Abstract
Numerous climatic fluctuations occurred during the Cenozoic (last 66 Ma BP); some of them were drastic (e.g., during the Eocene-Oligocene boundary) while others were more gradual (e.g., late Tertiary cooling), but both have deep effect on the biotas. Armadillos are exclusively from the Americas; they have an old evolutionary history in South America and faunal replacement and/or local extinctions were detected, linked with climatic fluctuations. The global cooling of the late Eocene - early Oligocene coincides with a well-documented faunal turnover of Dasypodinae by Euphractinae in Patagonia. During cold and arid periods of the Quaternary, Euphractinae and Tolypeutinae moved more than once to the eastern Pampean Region, and Dasypodinae moved northward to central Brazil or even further north to the Guyana Region. During interglacial periods some armadillos went extinct locally and/or moved to Patagonia (Zaedyus), central Argentina (Tolypeutes matacus, Chaetophractus vellerosus), or from the north to Mesopotamia and the Pampean Region (Dasypus). Since the end of the Pleistocene/early Holocene, human activity has strongly impacted armadillo populations. Currently, the eastern Pampean Region (Argentina) is characterized by the presence of the couple C. villosus - D. hybridus (probably established since the late Holocene), but during the Pleistocene was Z. pichiy – T. matacus while Z. pichiy - C. villosus characterized early-middle Holocene. This work serves as evidence that paleozoological studies can be used to assess responses of biological systems to large scale perturbations and is the basis for studying future species distributions, in order to identify species in danger of extinction and establish management actions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Armadillos are the only extant Cingulata (Xenarthra, Dasypodidae), mammals characterized by bony shielded regions that protect their head, body, and tail. The body carapace of cingulates consists of three regions (scapular and pelvic shields separated by movable bands), all of which are composed of small bones known as osteoderms. The morphology and ornamentation of the dorsal surface of an osteoderm has great taxonomic value, which is especially useful for identifying isolated remains found in paleontological and/or archaeological contexts (see Vizcaíno et al. 1995; Soibelzon et al. 2010, 2013, 2015, Soibelzon and Leon 2017; Loponte and Acosta 2012; Ciancio et al. 2013). As a result, cingulates have a good fossil record, particularly compared to other xenarthrans (Gaudin and Croft 2015).
All extant armadillos have traditionally been included in a single family, Dasypodidae (and so they will be considered in this paper), but recent molecular studies aim to divide them into two families, Dasypodidae and Chlamyphoridae, the former containing a single extant subfamily (Dasypodinae: long-nosed, nine- and seven-banded armadillos) and the latter comprising three subfamilies (Chlamyphorinae: fairy armadillos; Euphractinae: hairy, dwarf, and six-banded armadillos; and Tolypeutinae: naked-tailed, giant, and three-banded armadillos) (Gibb et al. 2016; Mitchell et al. 2016). Armadillos have been found exclusively in the Americas, from tropical forest to cold-open grasslands, and comprise approximately 20 extant species (approximately 67% of living xenarthrans). Their physiological characteristics (e.g., low basal rates of metabolism and body temperature, high thermal conductance, and the possibly of entering torpor; McNab 1980) largely determine their distribution, and they are more diverse in tropical regions than temperate ones (Fig. 1). Their oldest fossil record comes from the São José de Itaboraí Basin, Brazil and was referred to the early Eocene or latest Paleocene (Bergqvist et al. 2004) but the temporal assignation is under discussion (Gaudin and Croft 2015). In North America, they have been recorded since near the Plio-Pleistocene boundary (USA, Blancan, ca. 2.4 Ma BP; Castro 2015) when the formation of the Isthmus of Panama between the Americas favored terrestrial faunal displacement (this phenomenon is known as “Great American Biotic Interchange” or “GABI”; see details in Cione et al. 2015; O’Dea et al. 2016).
The aim of this study is to analyze the distribution of armadillos during the Cenozoic with emphasis in the Quaternary records, and its relationship with paleoclimates.
Paleoclimates and Paleogeographic Changes during the Cenozoic
Climatic oscillations occurred repeatedly during the Cenozoic (Fig. 2) and, together with some paleogeographic changes, have had profound effects on biotas. Especially during the early to middle Cenozoic (Paleogene and Neogene periods), xenarthrans experienced a great radiation (Delsuc et al. 2001). The main paleoclimatic conditions and paleogeographic changes that have strongly influenced their evolution and biogeographic patterns can be summarized as follows: 1- Eocene - Oligocene boundary: the shift from the “Greenhouse” world into the “Icehouse” world (see Goin et al. 2015) and the isolation and separation of South America from Antarctica (which favored the Antarctic Circumpolar Current); 2- Miocene: marine transgressions, epeirogenic uplifting of the central Brazilian shield, Andean orogeny (which produced Patagonian desertification); 3- Quaternary: more than 15 glacial cycles have been detected during the Pleistocene (Rabassa et al. 2005), the three with the greatest development at about 1 Ma, 0.78 Ma, and 26 to19 ka BP (Great Patagonian Glaciation or GPG, Matuyama/Brunhes Glaciation, and Last Glacial Maximum or LGM, respectively; Soibelzon and Tonni 2009). The LGM ends with colder and arid conditions of the Younger Dryas (ca. 11.7 ka; Broecker et al. 2010) (Fig. 2). The glacial periods were temporarily interrupted by short duration interglacial conditions (e.g., MIS 11, MIS5e) (Cione et al. 2015). During the Holocene (11.7 ka BP until the present day), the climate slowly turned warm, producing deglaciation and sea level rise (Ponce and Rabassa 2012). These processes were drastically interrupted by some episodes such as: the warmer and drier Holocene Thermal Maximum (HTM) or Holocene Climatic Optimum (HCO) during the middle Holocene (between 7 to 5 ka BP in the Southern Hemisphere) (Renssen et al. 2012); and the mid/low-latitude aridification (M-LA) event during the middle–late Holocene boundary (4.2 ka BP). During the last 1000 years, two significant climatic events were recorded: the Medieval Thermal Maximum or MTM (developed during the 800–1200 AD; Broecker 2001) and the Little Ice Age or LIA (between 1550 and 1900 AD) (de Menocal 2001; Tonni 2006; Marcott et al. 2013).
Time scale for the last 60 Ma BP with main global climatic events mentioned in the text. Redrawn after Zachos et al. 2001 (left and center) and Folland et al. 1990 (right). B-MG Matuyama/Brunhes Glaciation, EECO Early Eocene Climatic Optimum, GPG Great Patagonian Glaciation, HTM Holocene Thermal Maximum, Kyr Thousand years before present, LEC Late Eocene Cooling, LGM Last Glacial Maximum, LIA Little Ice Age, LTC Late Tertiary Cooling, Ma Million years before present, M-LA mid/low-latitude aridification, MMCO Middle Miocene Climatic Optimum, Pleist Pleistocene, 11 and 5 Marine Isotopic Stage
Materials and Methods
In order to analyze the distribution of Dasypodidae during the Quaternary (last 2.6 Ma, Pleistocene and Holocene epochs sensu Cohen et al. 2013), distributional maps were created based on specimens housed in the following paleontological, archaeological, and mastozoological collections: 1-Argentina: Museo de La Plata (La Plata); Museo Argentino de Ciencias Naturales “Bernardino Rivadavia” (Buenos Aires); Museo Municipal de Ciencias Naturales de Mar del Plata “Lorenzo Scaglia” (Mar del Plata); 2-Brazil: Coleção de Mamíferos Fósseis do Laboratório de Mastozoologia (Universidade Federal do Estado do Rio de Janeiro); Museu Nacional (Rio de Janeiro); Museu de Ciências Naturais (Pontifícia Universidade Católica de Minas Gerais); 3-USA: American Museum of Natural History (New York); Florida Museum of Natural History (Gainesville); Princeton Collection of Yale Peabody Museum (New Haven); 4-Bolivia: Museo Nacional Paleontológico-Arqueológico de Tarija (Tarija). The database was supplemented with information from FAUNMAP (Graham and Lundelius, 2010), published fossil records in paleontological and archaeological contexts (e.g., Vizcaíno et al. 1995; Soibelzon et al. 2006, 2010, 2013, 2015; Soibelzon and León 2017; Loponte and Acosta 2012; Rodriguez-Bualó et al. 2014; Castro 2015; Francia et al. 2015; Ciancio 2016), and new records from recent field investigations in Buenos Aires Province, Argentina (e.g., southern coastal cliffs, San Pedro and Marcos Paz counties). The datasets analyzed during the current study are available in the repository mentioned above and are available from the corresponding author on reasonable request.
The maps of South American species richness and distributional maps were created using IUCN 2016 Red List Spatial Data managed in DIVA-GIS 7.5.0.0.
Abbreviations
Institutions, Argentina: MACN, Museo Argentino de Ciencias Naturales “Bernardino Rivadavia”; MLP, Museo de La Plata; MMP-S, Museo Municipal de Ciencias Naturales de Mar del Plata “Lorenzo Scaglia”; Bolivia: MUT, Museo Nacional Paleontológico-Arqueológico de Tarija; France: MNHN-PAM, Museum National d’Histoire Naturelle; USA: YPM PU, Princeton Collection of Yale Peabody Museum.
B-MG Matuyama/Brunhes Glaciation, BP Before Present, EECO Early Eocene Climatic Optimum, GABI Great American Biotic Interchange, GPG Great Patagonian Glaciation, HTM Holocene Thermal Maximum, IUCN International Union for Conservation of Nature, Kyr Thousand years before present, LEC Late Eocene Cooling, LGM Last Glacial Maximum, LIA Little Ice Age, LTC Late Tertiary Cooling, Ma Million years before present, MIS Marine Isotopic Stage, M-LA mid/low-latitude aridification, MMCO Middle Miocene Climatic Optimum, MTM Medieval Thermal Maximum, Pleist Pleistocene, YD Younger Dryas.
Results
The family’s oldest fossil record is from Patagonia of Argentina, when tropical to subtropical forest covered southern South America, favored by the prevalent climatic conditions of the Greenhouse phase, especially during Early Eocene Climatic Optimum (Fig. 2) (Ciancio et al. 2013; Ciancio 2016). The beginning of the “Icehouse” phase coincides with the last record of Dasypodinae (a subfamily clearly adapted to tropical - subtropical climates) in Patagonia, and thereafter the family has been recorded during the “Middle Miocene Climatic Optimum” of equatorial South America (Ecuador, Colombia) and late Miocene of Brazil (Castro 2015). The oldest record of a Euphractinae corresponds to the late Eocene of Patagonia (Ciancio et al. 2006), when a great faunal turnover occurred during the Eocene-Oligocene transition, linked with a global cooling (Fig. 2). Thereafter, a great diversity of Euphractinae adapted to temperate climates is recorded (Ciancio 2016). The tolypeutines are poorly represented in pre-Quaternary times, even though their fossil record includes the late Oligocene of Bolivia and middle Miocene of Colombia (Billet et al. 2011; Ciancio 2016).
The Quaternary records of Dasypodidae in South America show differences in both taxonomic composition and geographical distribution for both fossil and extant species. Members of Dasypodinae, Euphractinae, and Tolypeutinae were recorded (no record of Chlamyphorinae was found) (Table 1).
The Dasypodinae are recorded since the early Pleistocene by the extinct genus Propraopus and since the late Pleistocene by Dasypus in Argentina, Bolivia, and Brazil (Tonni et al. 2009; Soibelzon et al. 2010; Castro 2015). During the Holocene, Dasypus was registered in some archaeological contexts of Argentina and Brazil (see Vizcaíno et al. 1995; Mazzanti and Quintana 2001; Soibelzon et al. 2013, and the bibliography cited therein).
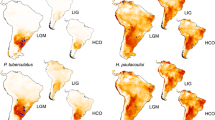
The Euphractinae are represented since the early Pleistocene in the Pampean Region by the extant genus Zaedyus and Chaetophractus (Fig. 3a, b) and the extinct Eutatus pasquali (Holotype MMPS-171, late Pliocene-early Pleistocene) and E. seguini (Holotype MNHN-PAM 273, late Pleistocene) (Krmpotic et al. 2009b). Chaetophractus villosus is also recorded in the Pleistocene of the Tarija Valley in Bolivia (MACN 1612, YPM PU 16612 and MUT-128; Rodriguez-Bualó et al. 2014). Finally, Euphractus is registered since the late Pleistocene of Argentina (northeastern Corrientes Province, Francia et al. 2015) and Brazil (Minas Gerais State, Bahia State, Tocantins; Soibelzon et al. 2015). Therefore, the Quaternary record of Euphractinae includes the early Holocene of Brazil (Euphractus, Ceará State), middle and late Holocene of Pampean Region (Chaetophractus and Zaedyus), and late Holocene of Uruguay (Chaetophractus, Eutatus; Ubilla et al. 2017). Its record in the middle Pleistocene of the Pampean Region (MLP 69-VIII-9-5) and in the late Holocene of Córdoba Province has been questioned (Soibelzon et al. 2010, 2013).
The Tolypeutinae are represented by the genus Tolypeutes from the early Pleistocene to late Holocene in numerous paleontological and archaeological sites of the northern and southern Pampean Region (Soibelzon et al. 2010, 2017; Beilinson et al. 2015) (Fig. 3c). The provenance of specimen MLP 69-IX-9-1 from “the sub-Andean Tertiary” of Salta is dubious. Deschamps et al. (2003) mentioned their presence during the LIA in Buenos Aires Province. In Brazil, there are a few records assigned to Tolypeutes tricinctus in the late Pleistocene of the states of Bahia, Ceará, Rio Grande do Norte, and Sergipe (Feijó et al. 2015).
Discussion
Pre-Quaternary records of armadillos shows faunal replacements during the Eocene-Oligocene boundary, biogeographic connections between Pampean Region and northwest Argentina during late Miocene, and displacements or local extinctions during the Pleistocene and Holocene.
The Quaternary is characterized by climatic oscillations, with a prevalence of cold and arid conditions alternating with warm events of short duration, some of them humid (Tonni et al. 1999). Given that every taxon inhabits certain areas with characteristic climatic attributes, changes in those attributes can have two outcomes for a taxon: local extinction or displacement to an area where the variables have not changed (Lyman 2006). Following Vivo and Carmignoto (2004), the colder and more arid glacial Pleistocene favored the development of grasslands and steppes in southern South America, open forests and savannas in the central Brazil and Central America (Fig. 4a), and xeric scrub and open wooded vegetation in eastern USA. On the other hand, interglacial periods (of short duration) were warm and wet and forced the development of evergreen forest in tropical-subtropical America and temperate paleoenvironments in southern South America (Fig. 4b).
Map of South America showing major vegetation types and lower sea levels during the Last Glacial Maximum (a) and Holocene Thermal Maximum (b). Redrawn after Vivo and Carmignotto (2004) and Ponce and Rabassa (2012). Solid arrows indicate the displacements of Central and Patagonian fauna (Euphractinae and Tolypeutinae) to the Pampean Region; upward diagonal fill arrow indicates the displacement of the Dasypodinae to the north. Dotted line: limits between Neotropical Region, South America transition zone, and Andean Region (sensu Morrone 2006). Black diamond: Aurora do Tocantins (see text). Scale bar = 1000 km
The Dasypodidae have a wide geographic range in America, but their greatest diversity is in tropical-subtropical climates of central South America (Fig. 1). They are adapted to different climatic conditions, and analysis of their past and current distributional records reveals geographic displacements (Fig. 3).
The Dasypodinae (commonly known as “mulitas” or “long-nosed armadillos”) are currently distributed along the Neotropical and Andean biogeographic regions (sensu Morrone 2006); only Dasypus novemcinctus has a distribution that extends into southern North America (Taulman and Robbins 2014). The extant genus Dasypus has been recorded in South America since the late Pleistocene, with a continuous distribution during the Holocene of the Pampean and Mesopotamia regions (Vizcaíno et al. 1995; Tonni 2003; Soibelzon et al. 2010, 2013; Abba and Vizcaíno 2011; Loponte and Acosta 2012). Strikingly, the oldest record of the genus corresponds to D. bellus in the late Pliocene-early Pleistocene of North America (Southeast USA and Mexico; Graham and Lundelius 2010), a species that became extinct in the late Pleistocene (Castro 2015). Apparently, the extant species D. novemcinctus reinvaded southern USA around 600 years ago and continues its expansion to the northeast (Humphrey 1974; Shapiro et al. 2015; Taulman and Robbins 2014). The distribution of D. bellus resembles the current range of D. novemcinctus, but the former probably had greater cold tolerance during the Quaternary (Feng et al. 2016). Several authors have proposed that cold and drought cause death in D. novemcinctus (Kalmbach 1944; Fitch et al. 1952; McDonough and Loughry 1997).
The Euphractinae (commonly known as “peludos” or “hairy armadillos”) are currently restricted to the southeast Neotropical region (especially along the “open dry diagonal” of South America, Floresta Atlántica and Pampa, sensu Zanella 2010), Neotropical transition zone, and Andean region (sensu Morrone 2006). The Quaternary record shows the presence of Zaedyus and Chaetophractus vellerosus (Fig. 3a, b) in the southeast Pampean Region (Soibelzon et al. 2006; Soibelzon and León 2017; Carlini et al. 2016), which went extinct locally, probably when interglacial conditions prevailed. A relictual population of C. vellerosus currently inhabits the area of Bahía Samborombón, (Buenos Aires Province); this disjunct distribution has been explained as a relic of a more extensive paleodistribution that included the entirety of the current territory of Buenos Aires Province (Soibelzon et al. 2007; Carlini et al. 2016). Phylogeographic studies performed by Poljak (2009) indicated that the ancestral haplotype of the species is located in central Argentina (Tucumán province) and the derived ones in Samborombón Bay. Chaetophractus villosus has one of the widest distributions in Argentina, excluding only Mesopotamia and the Puna of Argentina; its range could be extended due to the expansion of agricultural frontiers (Abba and Vizcaíno 2011). Following Carlini and Scillato-Yané (1999), its fossil record dates back to the Chapadmalalan Age (late Pliocene), while Cione et al. (2015) referred it at most to the Marplatan Age (late Pliocene-early Pleistocene). Pleistocene records include Bolivia (Tarija Valley; Rodriguez-Bualó et al., 2014), Argentina (Buenos Aires and Corrientes provinces; Soibelzon et al. 2010; Francia and Ciancio 2013; Francia et al. 2015), and Uruguay (Sopas Formation, late Pleistocene; Ubilla and Marinez 2016; Ubilla et al. 2017), and numerous archaeological sites document its presence during the Holocene (Vizcaíno et al. 1995; Loponte and Acosta 2012; Soibelzon and León 2017). Poljak et al. (2010), based on molecular markers, suggested an ancestral lineage in the Pampean Region with a later dispersion to Patagonia after the retraction of the Pleistocene glaciers (Fig. 4b). Finally, in recent times, humans introduced the species in the southernmost province of Argentina (Tierra del Fuego; Poljak et al. 2007). The last member of Euphractinae recorded during the Quaternary is Euphractus. Following Carlini and Scillato-Yané (1999), it has been recorded since the Ensenadan Age (early Pleistocene) based on some specimens with dubious geographic and/or stratigraphic provenance (e.g., MLP 69-VIII-9-5). For this reason, its biochron has been restricted to the late Pleistocene of Brazil (Soibelzon et al. 2015) and Argentina (Francia et al. 2015; Gasparini et al. 2016) to Recent. Finally, eutatines, which are recorded since the late Eocene of Patagonia, are the most hairy armadillos, probably in connection with the adaptation to colder climates (Krmpotic et al. 2009a; Scillato-Yané et al. 2010). Quaternary records are restricted to Eutatus pascuali (early to middle Pleistocene) and E. seguini (middle Pleistocene to early Holocene) distributed only in Argentina and Uruguay.
The Tolypeutinae (commonly known as “mataco bola” or “southern three-banded armadillo”) are currently restricted to warm-temperate climates in desert or semi-desert regions (Feijó et al. 2015). In Argentina, the Quaternary records corresponds to Tolypeutes matacus, which has been recorded from the early Pleistocene to late Holocene (including during the LIA) in numerous localities of the Pampean Region (with the exception of the specimen from Salta mentioned above) where it is currently absent, up to 800 km outside its current distribution (Fig. 3c). In Brazil, only a few records attributed to T. tricinctus are known. Following Feijó et al. (2015), its allopatric distribution could be explained as vicariant, linked to Miocene marine transgressions and uplift of the Brazilian Shield.
The expansion from central Argentina to the southeast Pampean Region (and vice versa) during glacial/interglacial cycles (C. vellerosus, T. matacus) was probably across the “Argentinean arid diagonal” (local expression of the “South America transition” zone sensu Morrone 2006) (Fig. 4). This arid diagonal has a long evolutionary history in South America (Monge-Nájera 1996; Iglesias et al. 2011), having its major development in the past and acting as a primary barrier to the dispersal of taxa from northeast to southwest (Bruniard 1982). Additionally, during glacial periods, sea level drops generated more continental conditions than those recorded today in the eastern Pampean Region and allowed armadillos to move to that region, where climatic conditions were probably similar to those prevalent in central Argentina and Patagonia during interglacial cycles. Following Ciancio et al. (2006), during the late Miocene (Chasicoan and Huayquerian stage), biogeographic connections between Pampean Region (Buenos Aires and La Pampa provinces) and northwest Argentina (San Juan and Catamarca provinces) were sustained in the diversity of Euphractini and Eutatini. Likewise, the development of open forests and savannas in lower latitudes allowed the latitudinal expansion of the Dasypodinae Propraopus and Dasypus to the Guiana Region of Brazil (e.g., Aurora do Tocantins in Brazil, Soibelzon et al. 2015) (Fig. 4a). Then, during interglacial cycles, warm and moist climates favored their expansion to the north, reaching southern USA (Shapiro et al. 2015) and to the south, into evergreen forest of central Brazil (Soibelzon et al. 2015), Mesopotamia, and Humid Chaco of Argentina.
Conclusions
Changes in the fossil record of Dasypodidae can be explained as displacements and/or local extinctions linked to past climate changes. The end of the “Greenhouse” phase and beginning of the “Icehouse” is connected with a faunal turnover of Dasypodinae by Euphractinae in Patagonia.
During cold and arid periods of the Quaternary, Euphractinae and Tolypeutinae moved to the eastern Pampean Region and Dasypodinae moved northward to the central Brazilian shield (Propraopus, Dasypus; Soibelzon et al. 2015) (Fig. 4a), or even farther north to the Guyana Region. This region could have acted as refugee, as has been proposed for other vertebrates. These geographic displacements surely have occurred more than once, probably during the early Pleistocene coinciding with the Great Patagonian Glaciation, the Brunhes/Matuyama boundary, the Last Glacial Maximum, and throughout the different cold and/or arid phases of the Holocene (i.e., Holocene Thermal Maximum, Little Ice Age) (Fig. 4a; Table 1). During interglacial periods (e.g., MIS 11, MIS 5e, current interglacial), armadillos went extinct locally and/or moved to Patagonia (Zaedyus), central Argentina (Tolypeutes matacus, C. vellerosus), or Mesopotamia and the Pampean Region (Dasypus) (Fig. 4b). Similarly, during these periods, some subtropical mammals expanded their distributions to higher latitudes (e.g., Tapirus in southeast Buenos Aires Province; Cione et al. 2015).
Taking into account the effects of the climatic and/or environmental perturbations on the organisms (see Walther et al. 2002, among others); and the physiological limitations of armadillos for living in temperate climates (McNab 1980); their presence in the eastern Pampean Region is due to climatic conditions in the past. Currently this area could be characterized by the couple C. villosus - D. hybridus (Abba and Vizcaíno 2011), probably established since the late Holocene (Tonni et al. 1992). But the characteristic association in the Pleistocene was Z. pichiy – T. matacus while Z. pichiy - C. villosus characterized early-middle Holocene (Tonni 1985; Vizcaíno et al. 1995). Today, the former association is recorded approximately 1000 km to the northwest of the Pampean Region (north of San Luis Province). Likewise, glacial conditions during the late Pleistocene and Holocene of Corrientes Province favored the expansion of C. villosus to that region, where they are currently absent. This is supported both by paleontological and archaeological records (Tonni 2003; Soibelzon et al. 2010, 2015, 2017; Francia et al. 2015) as well as molecular data (Poljak 2009; Poljak et al. 2010). The presence of D. hybridus in the Pampean Region during the LIA could be explained by particular microenvironments with wet conditions, as was proposed for other species (Teta and Medina 2005).
As detailed above, climate has had an important role in the distribution of armadillos but, since the end of the Pleistocene/early Holocene, human activity has strongly impacted armadillo populations. Archaeological evidence demonstrates the exploitation of armadillos as a food resource and the beginning of the transformation of native forests and grasslands for agricultural purposes by early humans (which entered South America as part of the GABI). Currently, armadillos suffer intensive hunting pressure for the damage caused to livestock by burrows, to prevent disease transmission, and/or for ornamental use (Chamberlain 1980; Feijó et al. 2015; Soibelzon and León 2017).
There is an urgent need for continuing data collection and revision of fossil (zooarchaeologic and paleontologic), historical, and current records of armadillos, in a way to elucidate how current climate change and anthropogenic disturbances will influence the biogeography of armadillos.
References
Abba AM, Vizcaíno SF (2011) Distribución de los armadillos (Xenarthra: Dasypodidae) en la provincia de Buenos Aires, Argentina. Mastozool Neotropical 18: 185–206
Beilinson E, Gasparini GM, Soibelzon LH, Soibelzon E (2015) Insights into Pleistocene palaeoenvironments and biostratigraphy in south-eastern Buenos Aires province (Argentina) from continental deposits. J South Am Earth Sci 60: 82–91
Bergqvist LP, Avilla LS, Abrantes EAL (2004) The Xenarthra of São José de Itaboraí basin (upper Paleocene, Itaboraian), Rio de Janeiro, Brasil. Geodiversitas 26: 323–337
Billet G, Hautier L, Muizon C de, Valentin X (2011) Oldest cingulate skulls provide congruence between morphological and molecular scenarios of armadillo evolution. Proc Royal Soc B 278:2791–2797
Broecker WS (2001) Was the Medieval warm period global? Science 291: 1497–1499
Broecker WS, Denton GH, Edwards RL, Cheng H, Alley RB, Putnam AE (2010) Putting the Younger Dryas cold event into context. Quaternary Sci Rev 29: 1078–1081
Bruniard ED (1982) La diagonal árida argentina: un límite climático real. Rev geográfica 95: 6–20
Carlini AA, Scillato-Yané GJ (1999) Evolution of Quaternary xenarthrans (Mammalia) of Argentina. In: Tonni EP, Cione AL (eds) Quaternary of South America and Antarctic peninsula. AA Balkema, Rotterdam, pp 149–175
Carlini AA, Soibelzon E, Glaz D (2016) Chaetophractus vellerosus (Cingulata: Dasypodidae). Mammal Species 937:73–82
Castro M (2015) Sistemática y evolución de los armadillos Dasypodini (Xenarthra, Cingulata, Dasypodidae). Rev Mus La Plata 15: 1–50
Chamberlain PA (1980) Armadillos: problems and control. Proceedings of the 9th vertebrate Pest conference: 163-169
Ciancio MR (2016) Los armadillos (Dasypodidae, Xenarthra) del Cenozoico temprano-medio de Argentina: Aspectos evolutivos, bioestratigráficos y biogeográficos. In: Agnolin FL, Lio GL, Egli FB, Chimento NR, Novas FE (eds) Historia evolutiva y paleobiogeográfica de los vertebrados de América del Sur. Contribuciones del MACN, Buenos Aires, pp 231–247
Ciancio MR, Carlini AA, Campbell KE, Scillato-Yané GJ (2013) New Palaeogene cingulates (Mammalia, Xenarthra) from Santa Rosa, Perú and their importance in the context of south American faunas. J Syst Palaeontol 11: 727–741
Ciancio MR, Krmpotic CM, Soibelzon E, Urrutia J (2006) Los Dasypodoidea (Mammalia, Xenarthra) de la Fm. Lomas de Las Tapias, San Juan, Argentina. Implicancias Paleobiogeográficas. Ameghiniana 43: 30R.
Cione AL, Gasparini GM, Soibelzon E, Soibelzon LH, Tonni EP (2015) The Great American Biotic Interchange in Southern South America: Land Mammal Biostratigraphy, Climatic Evolution and Faunal Integration. SpringerBriefs in Earth System Sciences, New York-London
Cohen KM, Finney SC, Gibbard PL, Fan JX (2013) The ICS international chronostratigraphic chart. Episodes 36: 199–204
De Menocal PB (2001) Cultural responses to climate change during the late Holocene. Science 292:667–673
Delsuc F, Catzeflis FM, Stanhope MJ, Douzery EJP (2001) The evolution of armadillos, anteaters and sloths depicted by nuclear and mitochondrial phylogenies: implications for the status of the enigmatic fossil Eurotamandua. Proc Roy Soc B 268: 1605–1615
Deschamps JR, Otero O, Tonni EP (2003) Cambio climático en la pampa bonaerense: las precipitaciones desde los siglos XVIII al XX. Universidad de Belgrano, Documentos de Trabajo 109: 1–18
Feijó A, Garbino GST, Campos BATP, Rocha PA, Ferrari SF, Langguth A (2015) Distribution of Tolypeutes Illiger, 1811 (Xenarthra: Cingulata) with comments on its biogeography and conservation. Zool Science 32:77–87
Feng X, Anacleto TCS, Papeş M (2016) Climatic similarity of extant and extinct Dasypus armadillos. J Mammal Evol DOI 10.1007/s10914-016-9336-y
Fitch HS, Goodrum P, Newman C (1952) The armadillo in the southeastern United States. J Mammal 33: 21–37
Folland CK, Karl TR, Vinnikov KYA (1990) Observed climate variations and change. In: Houghton JT, Jenkins GJ, Ephraums JJ (eds) Climate Change: The IPCC Scientific Assessment. Cambridge University Press, Cambridge, New York, and Melbourne, pp 195-238
Francia A, Ciancio MR (2013) First record of Chaetophractus villosus (Mammalia, Dasypodidae) in the late Pleistocene of Corrientes Province (Argentina). Rev Mus La Plata 13: 1–9
Francia A, Zurita AE, Carlini AA (2015) How marine Isotope stage 3 (MIS3) is reflected in northern Mesopotamia faunal assemblage of Argentina: the Xenarthra Cingulata case. Quaternary Internatl 377: 126–139
Gasparini GM, Soibelzon E, Deschamps CM, Francia A, Beilinson E, Soibelzon LH, Tonni EP (2016) Continental vertebrates during the marine Isotope stage 3 (MIS 3) in Argentina. In: Gasparini GM, Rabassa J, Deschamps CM, Tonni EP (eds) Marine Isotope Stage 3 in Southern South America, 60 Ka B.P.-30 Ka B.P. Springer Earth System Sciences, New York, pp 227-247
Gaudin TJ, Croft DA (2015) Paleogene Xenarthra and the evolution of South American mammals. J Mammal 96:622–634
Gibb GC, Condamine FL, Kuch M, Enk J, Moraes-Barros N, Superina M, Poinar HN, Delsuc F (2016) Shotgun mitogenomics provides a reference phylogenetic framework and timescale for living xenarthrans. Mol Biol Evol 33: 621–642
Goin FJ, Woodburne MO, Zimicz AN, Martin GM, Chornogubsky L (2015) A Brief History of South American Metatherians. Evolutionary Contexts and Intercontinental Dispersals. SpringerBriefs in Earth System Sciences, New York-London
Graham RW, Lundelius EL Jr (2010) FAUNMAP II: new data for North America with a temporal extension for the Blancan, Irvingtonian and early Rancholabrean. FAUNMAP II Database, version 1.0
Humphrey SR (1974) Zoogeography of the nine-banded armadillo (Dasypus novemcinctus) in the United States. BioScience 24: 457–462
Iglesias A, Artabe AE, Morel EM (2011) The evolution of Patagonian climate and vegetation from the Mesozoic to the present. Biol J Linn Soc 103: 409–422
IUCN (2016) The IUCN red list of threatened species. Version 2016-1.http://www.iucnredlist.org. Accessed 26 August 2016
Kalmbach ER (1944) The Armadillo: Its Relation to Agriculture and Game. Texas Game Fish and Oyster Commission, Austin, 60 pp
Krmpotic CM, Carlini AA, Scillato-Yane GJ (2009a) The species of Eutatus (Mammalia, Xenarthra): assessment, morphology and climate. Quaternary Internatl 210: 66–75
Krmpotic CM, Ciancio MR, Barbeito C, Mario RC, Carlini AA (2009b) Osteoderm morphology in recent and fossil euphractine xenarthrans. Acta Zool 90: 339–351
Loponte D, Acosta A (2012) Nuevos registros de armadillos (Xenarthra: Dasypodidae) del Holoceno tardío en la Región Pampeana, Argentina. Mastozool Neotropical 19: 163–178
Lyman R (2006) Paleozoology in the service of conservation biology. Evol Anthropol 15:11–19
Marcott SA, Shakun JD, Clark PU, Mix AC (2013) A reconstruction of regional and global temperature for the past 11,300 years. Science 339: 1198–1201
Mazzanti DL, Quintana CA (2001) Cueva Tixi. Cazadores y Recolectores de las Sierras de Tandilia Oriental: 1. geología, paleontología y zooarqueología. Laboratorio de Arqueología, Universidad Nacional de Mar del Plata, Buenos Aires
McDonough CM, Loughry WJ (1997) Patterns of mortality in a population of nine-banded armadillos, Dasypus novemcinctus. Am Midl Nat 138: 299–305
McNab BK (1980) Energetics and the limits to a temperate distribution in armadillos. J Mammal 61: 606–627
Mitchell KJ, Scanferla A, Soibelzon E, Bonini R, Ochoa J, Cooper A (2016) Ancient DNA from the extinct South American giant glyptodont Doedicurus sp. (Xenarthra: Glyptodontidae) reveals that glyptodonts evolved from Eocene armadillos. Mol Ecol 25: 3499–3508
Monge-Nájera J (1996) Jurassic-Pliocene biogeography: testing a model with velvet worm (Onychophora) vicariance. Rev Biol Trop 44: 159–175
Morrone JJ (2006) Biogeographic areas and transition zones of Latin America and the Caribbean Islands based on panbiogeographic and cladistic analyses of the entomofauna. Annu Rev Entomol 51:467–494
O’Dea A, Lessios HA, Coates AG, Eytan RI, Restrepo-Moreno SA, Cione AL, Collins LS, de Queiroz A, Farris DW, Norris RD, Stallard RF, Woodburne MO, Aguilera O, Aubry MP, Berggren WA, Budd AF, Cozzuol MA, Coppard SE, Duque-Caro H, Finnegan S, Gasparini GM, Grossman EL, Johnson KG, Keigwin LD, Knowlton N, Leigh EG, Leonard-Pingel JS, Marko PB, Pyenson ND, Rachello-Dolmen PG, Soibelzon E, Soibelzon LH, Todd JA, Vermeij GJ, Jackson JBC (2016) Formation of the isthmus of Panama. Sci Adv 2. doi: 10.1126/sciadv.1600883
Peel MC, Finlayson BL, McMahon TA (2007) Updated world map of the Köppen-Geiger climate classification. Hydrol Earth Syst Sci 11: 1633–1644
Poljak S (2009) Estudios filogeográficos en Dasypodidae [Mammalia Xenarthra] de Argentina: Chaetophractus villosus y Chaetophractus vellerosus como modelos de análisis. Tesis Doctoral. Facultad de Ciencias Naturales y Museo (UNLP), La Plata, Buenos Aires, Argentina
Poljak S, Confalonieri V, Fasanella M, Gabrieli M and Lizarralde MS (2010) Phylogeography of the armadillo Chaetophractus villosus (Dasypodidae Xenarthra): post-glacial range expansion from pampas to Patagonia (Argentina). Mol Phylogen Evol 55: 38–46
Poljak S, Escobar J, Deferrari G, Lizarralde M (2007) Un nuevo mamífero introducido en la Tierra del Fuego: el “peludo” Chaetophractus villosus (Mammalia, Dasypodidae) en Isla Grande. Rev Chilena Hist Nat 80: 285–294
Ponce JF, Rabassa J (2012) La plataforma submarina y la costa atlántica argentina durante los últimos 22.000 años. Ciencia Hoy 22: 37–43
Rabassa J, Coronato AM, Salemme, M (2005) Chronology of the late Cenozoic Patagonian glaciations and their correlation with biostratigraphic units of the Pampean region (Argentina). J South Am Earth Sci 20:81–103
Renssen H, Seppä H, Crosta X, Goosse H, Roche DM (2012) Global characterization of the Holocene Thermal Maximum. Quaternary Sci Rev 48: 7–19
Rodriguez-Bualó S, Soibelzon E, Scarano AC, Zurita AE (2014) Chaetophractus tarijensis (Xenarthra, Dasypodidae) ¿Un endemismo del Valle De Tarija (Bolivia)? Implicancias paleobiogeográficas. Rev bras paleontol 17:59–68
Scillato-Yané GJ, Krmpotic CM, Esteban GI (2010) The species of genus Chasicotatus Scillato-Yané (Eutatini, Dasypodidae). Rev Mex Cienc Geol 27: 43–55
Shapiro B, Graham RW, Letts B (2015) A revised evolutionary history of armadillos (Dasypus) in North America based on ancient mitochondrial DNA. Boreas 44: 14–23
Soibelzon E, Avilla LS, Castro M (2015) The cingulates (Mammalia: Xenarthra) from the late Quaternary of northern Brazil: fossil records, paleoclimates and displacements in America. Quaternary Internatl 377: 118–125
Soibelzon E, Carlini AA, Tonni EP, Soibelzon LH (2006) Chaetophractus vellerosus (Mammalia: Dasypodidae) in the Ensenadan (lower to middle Pleistocene) of southeastern Pampean region (Argentina). Paleozoogeographical and paleoclimatic aspects. Neues Jahrb Geol Paläontol 12: 734–748
Soibelzon E, Daniele G, Negrete J, Carlini AA, Plischuk S (2007). Annual diet of little hairy armadillo Chaetophractus vellerosus gray, 1865 (Mammalia, Dasypodidae) in Buenos Aires province, Argentina. J Mammal 88, 1319–1324
Soibelzon E, León DC (2017) Effects of climatic oscillations on the faunas. The Holocene Thermal Maximum and the displacement of armadillos in Argentina: anatomical features and conservation. J Archaeol Sci: Rep 11: 90–98
Soibelzon E, Medina M, Abba AM (2013) Late Holocene armadillos (Mammalia, Dasypodidae) of the sierras of Córdoba, Argentina: zooarchaeology, diagnostic characters and their paleozoological relevance. Quaternary Internatl 299: 72–79
Soibelzon E, Miño-Boilini AR, Zurita AE, Krmpotic CM (2010) Los Xenarthra (Mammalia) del Ensenadense (Pleistoceno Inferior a Medio) de la Región Pampeana (Argentina). Rev Mex Cienc Geol 27: 449–469
Soibelzon E, Tonni EP (2009) Early Pleistocene glaciations in Argentina (South America) and the response of the mammals: the case of the Pampean region. Current Research in the Pleistocene 26: 175–177
Taulman JF, Robbins LW (2014) Range expansion and distributional limits of the nine-banded armadillo in the United States: an update of Taulman and Robbins (2014). J Biogeogr 41: 1626–1630
Teta P, Medina M (2005) Holochilus brasiliensis (Rodentia, Cricetidae) en conjuntos arqueofaunísticos del Holoceno tardío de la Provincia de Córdoba (Argentina). Mastozool Neotropical 12: 271–275
Tonni EP (1985) Mamíferos del Holoceno del Partido de Lobería, Provincia de Buenos Aires. Aspectos paleoambientales y bioestratigráficos del Holoceno del Sector Oriental de Tandilia y Área Interserranassss. Ameghiniana 22: 283–288
Tonni EP (2003) Faunas y Clima en el Cuaternario de la Mesopotamia Argentina. Temas de la Biodiversidad del Litoral fluvial argentino INSUGEO. Miscelánea 12: 5–12
Tonni EP (2006) Cambio climático en el Holoceno tardío de la Argentina. Una síntesis con énfasis en los últimos 1000 años. Folia Histórica del Nordeste, 16:187e–195
Tonni EP, Alberdi MT, Prado JL, Bargo MS, Cione AL (1992) Changes of mammal assemblages in the Pampean region (Argentina) and their relation with the Plio-Pleistocene boundary. Palaeogeogr Palaeoclimatol Palaeoecol 95:179–194
Tonni EP, Cione AL, Figini AJ (1999) Predominance of arid climates indicated by mammals in the pampas of Argentina during the late Pleistocene and Holocene. Palaeogeogr Palaeoclimatol Palaeoecol 147: 257–281
Tonni EP, Soibelzon E, Cione AL, Carlini AA, Scillato-Yané GJ, Zurita AE, Paredes Ríos F (2009) Mammals from the Pleistocene of the Tarija Valley (Bolivia). Correlation with the Pampean chronological standard. Quaternary Internatl 210: 57–65
Ubilla M, Marinez S (2016) Geology and Paleontology of the Quaternary of Uruguay. SpringerBriefs in Earth System Sciences, New York-London
Ubilla M, Rinderknecht A, Corona A, Perea D (2017) Mammals in last 30 to 7 ka interval (late Pleistocene-early Holocene) in southern Uruguay (Santa Lucía River basin): last occurrences, climate, and biogeography. J Mammal Evol DOI 10.1007/s10914-017-9380-2
Vivo M, Carmignotto AP (2004) Holocene vegetation change and the mammal faunas of South America and Africa. J Biogeogr 31:943–957
Vizcaíno SF, Pardiñas UFJ, Bargo MS (1995) Distribución de los armadillos (Mammalia, Dasypodidae) en la Región Pampeana (República Argentina) durante el Holoceno. Mastozool Neotropical 2: 149–165
Walther GR, Post E, Convey P, Menzel A, Parmesan C, Beebee TJ (2002) Ecological responses to recent climate change. Nature 416: 389–395
Zachos JC, Pagani M, Sloan L, Thomas E, Billups K (2001) Trends, rythyms, and aberrations in global climate 65 ma to present. Science 292: 686–693
Zanella FCV (2010) Evolução da biota da diagonal de formações abertas secas da América do Sul. In: Carvalho CJB, Almeida EAB (eds) Biogeografia da América do Sul. Padrões e Processos, São Paulo, pp 198–220
Acknowledgements
I wish to thank the curators of museums who facilitated access to specimens used in this study, M. Reguero, D. Verzi (Museo de La Plata, Argentina), S. Hochgraf (Department of Ecology and Evolutionary Biology, University of Connecticut, USA), E. Westwig (American Museum of Natural History, USA), C. Cartelle (Museo de Historia Natural da Pontifícia Universidade Católica de Minas Gerais, Belo Horizonte, Brazil), J. A. de Olivera (Museu Nacional, Rio de Janeiro, Brazil), L. Avilla (Laboratorio de Mastozoología, UNIRIO, Brazil), F. Paredes Ríos (Museo Arqueológico Paleontológico de Tarija, Bolivia), D. Brinkman (Yale Paleontology Museum, USA). D.N. Ciai and D. Croft are thanked for improving the English. I especially thank D.A. Croft, E.P. Tonni, and two anonymous reviewers for comments that improved this manuscript. Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), Universidad Nacional de La Plata (UNLP) and Agencia de Promoción Científica y Tecnológica (ANPCyT) are acknowledged for financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Soibelzon, E. Using Paleoclimate and the Fossil Record to Explain Past and Present Distributions of Armadillos (Xenarthra, Dasypodidae). J Mammal Evol 26, 61–70 (2019). https://doi.org/10.1007/s10914-017-9395-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10914-017-9395-8