RD, McKenzie NL (2002) Scaling bat wingbeat frequency and amplitude. J Exp Biol 205(17): 2615–2626
Calder WA (1996) Size, Function, and Life History. Dover Publications, New York
Carter RT, Adams RA (2015) Postnatal ontogeny of the cochlea and flight ability in Jamaican fruit bats (Phyllostomidae) with implications for the evolution of echolocation. J Anat 226(4): 301–308
Cooper N, Purvis A (2010) Body size evolution in mammals: complexity in tempo and mode. Am Nat 175(6): 727–738
Cooper N, Thomas GH, Venditti C, Meade A, Freckleton RP (2016) A cautionary note on the use of Ornstein Uhlenbeck models in macroevolutionary studies. Biol J Linn Soc 118: 64–77
Churchill S (1998) Australian Bats. Reed New Holland, Sydney
Dammhahn M, Goodman SM (2014) Trophic niche differentiation and microhabitat utilization revealed by stable isotope analyses in a dry-forest bat assemblage at Ankarana, northern Madagascar. J Trop Ecol 30: 97–109
Decher J, Fahr J (2007) A conservation assessment of bats (Chiroptera) of Draw River, Boi-Tano, and Krokosua Hills forest reserves in the western region of Ghana. Myotis 43: 5–30
dos Reis NR, Peracchi AL, Pedro WA, de Lima IP (eds) (2007) Morcegos do Brasil. Londrina
Eick GN, Jacobs DS, Matthee CA (2005) A nuclear DNA phylogenetic perspective on the evolution of echolocation and historical biogeography of extant bats (Chiroptera). Mol Biol Evol 22(9): 1869–1886
Eiting TP, Gunnell GF (2009) Global completeness of the bat fossil record. J Mammal Evol 16(3): 151–173
Fenton B, Ratcliffe J (2004) Animal behavior: eavesdropping on bats. Nature 429(6992): 612–613
Fenton MB (1990) The foraging behaviour and ecology of animal-eating bats. Canadian J Zool 68(3): 411–422
Gannon MR, Kurta A, Rodríguez-Durán A, Willig MR (2005) Bats of Puerto Rico: An Island Focus and a Caribbean Perspective. Texas Tech University Press, Lubbock
Garbutt N (2007) Mammals of Madagascar: A Complete Guide. Yale University Press, New Haven and London
Giannini NP (2003) Canonical phylogenetic ordination. Syst Biol 52(5): 684–695
Giannini NP, Gunnell GF, Habersetzer J, Simmons NB (2012) Early evolution of body size in bats. In: Gunnell GF, Simmons NB (eds) Evolutionary History of Bats: Fossils, Molecules, and Morphology. Cambridge University Press, Cambridge, pp 530–555
Goloboff PA, Farris JS, Nixon KC (2008) TNT, a free program for phylogenetic analysis. Cladistics 24(5): 774–786
Goloboff PA, Mattoni CI, Quinteros AS (2006) Continuous characters analyzed as such. Cladistics 22: 589–601
Goodman SM, Cardiff SG, Ranivo J, Russell AL, Yoder AD (2006) A new species of Emballonura (Chiroptera: Emballonuridae) from the dry regions of Madagascar. Am Mus Novitates: 1–24
Gould GC, MacFadden BJ (2004) Gigantism, dwarfism, and Cope’s rule: nothing in evolution makes sense without a phylogeny. Bull Am Mus Nat Hist 285: 219–237
Gunnell GF, Simmons NB (2005) Fossil evidence and the origin of bats. J Mammal Evol 12: 209–246
Harvey MJ, Altenbach JS, Best TL (2011) Bats of the United States and Canada. The Hopkins University Press, Baltimore
Hasan NH, Khan FAA, Senawi J, Ketol B, Sait I, Abdullah MT (2012) A report on bats survey at Air Panas-GuaMusang, Kelantan, Malaysia. J Trop Biol Conserv 9(2): 156-162
Heller KG (1995) Echolocation and body size in insectivorous bats: the case of the giant naked bat Cheiromeles torquatus (Molossidae). Le Rhinolophe 11: 27–38
Huang JCC, Jazdzyk EL, Nusalawo M, Maryanto I, Wiantoro S, Kingston T (2014) A recent bat survey reveals Bukit Barisan Selatan Landscape as a chiropteran diversity hotspot in Sumatra. Acta Chiropt 16(2): 413–449
Hutcheon JM, Garland T (2004) Are megabats big? J Mammal Evol 11(3): 257–277
Isaac NJ, Jones KE, Gittleman JL, Purvis A (2005) Correlates of species richness in mammals: body size, life history, and ecology. Am Nat 165(5): 600–607
Jones G (1996) Does echolocation constrain the evolution of body size in bats?. Symp Zool Soc Lond 69: 111–128
Jones G (1999) Scaling of echolocation call parameters in bats. J Exp Biol 202(23): 3359–3367
Kalko EK, Schnitzler HU (1998) How echolocating bats approach and acquire food. In: Kunz TH, Racey PA (eds) Bat Biology and Conservation. Smithsonian Institution Press. Washington, D.C., pp 197–204
Kawai K, Mikhail P, Kondo N, Maksim A, Victor N, Ohtanishi N, Dewa H (2014) Bats from Kunashir and Iturup Island. Bull Hokkaido Univ Mus 4: 74–81
Kruskop SV (2013) New record of poorly known bat Myotis phanluongi (Mammalia, Chiroptera) from southern Vietnam. Russian J Theriol 12: 79–81
Kunz TH, Pierson ED (1994) Bats of the world: an introduction. In: Novak RM, Walker’s Bats of the World. Johns Hopkins University Press, Baltimore, pp 1–46
Lim BK, Engstrom MD, Reid FA, Simmons NB, Voss RS, Fleck DW (2010) A new species of Peropteryx (Chiroptera: Emballonuridae) from western Amazonia with comments on phylogenetic relationships within the genus. Am Mus Novitates 3686: 1–20
Lu D, Zhou CQ, Liao WB (2014) Sexual size dimorphism lacking in small mammals. North-Western J Zool 10(1): 53–59
Magalhaēs JP, Costa J (2009) A database of vertebrate longevity records and their relation to other life-history traits. J Evol Biol 22: 1770–1774
McNab BK (2007) The evolution of energetics in birds and mammals. In: Kelt ED, Lessa EP, Salazar-Bravo J, Patton JL (eds) The Quintessential Naturalist: Honoring the Life and Legacy of Oliver P. Pearson, Univ Calif Publ Zool 134: 67–110
Mendes P (2011) Prioridades globais para a aconservação e características biológicas associadas ao risco de extinção em morcegos (Chiroptera: Mammalia). Instituto de Ciências Biológicas. Programa de Pós-graduação em Ecología e Evolução. Universidade Federal de Goiás. Goiânia
Meredith RW, Janečka JE, Gatesy J, Ryder OA, Fisher CA, Teeling EC, Goodbla A, Eizirik E, Simão TLL, Stadler T, Rabosky DL, Honeycutt RL, Flynn JJ, Ingram CM, Steiner C, Williams TL, Robinson TJ, Burk-Herrick A, Westerman M, Ayoub NA, Springer MS, Murphy WJ (2011) Impacts of the Cretaceous terrestrial revolution and KPg extinction on mammal diversification. Science 334(6055): 521–524
Monadjem A, Richards L, Taylor PJ, Stoffberg S (2013) High diversity of pipistrelloid bats (Vespertilionidae: Hypsugo, Neoromicia and Pipistrellus) in a West African rainforest with the description of a new species. Zool J Linn Soc 167: 191–207
Norberg UM (1990) Vertebrate Flight. Springer, Berlin Heidelberg New York
Norberg UM (1994) Wing design, flight performance, and habitat use in bats. In: Wainwright PC, Reilly SM (eds) Ecological Morphology: Integrative Organismal Biology. University of Chicago Press, Chicago, pp 205–239
Norberg UM, Rayner JMV (1987) Ecological morphology and flight in bats (Mammalia: Chiroptera): wing adaptations, flight performance, foraging strategy and echolocation. Philos Trans R Soc Lond 316: 337–419
Norberg UML, Norberg RA (2012) Scaling of wingbeat frequency with body mass in bats and limits to maximum bat size. J Exp Biol 215(5): 711–722
Novacek MJ (1987) Auditory features and affinities of the Eocene bats Icaronycteris and Palaeochiropteryx (Microchiroptera, incertae sedis). Am Mus Novitates 2877: 1–18
O'Leary MA, Bloch JI, Flynn JJ, Gaudin TJ, Giallombardo A, Giannini NP, Goldberg SL, Kraatz BP, Luo ZX, Meng J, Ni X, Novacek MJ, Perini FA, Randall ZS, Rougier GW, Sargis EJ, Silcox MT, Simmons NB, Spaulding M, Velazco PM, Weksler M, Wible JR, Cirranello AL (2013) The placental mammal ancestor and the post–K-Pg radiation of placentals. Science 339(6120): 662–667
Pacheco V, Cadenillas R, Velazco S, Salas E, Fajardo U (2007) Noteworthy bat records from the Pacific Tropical rainforest region and adjacent dry forest in northwestern Peru. Acta Chiropt 9(2): 409–422
Parsons S, Riskin DK, Hermanson JW (2010) Echolocation call production during aerial and terrestrial locomotion by New Zealand's enigmatic lesser short-tailed bat, Mystacina tuberculata. J Exp Biol 213(4): 551–557
Patterson BD, Webala PW (2012) Keys to the Bats (Mammalia: Chiroptera) of East Africa. Fieldiana Life and Earth Sci 6: 1–60
Pennycuick CJ (1975) Mechanics of flight. In: Farner DS, King JR (eds) Avian Biology. Academic Press, London, New York, San Francisco, pp 1–75
Rao CR (1964) The use and interpretation of principal component analysis in applied research. Sankhya 26: 329–358
Rayner JMV (1986) Vertebrate flapping flight mechanisms and aerodynamics, and the evolution of flight in bats. In: Natctigall W (ed) Biona Report no. 5: Bat flight. Fledermausflug, pp 27–74
Rinderknecht A, Blanco RE (2008) The largest fossil rodent. Proc R Soc Lond [Biol] 275(1637): 923–928
Safi K, Meiri S, Jones KE (2013) Evolution of body size in bats. In: Smith FA, Lyons SK (eds) Animal Body Size: Linking Pattern and Process across Space, Time, and Taxonomic Group. University of Chicago Press, Chicago and London, pp 95–115
Safi K, Seid MA, Dechmann DK (2005) Bigger is not always better: when brains get smaller. Biol Lett 1(3): 283–286
Sánchez-Villagra MR, Aguilera O, Horovitz I (2003) The anatomy of the world's largest extinct rodent. Science 301(5640): 1708–1710
Schober W, Grimmberger E (1997) The Bats of Europe and North America; Knowing Them, Identifying Them, Protecting Them. TFH Publications, Neptune City
Sedlock JL, Jose RP, Vog JM, Paguntalan LMJ, Cariño AB (2014) A survey of bats in a karst landscapte in the central Philippines. Acta Chiropt 16(1): 197–211
Simmons NB (2005) Order Chiroptera. In: Wilson DE, Reeder DM (eds) Mammals Species of the World: A Taxonomic and Geographic Reference, 3rd edn. John Hopkins University Press, Baltimore, pp 312–529
Simmons NB, Seymour KL, Habersetzer J, Gunnell GF (2008) Primitive Early Eocene bat from Wyoming and the evolution of flight and echolocation. Nature 451(7180): 818–821
Simmons NB, Seymour KL, Habersetzer J, Gunnell GF (2010) Inferring echolocation in ancient bats. Nature 466(7309): 939–942
Smith FA, Lyons SK, Ernest SM, Jones KE, Kaufman DM, Dayan T, Marquet PA, Brown JH, Haskell JP (2003) Body mass of Late Quaternary mammals: ecological archives. Ecology 84(12): 3403
Smith T, Habersetzer J, Simmons NB, Gunnell GF (2012) Systematics and paleobiogeography of early bats. In: Gunnell GF, Simmons NB (eds) Evolutionary History of Bats. Cambridge University Press, Cambridge, pp 23–66
Smith FA, Lyons SK, Jones KE, Maurer BA, Brown JH (2013) The influence of flight on patterns of body size diversity and heritability. In: Smith FA, Lyons SK (eds) Animal Body Size: Linking Pattern and Process Across Space, Time, and Taxonomic Group. Chicago University Press, Chicago and London, pp 187–205
Speakman JR, Racey PA (1991) No cost of echolocation for bats in flight. Nature 350(6317): 421
Teeling EC, Dool S, Springer MS (2012) Phylogenies, fossils and functional genes: the evolution of echolocation in bats. In: Gunnell GF, Simmons NB (eds) Evolutionary History of Bats. Cambridge University Press, Cambridge, pp 1–22
Teeling EC, Springer MS, Madsen O, Bates P, O'brien SJ, Murphy WJ (2005) A molecular phylogeny for bats illuminates biogeography and the fossil record. Science 307(5709): 580–584
Ter Braak CJF (1995) Ordination. In: Jongman RHG, ter Braak CFJ, van Tongeren OFR (eds) Data Analysis in Community and Landscape Ecology. Pudoc, Wageningen, pp 91–173
Ter Braak CFJ, Smilauer P (1998) CANOCO reference manual and user’s guide to CANOCO for Windows: software for canonical community ordination, version 4.0. Microcomputer Power, Ithaca
Thiagavel J, Cechetto C, Santana SE, Jakobsen L, Warrant EJ, Ratcliffe JM (2018) Auditory opportunity and visual constraint enabled the evolution of echolocation in bats. Nat Commun 9(1): 98
Thong VD, Puechmaille SJ, Denzinger A, Bates PJ, Dietz C, Csorba G, Bates PJJ, Teeling EC, Schnitzler HU (2012) Systematics of the Hipposideros turpis complex and a description of a new subspecies from Vietnam. Mammal Rev 42(2): 166–192
Threlfall C, Law B, Penman T, Banks PB (2011) Ecological processes in urban landscapes: mechanisms influencing the distribution and activity of insectivorous bats. Ecography 34(5): 814–826
Uyeda JC, Harmon L (2014) bayou: Bayesian Fitting of Ornstein-Uhlenbeck Models to Phylogenies. R package version 1.0.1.
Uyeda, J. C., J. Eastman, and L. Harmon (2014) "bayou: Bayesian fitting of Ornstein-Uhlenbeck models to phylogenies." R package version 1.1.
Van Den Bussche RA, Hoofer SR (2004) Phylogenetic relationships among recent chiropteran families and the importance of choosing appropriate out-group taxa. J Mammal 85(2): 321–330
Venditti C, Meade A, Pagel M (2011) Multiple routes to mammalian diversity. Nature 479(7373): 393–396
Veselka N, McErlain DD, Holdsworth DW, Eger JL, Chhem RK, Mason MJ, Brain KL, Faure PA, Fenton MB (2010) A bony connection signals laryngeal echolocation in bats. Nature 463(7283): 939–942
Wang Z, Zhu T, Xue H, Fang N, Zhang J, Zhang L, Pang J, Teeling EC, Zhang S (2017) Prenatal development supports a single origin of laryngeal echolocation in bats. Nat Ecol Evol 1(0021)
Zhang JS, Han NJ, Jones G, Lin LK, Zhang JP, Zhu GJ, Huang DW, Zhang SY (2007) A new species of Barbastella (Chiroptera: Vespertilionidae) from north China. J Mammal 88(6): 1393–1403
Acknowledgements
We thank Santiago Catalano for his help with handling TNT in the body mass optimization analyses. We thank the support from Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), Argentina, PICT 2015-2389 and PICT 2016-3682.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Moyers Arévalo, R.L., Amador, L.I., Almeida, F.C. et al. Evolution of Body Mass in Bats: Insights from a Large Supermatrix Phylogeny. J Mammal Evol 27, 123–138 (2020). https://doi.org/10.1007/s10914-018-9447-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10914-018-9447-8