Abstract
This study contributes to the growing complexity of the impala fossil record through a morphological description and analysis of Aepyceros fossils from late Pleistocene deposits in Kenya’s Lake Victoria Basin. We show that the Lake Victoria impala belongs to an extinct species that differs from modern impala and its fossil predecessors by a combination of exceptionally deep mandibles and teeth characterized by greater hypsodonty and occlusal lengths. Whereas modern impala (A. melampus) displays substantial ecological flexibility, these traits in the extinct species suggest a more dedicated adaptation to grazing in open and dry environments. Previous phylogeographic observations indicate that A. melampus was extirpated from East Africa, perhaps during the middle-to-late Pleistocene, and later recolonized from southern Africa. The Lake Victoria impala raises the possibility that the evidence interpreted as extirpation may instead reflect speciation, with A. melampus giving rise to a novel East African species while persisting unchanged in southern Africa. Increased rainfall and rising atmospheric CO2 concentrations at the end of the Pleistocene may have played a role in the disappearance of the extinct form via habitat loss and possibly competition with the more versatile A. melampus.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Impalas (Aepyceros spp.) are distinguished among African bovids by their long-term evolutionary success (Gentry 1978; Vrba 1980, 1984), persisting with relatively little morphological change for the last >7 million years (Harris 2003). The continuity of Aepyceros has been linked to ecological flexibility (Vrba 1980, 1984). Modern impala (Aepyceros melampus) are mixed feeders (30–70 % dicots/grasses) known to exhibit massive dietary shifts in response to local resource availability (Meissner et al. 1996; Wronski 2002; Cerling et al. 2003; Sponheimer et al. 2003a, b; Codron et al. 2006). Their preferred habitat includes the lightly wooded ecotone between open grasslands and dense woodlands, where forage from both environments can be exploited (Kingdon 1982; Skinner and Chimimba 2005). Although the distribution of grasslands and woodlands expanded and contracted during past climate oscillations (e.g., DeMenocal 2004), the ecotones between them are likely to have persisted, providing suitable habitat under a range of paleoclimatic conditions.
Initial interpretations of the fossil record suggested that only a single species of impala existed at any time (Gentry 1978; Vrba 1980). However, recent evidence documents a more speciose evolutionary history (Fig. 1). The earliest representatives of the lineage (Aepyceros premelampus) are known from the late Miocene of Kenya (Harris 2003), and it is now apparent that a handful of younger species overlapped during the Pliocene (Geraads et al. 2009a, b, 2012; Gentry 2011) and early Pleistocene (Brink et al. 2012). Fossils attributed to the modern impala (A. melampus) first appear in East Africa ~2 million years ago (Harris 1991; Gentry 2010) and their successors are abundant and widespread throughout southern and East Africa today (East 1999) (Fig. 1).
a The geographic distribution of impala (Aepyceros melampus) in Africa (IUCN 2008). b The temporal distribution of fossil Aepyceros
Paralleling the growing complexity observed in the fossil record, recent phylogeographic evidence also indicates a complex history for A. melampus. Genetic data suggest impala was extirpated from East Africa one or more times during the Pleistocene and later recolonized from a southern African refugium (Nersting and Arctander 2001; Lorenzen et al. 2006, 2012). This scenario is supported by patterns of morphological variation among modern impala (Reynolds 2010), and is also seen in the phylogeographic histories of wildebeest (Connochaetes taurinus) and eland (Taurotragus oryx) (Arctander et al. 1999; Lorenzen et al. 2010). The extirpation of impala and other ungulates from East Africa is thought to be related to habitat changes driven by Pleistocene glacial cycles (Lorenzen et al. 2006, 2012; Reynolds 2007, 2010).
Here we contribute to the increasingly complex picture of impala evolution through the documentation of an extinct species from late Pleistocene deposits in Kenya’s Lake Victoria Basin. This study provides a morphological description of the fossil remains and a discussion of their biogeographic and evolutionary implications.
Geological Context
The fossils discussed here were recovered from Rusinga Island and Karungu in the Lake Victoria Basin (Fig. 2). Rusinga Island is located within Lake Victoria and, although now connected to the mainland by a causeway, was historically separated by a narrow channel ~350 m wide and ~5 m deep. Pleistocene archaeological and paleontological deposits have been noted since the 1930s (Kent 1942; MacInnes 1956; Van Couvering 1972; Leakey 1974; Pickford 1984, 1986) and are now the subject of renewed investigation focusing on the environmental and ecological context of the Middle Stone Age (MSA) archaeological sites (Tryon et al. 2010, 2012, in press; Faith et al. 2011). The poorly consolidated Pleistocene deposits on Rusinga Island, known as the Wasiriya Beds, are characterized by weakly-developed paleosols and tuffaceous fluvial sediments recording a complex cut-and-fill system. The age of the Wasiriya Beds is constrained to between 100,000 and 33,000 years ago. The maximum age is provided by geochemical analysis of tephra deposits, which suggests a derivation from East African Rift System volcanic sources that began erupting ~100,000 years ago (Tryon et al. 2010). The minimum age is provided by a suite of calibrated radiocarbon age estimates on the shells of gastropods that most likely burrowed into the sediments at some point after deposition (Tryon et al. 2010, 2012).
The fossil mammals from Rusinga Island suggest an expansion of grasslands distinct from the bushland, thicket, and forest found in the region today (Faith et al. 2011, 2012; Tryon et al. 2012). Open grassland species such as alcelaphine antelopes (wildebeest and allies) are dominant and several extinct specialized grazers are present, including Rusingoryx atopocranion, Damaliscus hypsodon, Megalotragus, and Syncerus antiquus. Fossil remains of arid-adapted oryx (Oryx beisa) and Grevy’s zebra (Equus grevyi) are found well outside of their contemporary ranges, suggesting greater aridity relative to the present (Faith et al. 2013). The combination of diminished rainfall together with the competitive advantage of C4 vegetation at lower atmospheric CO2 concentrations probably accounts for the grassy paleoenvironment implied by the fauna, consistent with paleo-vegetation models for Pleistocene glacial phases (e.g., Cowling et al. 2008; Prentice et al. 2011). In light of lake level fluctuations observed historically (Nicholson 1998) and documented in the late Pleistocene and Holocene geological record (Johnson et al. 1996; Stager et al. 2002, 2011; Stager and Johnson 2008), the presence of large gregarious grazers and arid-adapted ungulates from Rusinga Island suggests a connection to the mainland (Faith et al. 2011). This is further supported by the presence of a similar fauna from roughly contemporaneous deposits on nearby Mfangano Island, which imply a >25 m decline in lake levels (Tryon et al. in press).
Karungu is located on the Kenyan mainland approximately 50 km south of Rusinga Island (Fig. 2). Initial archaeological and paleontological explorations were conducted by Owen (1937, 1938) and later by Pickford (1986), who surveyed and mapped the Pleistocene deposits. Recent investigations of these deposits in 2011–2012 documented MSA artifacts and a rich fossil assemblage similar to that recovered from Rusinga Island (see appendix in Faith et al. 2013). The Pleistocene deposits from Karungu are lithologically similar to those from Rusinga Island (Beverly et al. 2012), and preliminary analyses of the tephra deposits at Karungu document stratigraphic correlations between the two sites (Tryon et al. 2013), suggesting that they are of the same age.
The Lake Victoria Impala
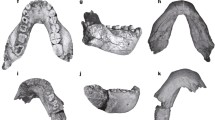
Fossil collections over the last several years on Rusinga Island and Karungu have yielded a modest sample of Aepyceros dental remains, including isolated teeth, partial mandibles, and a partial maxilla, that are morphologically distinct from modern and fossil A. melampus (Table 1, Fig. 3). Due to the lack of a complete specimen worthy of designation as a type, we refrain from providing a formal species description here. However, the existing sample is sufficient to document substantial morphological departures from A. melampus and to indicate the presence of an extinct late Pleistocene impala.
The following analyses make use of comparative measurements on modern impala and Pliocene-to-Pleistocene fossil remains from West Turkana (Harris et al. 1988), East Turkana (Harris 1991), and Lainyamok (Potts and Deino 1995) (Table 2), all housed at the National Museums of Kenya in Nairobi (NMK). This sample includes representatives of both A. melampus and its slightly smaller predecessor A. shungurae, in addition to specimens identified to genus only. The modern and fossil samples include individuals from a range of age classes, with the youngest individuals represented by specimens with the m2 in eruption and the oldest individuals including specimens with all molars in an advanced state of wear (i.e., obliteration of the internal enamel cavities). Although our sample of modern impala includes only East African individuals, Reynolds (2007) showed that East and southern African impala have comparable cranial-dental measurements.
The Lake Victoria impala shares several features with modern A. melampus that distinguish their teeth from similarly-sized Antilopini, such as Gazella or Antidorcas, including a tendency toward more evenly rounded lingual lobes in upper molars of middle-to-late wear, outbowing of the lingual walls and centrally constricted internal enamel cavities of lower molars, and thickened enamel on the internal enamel cavities of upper and lower molars (Fig. 4). The tooth enamel of the Lake Victoria impala tends to be thicker than in A. shungurae or early Pleistocene A. melampus. There is some overlap in tooth size between the Lake Victoria impala and smaller Alcelaphini, including blesbok (Damaliscus dorcas) and extinct D. hypsodon, but it can be distinguished from these and other alcelaphines by its smooth (not rugose) enamel surfaces, more triangular (less circular) lobes of lower molars, flatter or concave labial walls of upper molars (paracone and metacone), and less complex internal enamel cavities of the upper and lower molars (Fig. 4). The concave wall of the metacone is also seen in earlier Aepyceros, including A. shungurae, A. dietrichi, and A. datoadeni, whereas it is more often convex or flat in modern A. melampus.
The Lake Victoria impala is distinguished from modern impala and fossil A. shungurae-melampus by exceptionally high-crowned teeth and correspondingly deep mandibles (Figs. 5 and 6). Figure 5 presents box-plots illustrating the depth of the mandible anterior to the m1, m2, and m3 for the Lake Victoria impala, modern impala, and fossil A. shungurae-melampus (measurements follow von den Driesch 1976). The mandibular depth anterior to the m1 and m2 falls outside the range of modern impala and its fossil predecessors, with little overlap for the depth anterior to the m3. One-way ANOVA shows that the mandibular depths anterior to the m2 and m3 differ significantly across groups (m2: F = 17.15, p < 0.001; m3: F = 7.82, p = 0.001), with Tukey’s pairwise comparisons showing that this is driven entirely by the greater mandibular depths of the Lake Victoria specimens, whereas modern impala and fossil A. shungurae-melampus are indistinguishable (Table 3). We are unable to provide a comparable analysis comparing mandibular depths anterior to the m1 as there is only one specimen where this measurement could be taken, but we note that this specimen falls 4.07 and 2.76 standard deviations above the mean for modern impala and fossil A. shungurae-melampus, respectively, suggesting that it is not likely part of the same population.
Mandibular depths of the Lake Victoria impala compared to fossil A. shungurae-melampus and modern Aepyceros melampus. Sample size in parentheses. See also Table 3
The deep mandibles of the Lake Victoria impala imply a substantial increase in dental crown height. This is clearly seen on mandibular specimen KNM-RU 56803 (Figs. 3 and 6), where exposure of the lingual surface of the m2 allows for a measurement of the paraconid height (41.5 mm). This specimen is in an early state of medium wear (Klein and Cruz-Uribe 1984) with the internal enamel cavities of both lobes separated, suggesting that a moderate amount of the crown has already worn away. Janis (1988) reports an unworn crown height for modern impala m3 of 35.2 mm. We lack any measurable m3s from the Lake Victoria impala, but assuming a comparable crown height as the m2, this implies a >18 % increase in crown height. This is likely a conservative estimate, given that m3s in Aepyceros tend to be more high-crowned than the m2, as indicated here by greater mandibular depths anterior to the m3 (Fig. 5).
Molar occlusal lengths show some overlap with modern impala and fossil A. shungurae-melampus, although they tend to be somewhat larger and with greater maximum dimensions (Table 3, Fig. 7). The occlusal widths of the mandibular teeth, however, are comfortably within the range of modern impala. Because the molars are more high-crowned, this indicates an increase in the hypsodonty index (tooth height divided by width). Based on our tentative estimate of an >18 % increase in crown height (41.5 mm) and assuming a comparable m3 width as modern impala (0.72 mm, after Janis 1988), this implies a hypsodonty index of >5.77, a value in excess of extant African bovids (Janis 1988).
Molar lengths and widths of the Lake Victoria impala compared to fossil A. shungurae-melampus and modern Aepyceros melampus. Sample size in parentheses. See also Table 3
The large molar occlusal lengths of the Lake Victoria impala raise the possibility that its deep mandibles and high-crowned teeth are related in part to greater body size. To examine mandibular depth relative to body size, Fig. 8 illustrates the relationship between m2 occlusal length, which is tightly correlated with body mass in ungulates (Janis 1990), and mandibular depth anterior to the m2. Excluding an outlier from East Turkana, these variables are correlated across modern impala and fossil A. shungurae-melampus (r = 0.486, p = 0.009). Compared to these samples, the Lake Victoria impala is characterized by deeper mandibles for its tooth size (i.e., body mass). The mandibular depth of the smaller specimen (KNM-RU 56802: 32.2 mm), which has a tooth size within the range of modern A. melampus (Fig. 8), is 4.9 mm (18 %) deeper than predicted by the regression for modern impala and A. shungurae-melampus (27.3 mm). A similar shift is seen in the mandibular depth of the larger specimen (KNM-RU 56803: 34.3 mm), which is 5.0 mm (17 %) greater than predicted (29.3 mm), although the predicted value should be treated with caution as its m2 occlusal length exceeds the limits of the regression. These patterns suggest that when body size is taken into account, the mandibles of the Lake Victoria impala are 17–18 % deeper than modern impala or its fossil predecessors, a figure that is in agreement with our estimate of a >18 % increase in hypsodonty.
Discussion
The Aepyceros remains from Lake Victoria differ from modern impala and its fossil predecessors by a combination of deep mandibles and molars characterized by elevated hypsodonty and greater occlusal lengths, with many of the measurements reported here falling well outside the range of modern and fossil impala. The fact that these morphological traits are not seen in modern impala or several million years of A. shungurae-melampus strongly suggests that the Lake Victoria impala represents an extinct late Pleistocene Aepyceros. The features characteristic of the Lake Victoria form have not been described in any previously known Aepyceros (Cooke 1974; Gentry 1985, 2011; Harris 1991, 2003; Geraads et al. 2009b, 2012; Brink et al. 2012), suggesting that it represents a novel species. The recovery of more complete specimens, particularly horn cores, will be needed to more fully understand how it differs from known impalas. There is no evidence that A. melampus is present in the Lake Victoria sample.
It was previously thought that only a single species of Aepyceros existed at any time in its evolutionary history (Gentry 1978; Vrba 1984), but this viewpoint has been overturned by recent discoveries of several temporally overlapping Pliocene species (Fig. 1) (Harris 2003; Geraads et al. 2009b, 2012; Gentry 2011) and the recognition of A. helmoedi in the early Pleistocene of southern Africa (Brink et al. 2012). Geraads et al. (2009b:68) note that rather than consisting of a single lineage leading to modern impala, fossil Aepyceros seems to have included numerous variants of a basic impala pattern. In light of the diversity of earlier Aepyceros, the presence of an extinct form in the late Pleistocene is not unexpected.
The increase in mandibular depth and hypsodonty implies significant ecological differences between the Lake Victoria impala and modern impala. Both features are strongly correlated with diet in African bovids (Janis 1988; Spencer 1995; Sponheimer et al. 2003b; Damuth and Janis 2011), and the increases in the Lake Victoria impala are suggestive of a greater reliance on grassy forage. Although a rough estimate, the estimated hypsodonty index (>5.77) falls solely within the range of grazing ungulates that inhabit open grasslands (Damuth and Janis 2011). A greater reliance on grasses and grassland habitats is consistent with the associated faunal communities at Rusinga Island and Karungu, which suggest the presence of open and seasonally arid grassland habitats (Tryon et al. 2010, 2012, in press; Faith et al. 2011, 2012, 2013).
Phylogeographic data suggest that A. melampus was extirpated from East Africa and later recolonized by a southern African population (Nersting and Arctander 2001; Lorenzen et al. 2006, 2012). The precise timing of these events remains unclear, but the phylogeographic histories of other ungulates show a convergent pattern of genetic substructuring between East and southern African populations dating to the middle-to-late Pleistocene (Flagstad et al. 2001; Brown et al. 2007; Lorenzen et al. 2012). The presence of A. melampus from late Pleistocene and Holocene deposits (Beds III and V: <57 ka, Gliganic et al. 2012) at Mumba rockshelter in Tanzania (Mehlman 1989) suggests that the extirpation probably predates 57,000 years ago. The extinct late Pleistocene impala raises the possibility that the proposed extirpation from East Africa instead reflects allopatric speciation, with East African A. melampus giving rise to a novel species and southern African A. melampus remaining unchanged (Fig. 9). This scenario is consistent with arguments that dynamic climate changes in East Africa contributed to high rates of evolutionary change (speciation and extinction) while relatively muted climate fluctuations in southern Africa fostered a refugium that conserved ungulate populations over evolutionary time-scales (Reynolds 2007; Lorenzen et al. 2010, 2012).
The grassland adaptations of the Lake Victoria impala suggest that vegetation change across East Africa may have played a role in its divergence from A. melampus. Paleo-vegetation models show that the combination of reduced precipitation and lower atmospheric CO2 concentrations during Pleistocene glacial phases would have contributed to an expansion of dry grasslands or shrublands and a contraction of forests in equatorial East Africa (Cowling et al. 2008; Prentice et al. 2011). These models are consistent with vegetation reconstructions derived from pollen records (e.g., Elenga et al. 2001; Prentice et al. 2011) and fossil leaf waxes (Sinninghe Damsté et al. 2011). Faunal evidence is consistent with these changes, with equatorial East African faunas from the last 400,000 years including several extremely hypsodont or large-bodied grassland specialists, such as Damaliscus hypsodon, Rusingoryx atopocranion, Megalotragus, and Syncerus antiquus (Marean and Gifford-Gonzalez, 1991; Marean, 1992; Potts and Deino, 1995; Faith et al., 2011, 2012; Tryon et al., 2012). Their presence alongside arid-adapted ungulates such as Grevy’s zebra and oryx is thought to signal a more arid environment characterized by dry grasses and scrub vegetation. The expansion of such vegetation during middle-to-late Pleistocene glacial phases may have translated to selective pressure for a grassland-adapted impala, a hypothesis consistent with the adaptive implications of elevated hypsodonty (Marean 1992; Damuth and Janis 2011; Faith et al. 2012; Jardine et al. 2012). Such evolutionary change could reflect a greater reliance on open habitats compared to A. melampus or the mechanical demands of consuming dry grasses and grit.
Although the extinction chronology is only secure for D. hypsodon and S. antiquus, it has been proposed that increased rainfall at the onset of the Holocene contributed to a loss of arid grasslands and increased competition with mesic-adapted species, leading to extinctions across the arid grassland community (Marean and Gifford-Gonzalez 1991; Marean 1992; Faith et al. 2011, 2012, 2013). In addition, the expansion of the equatorial forest belt (e.g., Cowling et al. 2008), driven by increased precipitation and rising atmospheric CO2 levels, may have fragmented populations of grassland species and increased the likelihood of their extinction (see also Lorenzen et al. 2012). The morphological evidence for dietary specialization in the Lake Victoria impala indicates a less flexible ecology, in which case competition with ecologically flexible A. melampus may have also contributed to its demise. However, a refined chronology is clearly needed to test these hypotheses in detail.
Conclusions
The Lake Victoria impala fossils represent an extinct late Pleistocene species of Aepyceros that is morphologically distinct from modern A. melampus and its fossil predecessors. In contrast to the ecological flexibility characteristic of modern impala, its deep mandibles and more hypsodont dentition are consistent with a specialized adaptation to grassland environments. The presence of an extinct late Pleistocene impala contributes to the increasingly speciose fossil record of impala evolution and is consistent with the dynamic history suggested by phylogeographic data. We suggest that the previously proposed extirpation of East African A. melampus may reflect cladogenetic speciation related to the expansion of seasonally arid grasslands during middle-to-late Pleistocene glacial phases. Habitat loss mediated by increased rainfall and rising atmospheric CO2 concentrations at the end of the Pleistocene may have played an important role in the extinction of the Lake Victoria impala, perhaps exacerbated by competition with more versatile A. melampus.
References
Arctander P, Johansen C, Coutellec-Vreto M-A (1999) Phylogeography of three closely related African bovids (tribe Alcelaphini). Mol Bio Evol 16:1724–1739
Beverly EJ, Faith JT, Peppe DJ, Tryon CA, Driese SG, Blegen N, Patterson D, Horner WH (2012) Paleoenvironmental context of Pleistocene archaeological and paleontological sites in Kenya’s Lake Victoria Basin. Geol Soc Am Abstracts with Programs 44:415
Brink JS, Herries AIR, Moggi-Cecchi J, Gowlett JAL, Bousman CB, Hancox JP, Grün R, Eisenmann V, Adams JW, Rossouw L (2012) Fossil hominine remains from a ~1.0 million year old bone bed at Cornelia-Uitzoek, Free State Province, South Africa. J Hum Evol 63:527–353
Brown DM, Brenneman RA, Koepfli K-P, Pollinger JP, Milá B, Gerogiadis NJ, Louis Jr EE, Grether GF, Jacobs DK, Wayne RK (2007) Extensive population genetic structure in the giraffe. BMC Biol 5:57
Cerling TE, Harris JM, Passey BH (2003) Diets of east African bovidae based on stable isotope analysis. J Mammal 84:456–470
Codron D, Codron J, Lee-Thorp JA, Sponheimer M, De Ruiter D, Brink JS (2006) Dietary variation in impala Aepyceros melampus recorded by carbon isotope composition of feces. Acta Zool Sinica 52:1015–1025
Cooke HBS (1974) The fossil mammals of Cornelia, OFS, South Africa. Mem Natl Mus Bloemfontein 9:63–84
Cowling SA, Cox PM, Jones CD, Maslin MA, Peros M, Spall SA (2008) Simulated glacial and interglacial vegetation across Africa: implications for species phylogenies and trans-African migration of plants and animals. Glob Change Biol 14:827–840
Damuth J, Janis CM (2011) On the relationship between hypsodonty and feeding ecology in ungulate mammals, and its utility in palaeoecology. Biol Rev 86:733–758
DeMenocal PB (2004) African climate change and faunal evolution during the Pliocene-Pleistocene. Earth Planet Sci Lett 220:3–24
East R (1999) African Antelope Database 1999. IUCN, Gland, Switzerland
Elenga H, Peyron O, Bonnefile R, Jolly D, Cheddadi R, Guiot J, Andrieu V, Bottema S, Buchet G, de Beaulieu J-L, Hamilton AC, Maley J, Marchant R, Perez-Obiol R, Reille M, Riollet G, Scott L, Straka H, Taylor D, Van Campo E, Vincens A, Laarif F, Jonson H (2001) Pollen-based biome reconstruction for southern Europe and Africa 18,000 yr BP. J Biogeogr 27:621–634
Faith JT, Choiniere JN, Tryon CA, Peppe DJ, Fox DL (2011) Taxonomic status and paleoecology of Rusingoryx atopocranion (Mammalia, Artiodactyla), an extinct Pleistocene bovid from Rusinga Island, Kenya. Quaternary Res 75:697–707
Faith JT, Potts R, Plummer TW, Bishop LC, Marean CW, Tryon CA (2012) New perspectives on middle Pleistocene change in the large mammal faunas of East Africa: Damaliscus hypsodon sp. nov. (Mammalia, Artiodactyla) from Lainyamok, Kenya. Palaeogeogr Palaeoclimatol Palaeoecol 361–362:84–93
Faith JT, Tryon CA, Peppe DJ, Fox DL (2013) The fossil history of Grévy’s zebra (Equus grevyi) in equatorial East Africa. J Biogeogr 40:359–369
Flagstad Ø, Syvertsen PO, Stenseth NC, Jakobsen KS (2001) Environmental change and rates of evolution: the phylogeographic pattern within the hartebeest complex as related to climatic variation. Proc R Soc B 268:667–677
Gentry AW (1978) Bovidae. In: Maglio VJ, Cooke HBS (eds) Evolution of African Mammals. Harvard University Press, Cambridge, pp 540–572
Gentry AW (1985) The Bovidae of the Omo Group deposits, Ethiopia. In: Coppens Y, Howell FC (eds) Les Faunes Plio-Pléistocènes de la Basse Vallée de l’Omo (Ethiope), I: Périssodactyles, Artiodactyles (Bovidae). CNRS, Paris, pp 119–191
Gentry AW (2010) Bovidae. In: Werdelin L, Sanders WJ (eds) Cenozoic Mammals of Africa. University of California Press, Berkeley, pp 741–796
Gentry AW (2011) Bovidae. In: Harrison T (ed) Paleontology and Geology of Laetoli, Volume 2. Springer, Dordrecth, pp 363–465
Geraads D, Blondel C, Mackaye HT, Likius A, Vignaud P, Brunet M (2009a) Bovidae (Mammalia) from the lower Pliocene of Chad. J Vertebr Paleontol 29:923–933
Geraads D, Melillo S, Haile-Selassie Y (2009b) Middle Pliocene Bovidae from the hominid-bearing sites in the Waronso-Mille area, Afar region, Ethiopia. Palaeontol Afr 44:57–68
Geraads D, Bobe R, Reed KE (2012) Pliocene Bovidae (Mammalia) from the Hadar Formation of Hadar and Ledi-Geraru, Lower Awash, Ethiopia. J Vertebr Paleontol 32:180–197
Gliganic LA, Jacobs Z, Roberts RG, Domínguez-Rodrigo M, Mabulla AZP (2012) New ages for Middle and Later Stone Age deposits at Mumba rockshelter, Tanzania: optically stimulated luminescence dating of quartz and feldspar grains. J Hum Evol 62:533–547
Harris JM (1991) Family Bovidae. In: Harris JM (ed) Koobi Fora Research Project. Volume 3. The Fossil Ungulates: Geology, Fossil Artiodactyls, and Palaeoenvironments. Clarendon Press, Oxford, pp 139–320
Harris JM (2003) Bovidae from the Lothagam succession. In: Leakey MG, Harris JM (eds) Lothagam: the Dawn of Humanity in Eastern Africa. Columbia University Press, New York, pp 531–579
Harris JM, Brown FH, Leakey MG (1988) Stratigraphy and paleontology of Pliocene and Pleistocene localities west of Lake Turkana, Kenya. Nat Hist Mus LA County Contrib Sci 399:1–128
IUCN SSC Antelope Specialist Group (2008) Aepyceros melampus. In: IUCN 2012. IUCN Red List of Threatened Species. Version 2012.2 <www.iucnredlist.org>
Janis CM (1988) An estimation of tooth volume and hypsodonty indices in ungulate mammals, and the correlation of these factors with dietary preference. In: Russell DE, Santoro JP, Sigogneau-Russell D (eds) Teeth Revisited: Proceedings of the VIIth International Symposium on Dental Morphology. Mém Mus Natl Hist Nat Ser C, Paris, pp 367–387
Janis CM (1990) Correlation of cranial and dental variables with body size in ungulates and macropodoids. In: Damuth J, MacFadden BJ (eds) Body Size in Mammalian Paleobiology: Estimation and Biological Implications. Cambridge University Press, Cambridge, pp 255–300
Jardine PE, Janis CM, Sahney S, Benton MJ (2012) Grit not grass: concordant patterns of early origin of hypsodonty in Great Plains ungulates and glires. Palaeogeogr Palaeoclimatol Palaeoecol 365–366:1–10
Johnson TC, Scholz CA, Talbot MR, Kelts K, Ricketts RD, Ngobi G, Beuning KR, Ssemmanda I, McGill JW (1996) Late Pleistocene desiccation of Lake Victoria and the rapid evolution of cichlid fishes. Science 273:1091–1093
Kent PE (1942) The country round the Kavirondo Gulf of Victoria Nyanza. Geogr J 100:22–31
Kingdon J (1982) East African Mammals. University of Chicago Press, Chicago
Klein RG, Cruz-Uribe K (1984) The Analysis of Bones from Archaeological Sites. University of Chicago Press, Chicago
Leakey LSB (1974) By the Evidence: Memoirs 1932–1951. Harcourt Brace Jovanovich, New York
Lorenzen ED, Arctander P, Siegismund HR (2006) Regional genetic structuring and evolutionary history of the impala Aepyceros melampus. J Hered 97:119–132
Lorenzen ED, Heller R, Siegismund HR (2012) Comparative phylogeography of African savannah ungulates. Mol Ecol 21:3656–3670
Lorenzen ED, Masembe C, Arctander P, Siegismund HR (2010) A long-standing Pleistocene refugium in southern Africa and a mosaic of refugia in East Africa: insights from mtDNA and the common eland antelope. J Biogeogr 37:571–581
MacInnes D (1956) Fossil Tubulidentata from East Africa. Fossil Mammal Afr 10:1–38
Marean CW (1992) Implications of late Quaternary mammalian fauna from Lukenya Hill (south-central Kenya) for paleoenvironmental change and faunal extinctions. Quaternary Res 37:239–255
Marean CW, Gifford-Gonzalez D (1991) Late Quaternary extinct ungulates of East Africa and palaeoenvironmental implications. Nature 350:418–420
Mehlman MJ (1989) Later Quaternary archaeological sequences in northern Tanzania. PhD Thesis, University of Illinois, Urbana-Champaign
Meissner HH, Pieterse E, Potgeiter JHJ (1996) Seasonal food selection and intake by male impala Aepyceros melampus in two habitats. S Afr J Wildl Res 26:56–63
Nersting LG, Arctander P (2001) Phylogeography and conservation of impala and greater kudu. Mol Ecol 10:711–719
Nicholson SE (1998) Historical fluctuations of Lake Victoria and other lakes in the northern rift valley of East Africa. In: Lehman JT (ed) Environmental Change and Responses in East African Lakes. Kluwer Academic Publishers, Dordrecht, pp 7–35
Owen WE (1937) Draft of tentative preliminary report on the July 1937 investigations at Ng’ira Karungu. Unpublished manuscript housed in the archives of the Natural History Museum, London
Owen WE (1938) The Kombewa Culture, Kenya Colony. Man 38:203–205
Pickford M (1984) Kenya Paleontology Gazeteer Volume 1: Western Kenya. National Museums of Kenya Department of Sites and Monuments Documentation, Nairobi.
Pickford M (1986) Cainozoic paleontological sites of Western Kenya. Münchner Geowissensch Abh 8:1–151
Potts R, Deino A (1995) Mid-Pleistocene change in large mammal faunas of East Africa. Quaternary Res 43:106–113
Prentice IC, Harrison SP, Bartlein PJ (2011) Global vegetation and terrestrial carbon cycle changes after the last ice age. New Phytol 189:988–998
Reynolds SC (2007) Mammalian body size changes and Plio-Pleistocene environmental shifts: implications for understanding hominin evolution in eastern and southern Africa. J Hum Evol 53:528–548
Reynolds SC (2010) Morphological evaluation of genetic evidence for a Pleistocene extirpation of eastern African impala. S Afr J Sci 106:1–7
Sinninghe Damsté JS, Verschuren D, Osssebaar J, Blokker J, van Houten R, van der Meer MTJ, Plessen B, Schouten S (2011) A 25,000-year record of climate-induced changes in lowland vegetation of eastern equatorial Africa revealed by stable carbon-isotopic composition of fossil plant leaf waxes. Earth Planet Sci Lett 302:236–246
Skinner JD, Chimimba CT (2005) The Mammals of the Southern African Subregion. Cambridge University Press, Cambridge
Spencer LM (1995) Morphological correlates of dietary resource partitioning in the African Bovidae. J Mammal 76:448–471
Sponheimer M, Grant CC, De Ruiter D, Lee-Thorp JA, Codron DM, Codron J (2003a) Diets of impala from Kruger National Park: evidence from stable carbon isotopes. Koedoe 46:101–106
Sponheimer M, Lee-Thorp JA, DeRuiter DJ, Smith JM, van der Merwe NJ, Reed K, Grant CC, Ayliffe LK, Robinson TF, Heidelbergery C, Marcus W (2003b) Diets of southern African bovidae: stable isotope evidence. J Mammal 84:471–479
Stager JC, Johnson TC (2008) The late Pleistocene desiccation of Lake Victoria and the origin of its endemic biota. Hydrobiologia 596:5–16
Stager JC, Mayewski PA, Meeker LD (2002) Cooling cycles, Heinrich event 1, and the desiccation of Lake Victoria. Palaeogeogr Palaeoclimatol Palaeoecol 183:169–178
Stager JC, Ryves DB, Chase BM, Pausata FSR (2011) Catastrophic drought in the Afro-Asian monsoon region during Heinrich Event 1. Science 331:1299–1302
Tryon CA, Faith JT, Peppe DJ, Fox DL, McNulty KP, Jenkins K, Dunsworth H, Harcourt-Smith W (2010) The Pleistocene archaeology and environments of the Wasiriya Beds, Rusinga Island, Kenya. J Hum Evol 59:657–671
Tryon CA, Peppe DJ, Faith JT, Van Plantinga A, Nightengale S, Ogondo J (2012) Late Pleistocene artefacts and fauna from Rusinga and Mfangano islands, Lake Victoria, Kenya. Azania 47:14–38
Tryon CA, Faith JT, Peppe DJ, Blegen N, Beverly E, Patterson D, Jacobs Z (2013) Pleistocene archaeology and paleoenvironments of the Lake Victoria basin in Kenya. PaleoAnthropology Abstracts
Tryon CA, Faith JT, Peppe DJ, Keegan WF, Keegan KN, Jenkins KH, Nightingale S, Patterson D, Van Plantinga A, Driese S, Johnson CR, Beverly EJ (In Press) Sites on the landscape: paleoenvironmental context of late Pleistocene archaeological sites from the Lake Victoria Basin, equatorial East Africa. Quaternary Internatl
Van Couvering J (1972) Geology of Rusinga Island and correlation of the Kenya Mid-Tertiary fauna. PhD Thesis, Cambridge University, Cambridge
von den Driesch A (1976) A Guide to the Measurement of Animal Bones from Archaeological Sites. Peabody Museum Press, Cambridge
Vrba ES (1980) Evolution, species and fossils: how does life evolve? S Afr J Sci 76:61–84
Vrba ES (1984) Evolutionary pattern and process in the sister-group Alcelaphini-Aepycerotini (Mammalia: Bovidae). In: Eldredge N, Stanley SM (eds) Living Fossils. Springer, New York, pp 62–79
Wronski T (2002) Feeding ecology and foraging behaviour of impala Aepyceros melampus in Lake Mburu National Park, Uganda. Afr J Ecol 40:205–211
Acknowledgments
Collection and study of the fossil remains was conducted under research permits NCST/RCD/12B/012/31 issued to JTF and NCST/5/002/R/576 issued to CAT and an exploration and excavation license issued by the National Museums of Kenya (NMK). Our fieldwork is made possible through the support of the NMK and the British Institute in East Africa and with financial support from the National Science Foundation (BCS-1013199 and BCS-1013108), the Leakey Foundation, the National Geographic Society (8762–10), University of Queensland, Baylor University, and New York University. Ogeto Mwebi kindly facilitated access to modern impala specimens at the NMK. We thank Eline Lorenzen for helpful discussions on impala phylogeography and Sally Reynolds and James Brink for their insights concerning the fossil record. John Wible (Editor) and two anonymous referees provided valuable feedback on a previous version of this paper.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Faith, J.T., Tryon, C.A., Peppe, D.J. et al. Biogeographic and Evolutionary Implications of an Extinct Late Pleistocene Impala from the Lake Victoria Basin, Kenya. J Mammal Evol 21, 213–222 (2014). https://doi.org/10.1007/s10914-013-9238-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10914-013-9238-1