Abstract
Mastitis represents one major constraint in dairy goat farms implicating adverse effects on milk yield and composition and, in some cases, public health constraints. Intramammary infection, the principal cause of mastitis, can reach high prevalence in dairy goat herds, commonly more than 30%. Coagulase-negative staphylococci and coagulase-positive staphylococci, with emphasis for Staphylococcus aureus, are the major bacterial species related with in intramammary infection. Milk pathogens overtake anatomical, physiological, and immunological local defenses of the mammary glands. However, some enzootic systemic disease, such as contagious agalaxia, among others, with systemic tropism for the mammary gland, can have a significant impact on the milk production and quality. At immune level, neutrophils play a major role in the healthy and infected mammary gland representing 45–75% of total leucocyte counts in milk. Apparently, the threshold for significant neutrophils increase is 700,000 cells/ml. Moreover, the continuous renewal of epithelial cells from apocrine glands, which have phagocytosis cytokine production properties, improves significantly the somatic cells in milk. All these topics are discussed in the present chapter providing key points to improve the udder health status in goats.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
1 Introduction
Mastitis is the inflammation of the mammary gland , a multifactorial disease characterized by physiological, chemical, and bacteriological changes in milk , along with pathological modifications in the glandular tissue (Contreras et al. 1997a; Matthews 2009). Normally, it is the response to an infection and aims to eliminate the pathogen involved, restoring the integrity of affected tissue, and functionality of the mammary gland (Contreras et al. 1997b).
To understand the significance of mastitis, the first concept that is important to interiorize is that the milk secretion, since correctly collected, should be sterile (Poutrel 1983; Leitner et al. 2012), even milk microbiota concept has recently been also reported in goats (McInnis et al. 2015; Li et al. 2017). Therefore, the milk flora that has so much interest in the cheese industry is formed by environmental agents, including commensal bacteria from skin teats (Tormo et al. 2007), which are incorporated into milk after leaving the udder (Contreras et al. 1997b). This flora is usually conveyed by the teats and air at the time of milking, or through the milking machine itself (Tormo et al. 2006), such as the total mesophilic aerobic flora (Muehlherr et al. 2003), or even psychrotrophic bacteria, apparently associated with problems in milk cooling or long periods of storage (Contreras et al. 1997a, 2003), among others microorganisms, including milk pathogens.
With the presence of a pathogen, usually bacterial, an intramammary infection (IMI) take place into the mammary gland with associated inflammatory processes which negatively modify the milk yield and composition (Merin et al. 2004; Leitner et al. 2007; Le Maréchal et al. 2011; Gelasakis et al. 2016). Each pathogen induces a specific modification in milk during mastitis (Le Maréchal et al. 2011). The milk from clinical mastitis (IMI with milk and udder macroscopic changes) is immediately rejected for human and animal consummation. Inversely, untreated goats presenting subclinical mastitis also contribute with their milk for the bulk milk tank or direct consummation. However, several aspects must be taken into account with emphasis for IMI diagnosis , mastitis control and prevention management , total bacterial count in milk , milk pasteurization, and cheese ripening, among others.
The milk of subclinical mastitis is pasteurized , which kills bacteria, and manufactured or directly used for raw milk cheese (with more than 60 days of ripening). However, the high prevalence of infected goats can pose some constraints promoting, production and manufacture losses. For example, Staphylococcus aureus , one of the most prevalent milk pathogen, can be present in a significant proportion of bulk tanks milk, such as the recently reported by Cortimiglia et al. (2015) in Italy (43.1%) and by Merz et al. (2016) in Switzerland (46%). On the other hand, milk raw and unpasteurized dairy products contaminated by S. aureus can cause food poisoning (Oliver et al. 2009) in human and animals due to several (exo) enterotoxins (Dinges et al. 2000; Le Loir et al. 2003; Johler et al. 2015; Jans et al. 2017). Enteropathogenic and Shiga toxin-producing Escherichia coli are other significant endotoxins related with dairy products and potential adverse impact on public health (Álvarez-Suárez et al. 2015). Besides, subclinical mastitis is difficult to detect, is of long duration and usually precedes the clinical form. This chapter aims to describe the particularities of mastitis in goats regarding their etiophysiopathology, and consequently a better understanding of the health concepts for a more profit milk production.
2 Clinical and Subclinical Mastitis Occurrence
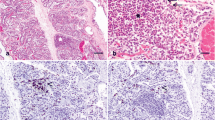
Clinical mastitis causes visible variable changes, ranging from a small change in the macroscopic characteristics of milk secretion, with or without local inflammatory changes, to manifestations of systemic disease (e.g., gangrenous mastitis) (Contreras et al. 1997b). In addition to the decrease in milk production, often the first sign detected, is the appearance of hungry kids or the increased mortality rate of suckling kids (Smith and Sherman 2009). Due to its faster evolution and the more intense severity of clinical symptoms, different categories have been proposed to classify clinical mastitis : hyperacute, acute, subacute, and chronic (Figs. 18.1 and 18.2).
In subclinical mastitis, which causes the greatest impact on dairy farms; the subsequent inflammation is not visible but changes in milk quality as well as, a decrease in production occur. In addition to these poorly perceptible but constant losses, the infected animals contaminate the milking teats and/or the milker hands, spreading the infection. Many of the agents involved in IMI, such as coagulase-negative Staphylococci (CNS), are able to persist in the mammary gland throughout lactation and even during the drying period. Thus, kidding goats with IMI will present subclinical mastitis during the new lactation period (Poutrel 1984; Contreras et al. 1997a; Bergonier et al. 2003). Although persistent, this subclinical mastitis does not alter the macroscopic appearance of the milk but can be detected through bacteriological isolation or associated cell recruitment, (i.e., somatic cells count (SCC) at the laboratory or Californian mastitis test at the farm) (Plummer and Plummer 2012).
In an appropriate sanitary context, the prevalence of clinical mastitis should not exceed 5% of the flock (Bergonier et al. 1997; Contreras et al. 2007) but this incidence can become greater sporadically. The presence of mycoplasmas in the herds may alter the relative proportions of the other bacteriological agents causing clinical mastitis. In endemic areas for contagious agalactia , the prevalence of clinical cases is generally low, but may increase drastically, especially in newly infected herds (Bergonier and Berthelot 2008). In Spain, the analysis of 820 milk samples from clinical mastitis allowed to identify 78.6% of IMI caused by (other) bacterial agents, 16.5% by mycoplasmas, and 4.9% caused simultaneously by mycoplasmas and other bacteria (Amores et al. 2012a, b). In these studies, staphylococci were the most prevalent bacteria (75.5%, of which 19% were Staphylococcus aureus ) followed by Gram-negative bacilli (11.7%), streptococci (7.7%) and other bacteria (5.1%). The most frequently identified mycoplasmas were Mycoplasma agalactiae (91.4%), M. mycoides subsp. capri (5.7%) and M. putrefaciens (3%).
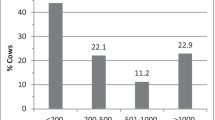
The prevalence of subclinical mastitis varies on average from 5 to 30% (Contreras et al. 2007), although some herds present higher values (Contreras et al. 1999; Rovai et al. 2014), reaching up to 70% in certain herds (Vega et al. 2004; Bazan et al. 2009). In this sense, according to Andrews et al. (1983). Kalogridou-Vassiliadou (1991) considers an animal as infected by a pathogen when the same microorganism is isolated in two of three consecutive examinations, and he found 65% of goats infected with pathogenic microorganisms with no clinical signs of mastitis in Greece . CNS are the main agents isolated from goats milk with subclinical mastitis, with a total average between 25 and 95% of isolates, followed by S. aureus (5–35%), streptococci (5–15%) and Enterobacteriaceae (2–12%) (Contreras et al. 2007). Nineteen staphylococcal species have been identified from subclinical intramammary infections in goats (Contreras et al. 2007). In Greece , even in low-input dairy goat farms, CNS reached 50.2% of isolates followed by coagulase-positive Staphylococci (e.g., S. aureus and S. intermedius) with 34.5% (Gelasakis et al. 2016). Similar high values (59.5%) of SCN prevalence were reported in China in dairy goats (Zhao et al. 2015).
3 Etiology
Caprine mastitis may be due to mechanical, traumatic (e.g., footprints, wounds, blows, etc.) or caused by bacterial toxins (Stehling et al. 1986), but in the overwhelming majority are due to bacterial infections, without excluding lentiviruses and fungi (Bergonier et al. 1997, 2003). Vega et al. (2004) isolated 86 different microorganisms in 166 goats from 16 goat herds. Most of these pathogens are reported in Table 18.1, according to each biological group. In most of the milk samples, only one of the microorganisms is isolated, but some samples contained two or more kinds of microorganisms.
The pathogens responsible for caprine mastitis can also be divided into two groups according to their virulence: major pathogens and minor pathogens (White et al. 2001; Bagnicka et al. 2011). Major pathogens induce more intense immune responses and consequently result in higher SCC and are often associated with clinical mastitis. As an example, IMI caused by S. aureus show higher SCC than those caused by CNS or other bacteria (Persson et al. 2015). In this, major pathogens class are included bacteria, such as S. aureus, M. agalactiae, M. mycoides subsp. capri, M. capricolum subsp. capricolum, M. putrefaciens, Corynebacterium pseudotuberculosis, Trueperella pyogenes, Streptococcus spp. (Strep. agalactiae, Strep. dysgalactiae, Strep. uberis, and Strep. bovis), Brucella spp., Pasteurella spp./Mannheimia spp., Aspergillus fumigatus, Nocardia asteroides, E. coli, Klebsiella spp., Pseudomonas aeruginosa, Enterococcus faecium, Enterococcus faecalis and CNS novobiocin-sensitive.
Minor pathogens would cause subclinical mastitis with low SCC. The inclusion of CNS as a minor pathogen is not consensual. Classically, due to their classification in cattle , they were considered as “minor” agents, but their importance in caprine mastitis led some authors to consider this as a non-proper classification (Maisi and Riipinen 1991; Contreras et al. 1995, 2003; Bergonier et al. 1997, 2003). In other words, subclinical mastitis caused by some CNS can cause, in goats, high SCC, considerable lesions in the mammary tissue, and significant economic losses due to production decrease and altered milk quality (Bergonier et al. 2003; Contreras et al. 2007; Le Maréchal et al. 2011). Thus, it is thought that the individual classification of the different CNS species (Sánchez et al. 1998; Bergonier et al. 2003), as proposed in sheep, consider the in vitro susceptibility to novobiocin as classification criterion in goats, since it seems to be associated with the virulence of CNS in this species (Sánchez et al. 1998).
The CNS resistant to novobiocin, i.e., S. xylosus, S. saprophyticus, S. lentus, S. sciuri and S. arlettae, behave as minor pathogens inducing small changes in SCC and a slightly reduction in milk production. Nevertheless, the novobiocin-susceptible CNS, i.e., S. epidermidis, S. simulans, S. chromogenes, S. warneri, and S. lugdunensis, are considered major pathogens inducing important changes in SCC and considerable breaks in milk production (Gonzalo et al. 1998; Sánchez et al. 1998). S. epidermidis and S. simulans would be the responsible for the higher SCC. However, these considerations should always be carefully analyzed due to the variations among isolation frequencies in different herds and from different studies, as well as the variations in the invasiveness and virulence of the numerous bacterial strains (Sánchez et al. 1998).
These pathogenic microorganisms can still be divided into (1) Contagious (Gelasakis et al. 2016): their main habitat is the mammary gland in such a way that infection occurs normally during milking operations. This group includes Strep. agalactiae and Mycoplasma spp., although its main habitat is not the internal udder , but the internal and external teat epithelium. This group also includes S. aureus, a major pathogens. Merz et al. (2016), which studied the S. aureus-specific staphylococcal protein A and clonal complexes, suggested that S. aureus isolated from milk samples of goats and ewes in Swiss farms are sufficiently genetically close to form distinct population from dairy cattle ; (2) Environmental (Gelasakis et al. 2016): infections do not occur during milking but rather with the contact of animals with contaminated materials (e.g., soil, bed, water , manure, food, among others). In this group Streptococcus spp., excluding Strep. agalactiae, are included, and some bacillus species and Gram-negative bacteria in general; and, (3) Opportunists: their natural habitat is the animal and human skin, mainly the Staphylococcus genus, and they compose the principal cause of subclinical mastitis in caprine herds: CNS (Corrales et al. 1997; Chu et al. 2012).
4 Pathogeny
Pathogens enter into the mammary gland through teats canal or by hematogenous dissemination, being the first the main route of entry to the main milk pathogens reported above. Other biological agents such as those belonging to the complex Mycobacterium tuberculosis , Brucella melitensis, Listeria monocytogenes , mycoplasmas, and lentivirus cause systemic infection and have tropism for the mammary gland (Contreras et al. 1995, 1997b). Therefore, IMI occurs when a pathogen can overcome anatomical defenses, multiply in the gland cistern and reach the alveoli. The inflammatory phenomena (i.e., mastitis) accompanying the infection may be visible or not (Radostits et al. 2007).
Mammary gland protection involves the innate and adaptive immune response (Leitner et al. 2000), which work together. The innate immune response consists of passive defense mechanisms, such as the physical barriers of the teat canal, and active, formed by the resident cells in the mammary gland (i.e., cellular component) and some factors soluble (i.e., humorous component) (Sordillo and Streicher 2002; Rainard and Riollet 2006; Sladek and Rysanek 2010).
The teat canal is the main barrier against bacterial infections (Zecconi et al. 2000; Sudhan and Sharma 2010; Ezzat Alnakip et al. 2014). The teat sphincter, consisting of smooth and elastic muscle cells, ensures the closure of the teat canal among milking, constituting an anatomical barrier to the entry of pathogens. Similarly, in the proximal part of the teat canal (Fasulkov et al. 2014; Vesterinen et al. 2015), the folds in the mucosa of the Fürstenberg rosette also play the same protective role similar to the reported in cows (Hibbitt et al. 1996; Ezzat Alnakip et al. 2014). The elimination of foreign agents is further favored by the downward flow of milk during milking and by the shedding of keratinized epithelial cells from the canal.
Keratin has the ability to bind to bacteria and is composed of substances with bacteriostatic properties that prevent bacterial multiplication (Capuco et al. 1992; Paape and Capuco 1997). Its constant renewal at each milking allows the elimination of the bacteria that keratin agglutinates. Similarly, continuous renewal of epithelial cells may also play an important role in defense against invading agents (Leitner et al. 2012).
Cellular defense is ensured by leukocytes, which vary according to the SCC apparently with the threshold at 700,000 cells/ml (Albenzio et al. 2015), and epithelial cells of the mammary gland (Table 18.2). Leukocytes include not only polymorphonuclear leukocytes (mainly neutrophils in goat milk , but also some eosinophils and basophils), monocytes/macrophages, natural killer (NK) cells, and dendritic cells (Paape and Capuco 1997; Oviedo-Boyso et al. 2006; Sladek and Rysanek 2010). In the mammary gland , recognition of foreign agents is performed by resident macrophages and epithelial cells, due to the presence of lipopolysaccharides, peptidoglycans, or lipoteichoic acid in the bacterial cell wall, for which these cells have receptors (Oviedo-Boyso et al. 2006).
The epithelial cells of the mammary gland have the ability to phagocyte and produce cytokines, behaving like macrophages (Monks et al. 2002; Atabai et al. 2007; Monks and Henson 2009) playing a central role in the proinflammatory response by secreting chemotactic factors (e.g., IL-8) and several acute phase proteins, such as serum amyloid A3 (SAA3), pentraxin 3 (PTX3), and antiproteinase alpha-1 (SERPINA1) (Brenaut et al. 2014). Thus, after coming into contact with the pathogens, epithelial cells, and macrophages produce proinflammatory cytokines (i.e., IL-8 signaling pathway) that promote the mobilization of neutrophils from the bloodstream to the mammary gland (Leitner et al. 2000; Sladek and Rysanek 2010; Brenaut et al. 2014) through the activation of intercellular and vascular adhesion molecules of the endothelial cells. These adhesion molecules promote neutrophil extravasation and migration (i.e., diapedesis) through the vascular endothelium to the mammary gland (Paape et al. 2002; Oviedo-Boyso et al. 2006). After that, the receptors present on neutrophils recognize the molecular pattern associated with the pathogen and begin the process of phagocytosis (Blagitz et al. 2011). Neutrophils (Fig. 18.3) contribute to contain bacterial invasion by phagocytizing the pathogens and by releasing compounds with high oxidative capacity (e.g., reactive oxygen species: ROS) (Paape et al. 2003; Rinaldi et al. 2007). The phagocytic and bactericidal properties of neutrophils are the main infection control element in the mammary gland (Fetherson et al. 2001).
In addition, due to several inflammatory mediators (Le Maréchal et al. 2011), the intervention of T and B lymphocytes and macrophages occurs, but neutrophils are always maintained as the most representative cell line. Macrophages, in addition to their phagocytic capacity, play a key role in IMI, through the secretion of a wide range of cytokines, such as interleukins (IL) 1β, IL-6, IL-8, tumor necrosis factor α (TNFα), and interferon γ (IFNγ), which induce the acute phase response and will successively attract more neutrophils into the mammary gland until resolution of the infection (Oviedo-Boyso et al. 2006; Rainard and Riollet 2006).
Neutrophils become 50–70% of the SCC in normal goat milk, in contrast to only 5–20% of the SCC in normal cow milk (Dulin et al. 1983; Poutrel and Lerondelle 1983). However, the number of neutrophils migrating to the healthy mammary glands is small compared to those that migrate in the case of an IMI (Paape et al. 2002, 2003). The massive recruitment of neutrophils in the udder leads to a marked increase in somatic cells in milk (Kehrli and Shuster 1994; Table 18.3) which support the use of this method for the diagnosis of subclinical mastitis in ruminants (Kehrli and Shuster 1994; Bergonier et al. 2003).
Therefore, IMI changes the number of leukocytes in milk . However, the response is not uniform: the bacterial species involved and the duration of infection play an important role in defining the percentage in which leukocytes are present in milk during mastitis (Leitner et al. 2012). The localization of the leukocyte populations is variable; the neutrophils are in a greater quantity in the milk, and the lymphocytes and macrophages in the tissues (Leitner et al. 2003). The vascular changes seen in this process are responsible for the cardinal signs of inflammation: increased vascular permeability, vasodilatation, and increased vascular flow (i.e., pain, heat, redness, and edema) and decreased milk synthesis capacity of the mammary gland (i.e., loss of function); this latter due to the combined action of bacterial toxins and inflammatory mediators that damage milk -producing acini cells (Oviedo-Boyso et al. 2006).
An effective innate immune response can quickly eliminate invading pathogens without major quantitative and qualitative changes in milk (Sordillo and Streicher 2002; Baumert et al. 2009). After removing pathogens, neutrophils undergo apoptosis and are phagocytized by macrophages, leading to resolution of the inflammatory process (Baumert et al. 2009; Sladek and Rysanek 2010). However, the significant increase of neutrophils in the mammary gland due to the infection can result in a large amount of neutrophils in apoptosis and exceed the phagocytic capacity of macrophages, leading to increased lysis, and necrosis of resident neutrophils (Paape and Capuco 1997; Kobayashi et al. 2003; Albenzio and Caroprese 2011). This may lead to the release of cytotoxic granules and oxygen free radicals that will damage the surrounding tissue and convert the matrix proteins into chemotactic factors that amplify the inflammatory process, attracting more cells (Paape and Capuco 1997; Paape et al. 2002; Kobayashi et al. 2003; Sladek and Rysanek 2006). The importance of this phenomenon is based on several factors: (a) the type of apocrine secretion, and the physiologically high SCC; (b) the fact that neutrophils are the most common cell population in goats’ milk (Paape and Capuco 1997; Paape et al. 2001; Bergonier et al. 2003; Tian et al. 2005; Gomes et al. 2006; Madureira and Gomes 2010); and, (c) the 30% of milk neutrophils that undergo apoptosis or necrosis in milk with low SCC (<300 × 103 cells/ml) (Tian et al. 2005).
Nevertheless, the ejection of milk during milking contributes to a constant supply of neutrophils to the gland and allows the removal of dead neutrophils, avoiding the release of toxic substances in the mammary parenchyma. In addition, frequent milking during clinical mastitis seems to favor the proper functioning of the immune system (Paape and Capuco 1997). The efficacy of neutrophils and macrophages in milk is decreased by ingestion of fat globules and casein particles (Paape and Wergin 1977; Cooray 1996; Amorena and Perez 1998; Tian et al. 2005). This fact, together with specific evasion mechanisms of some bacteria, can justify the persistence of some mastitis outbreaks.
Finally, the presence of caprine arthritis encephalitis virus in farms should be taken into account. According to Kaba et al. (2012), goats infected by this lentivirus only show a small decrease in total protein (0.05%), fat (0.15%), and lactose (0.05%), without significant yield milk variation. A decrease of 4.6 g per 1 kg milk from infected goats was observed after manufacturing fresh cheese (Nowicka et al. 2015) in accordance with the previous study.
There are some evidence that caprine arthritis encephalitis virus improves the SCC (Ryan et al. 1993; Nord and Adnøy 1997; Sánchez et al. 2001; Turin et al. 2005) but this response is not consensual (Leitner et al. 2010; Kaba et al. 2012) or can interact with other milk pathogens (Sánchez et al. 1998, 2001; Martínez 2000). The virus replication takes place in macrophages, at the low number in milk during the normal lactation, which increases in infected goats (Lerondelle et al. 1995). However, the SCC increase appears to be similar to an IMI caused by SCN (Ryan et al. 1993; Paape et al. 2007) and apparently nonadditive effects are observed (Sánchez et al. 1998, 2001).
5 Concluding Remarks
A “normal” milk flora mainly constituted by nonpathogenic environmental agents and commensal bacteria from the skin is essential for a goat udder health and for the cheese industry. For a correct cheese processing, the milk should be originated from goat farms with low IMI prevalence. Especial attention should be given to milk pathogens provoking a high inflammatory response as well as potential toxins production (e.g., S. aureus and E. coli.). Moreover, systemic pathogens with tropism for mammary gland , normally in endemic diseases, also can play a significant role.
Polymorphonuclear leukocytes (neutrophils) represents the major part of total leucocytes, even in noninfected mammary glands; and assume a great importance in cellular response to milk pathogens. However, little information about the key role of these cells in the caprine mastitis has been found. In consequence, further researches about leucocyte distribution and their relation with other inflammatory mediators are needed for a better understanding of the response to IMI in goats.
References
Albenzio M, Caroprese M (2011) Differential leukocyte count for ewe milk with low and high somatic cell count. J Dairy Res 78:43–48
Albenzio M, Santillo A, Kelly AL et al (2015) Activities of indigenous proteolytic enzymes in caprine milk of different somatic cell counts. J Dairy Sci 98(11):7587–7594
Álvarez-Suárez ME, Otero A, García-López ML et al (2015) Microbiological examination of bulk tank goat’s milk in the Castilla y León region in Northern Spain. J Food Prot 78(12):2227–2232
Amorena B, Perez M (1998) Dinamica molecular y celular en la defensa inmune de la glandula mamária caprina. Ovis 54:69–82
Amores J, Sánchez A, Gómez-Martín A et al (2012) Surveillance of Mycoplasma agalactiae and Mycoplasma mycoides subsp. capri in dairy goat herds. Small Rumin Res 102:89–93
Andrews RJ, Kitchen BJ, Kwee WS et al (1983) Relationship between individual cow somatic cell counts and the mastitis infection status of the udder. Aust J Dairy Technol 38:71–74
Atabai K, Sheppard D, Werb Z (2007) Roles of the innate immune system in mammary gland remodeling during involution. J. Mammary Gland Biol 12:37–45
Bagnicka E, Winnicka A, Jóźwik A et al (2011) Relationship between somatic cell count and bacterial pathogens in goat milk. Small Rumin Res 100(1):72–77
Baumert A, Bruckmaier RM, Wellnitz O (2009) Cell population, viability, and some key immunomodulatory molecules in different milk somatic cell samples in dairy cows. J Dairy Res 76(3):356–364
Bazan R, Cervantes E, Salas G et al (2009) Prevalencia de mastitis subclínicas en cabras lecheras en Michoacán. México Revista Científica 19(4):334–338
Bergonier D, Berthelot X (2008) Mycoplasmoses des petits ruminants: le syndrome de l’agalactie contagieuse. Bull Acad Vét Fr 161(2):167–177
Bergonier D, Blanc M-C, Fleury P et al (1997) Les mammites des ovins et des caprins laitiers: étiologie, épidémiologie, contrôle. Renc Rech Rum 4:251–260
Bergonier D, de Crémoux R, Rupp R et al (2003) Mastitis of dairy small ruminants. Vet Res 34:689–716
Blagitz MG, Souza FN, Gomes V et al (2011) Apoptosis and necrosis of polymorphonuclear leukocytes in goat milk with high and low somatic cell counts. Small Rumin Res 100:67–71
Boutinaud M, Jammes H (2002) Potential uses of milk epithelial cells: a review. Reprod Nutr Dev 42 (2):133–147
Brenaut P, Lefèvre L, Rau A et al (2014) Contribution of mammary epithelial cells to the immune response during early stages of a bacterial infection to Staphylococcus aureus. Vet Res 45:16. https://doi.org/10.1186/1297-9716-45-16
Capuco AV, Bright SA, Pankey JW et al (1992) Increased susceptibility to intramammary infection following removal of teat canal keratin. J Dairy Sci 75:2126–2130
Chu C, Yu C, Lee Y et al (2012) Genetically divergent methicillin-resistant Staphylococcus aureus and sec-dependent mastitis of dairy goats in Taiwan. BMC Vet Res 8:39. https://doi.org/10.1186/1746-6148-8-39
Contreras A, Corrales JC, Sierra D et al (1995) Prevalence and aetiology of non-clinical intramammary infection in Murciano-Granadina goats. Small Rumin Res 17:71–78
Contreras A, Corrales JC, Sanchez A et al (1997a) Persistence of subclinical intramammary pathogens in goats throughout lactation. J Dairy Sci 80(11):2815–2819
Contreras A, Sanchez A, Corrales J, et al (1997b) Concepto e importância de las mamitis caprinas. In: Mamitis caprina, Ovis (España), No. 53 (Mamitis Caprina I), pp 11–31
Contreras A, Paape MJ, Miller RH (1999) Prevalence of subclinical intramammary infection caused by Staphylococcus epidermidis in a commercial dairy goat herd. Small Rum Res 31:203–208
Contreras A, Luengo C, Sánchez A et al (2003) The role of intramammary pathogens in dairy goats. Livestock Prod Sci 79:273–283
Contreras A, Sierra D, Sánchez A et al (2007) Mastitis in small ruminants. Small Rum Res 68:145–153
Cooray R (1996) Casein effects on the myeloperoxidase-mediated oxygen-dependent bactericidal activity of bovine neutrophils. Vet Immunol Immunopathol 51(1–2):55–65
Corrales J, Contreras A., Sanchez, et al (1997) Etiologia y diagnostico microbiologico de las mamitis caprinas. Ovis 53:33–65
Cortimiglia C, Bianchini V, Franco A et al (2015) Short communication: prevalence of Staphylococcus aureus and methicillin-resistant S. aureus in bulk tank milk from dairy goat farms in Northern Italy. J Dairy Sci 98(4):2307–2311
Dinges MM, Orwin PM, Schlievert PM (2000) Exotoxins of Staphylococcus aureus. Clin Microbiol Rev 13(1):16–34
Doğruer G, Mk Saribay, Aslantaş O et al (2016) The prevalance, etiology and antimicrobial susceptibility of the microorganisms in subclinical mastitis in goats. Atatürk Üniversitesi Vet Bil Derg 11(2):138–145
Dore S, Liciardi M, Amatiste S et al (2016) Survey on small ruminant bacterial mastitis in Italy, 2013–2014. Small Rum Res 141:91–93
Dulin AM, Paape MJ, Schultze WD et al (1983) Effect of parity, stage of lactation, and intramammary infection on concentration of somatic cells and cytoplasmic particles in goat milk. J Dairy Sci 66:2426–2433
Ezzat Alnakip M, Quintela-Baluja M, Böhme K et al (2014) The immunology of mammary gland of dairy ruminants between healthy and inflammatory conditions. J Vet Med 2014:659801. https://doi.org/10.1155/2014/659801
Fasulkov M, Karadaev M, Djabirova M (2014) Ultrasound measurements of teat structures in goats. Revue Méd Vét 165(5–6):188–192
Fetherson CM, Lee C, Hartmann PE (2001) Mammary gland defense: the role of colostrums, milk and involution secretion. Adv Nutr Res 10(8):167–198
Gelasakis AI, Angelidis AS, Giannakou R et al (2016) Bacterial subclinical mastitis and its effect on milk yield in low-input dairy goat herds. J Dairy Sci 99(5):3698–3708
Göçmen H, Rosales RS, Ayling RD et al (2016) Comparison of PCR tests for the detection of Mycoplasma agalactiae in sheep and goats. Turk J Vet Anim Sci 40:421–427
Gomes V, Libera AM, Paiva M et al (2006) Effect of the stage of lactation on somatic cell counts in healthy goats (Caprae hircus) breed in Brazil. Small Rumin Res 64(1–2):30–34
Gonzalo C, Ariznabarreta A, Tardáguila JA et al (1998) Factores infecciosos de variación del recuento celular de la leche de oveja. Ovis 56:27–34
Hibbitt KG, Craven N, Batten H (1996) Anatomy, physiology and immunology of theudder. In: Andrews AH, Blowey RH, Boyd H, et al (eds) Bovine medicine: diseases and husbandry of cattle. Blackwell, Oxford, pp 273–278
Ilhan Z, Eking IH, Koltas S et al (2016) Occurrence of fungal agents in mastitis in dairy goats. J Anım Plant Scı 29(3):4691–4700
Jans C, Merz A, Johler S et al (2017) East and West African milk products are reservoirs for human and livestock-associated Staphylococcus aureus. Food Microbiol 65:64–73
Johler S, Giannini P, Jermini M et al (2015) Further evidence for staphylococcal food poisoning outbreaks caused by egc-encoded enterotoxins. Toxins (Basel) 7(3):997–1004
Kaba J, Strzałkowska N, Jóźwik A et al (2012) Twelve-year cohort study on the influence of caprine arthritis-encephalitis virus infection on milk yield and composition. J Dairy Sci 95(4):1617–1622
Kalogridou-Vassiliadou D (1991) Mastitis-related pathogens in goat milk. Small Rum Res 4(2):203–212
Kehrli ME, Shuster DE (1994) Factors affecting milk somatic cells and their role in health of the bovine mammary gland. J Dairy Sci 77:619–627
Kobayashi SD, Voyich JM, DeLeo FR (2003) Regulation of the neutrophil-mediated inflammatory response to infection. Microbes Infect 5(14):1337–1344
Koltas S, Ilhan Z (2016) Isolation of some aerobic bacteria and Mycoplasma spp. Van Vet J 27(2):74–78
Le Loir Y, Baron F, Gautier M (2003) Staphylococcus aureus and food poisoning. Genet Mol Res 312(1):63–76
Le Maréchal C, Thiéry R, Vautor E et al (2011) Mastitis impact on technological properties of milk and quality of milk products—a review. Dairy Sci Techno 91:247–282
Leitner G, Shoshani E, Krifucks O et al (2000) Milk leukocyte population patterns in bovine udder infections of different aetiology. J Vet Med B Infect Dis Vet Public Health 47(8):581–589
Leitner G, Eligulashvily R, Krifucks O et al (2003) Immune cell differentiation in mammary gland tissues and milk of cows chronically infected with Staphylococcus aureus. J Vet Med B Infect Dis Vet Public Health 50(1):45–52
Leitner G, Merin U, Lavi Y et al (2007) Aetiology of intramammary infection and its effect on milk composition in goat flocks. J Dairy Res 74(2):186–193
Leitner G, Krifucks O, Weisblit L et al (2010) The effect of caprine arthritis encephalitis virus infection on production in goats. Vet J 183:328–331
Leitner G, Merin U, Krifucks O et al (2012) Effects of intra-mammary bacterial infection with coagulase negative staphylococci and stage of lactation on shedding of epithelial cells and infiltration of leukocytes into milk: comparison among cows, goats and sheep. Vet Immunol Immunopathol 147(3–4):202–210
Lerondelle C, Greenland T, Jane M, Mornex JF (1995) Infection of lactating goats by mammary instillation of cell-borne caprine arthritis-encephalitis virus. J Dairy Sci 78:850–855
Li Z, Wright A-DG, Yang Y et al (2017) Unique bacteria community composition and co-occurrence in the milk of different ruminants. Sci Rep 7:40950. https://doi.org/10.1038/srep40950
Madureira KM, Gomes V (2010) Total and differential leukocyte counts in the milk of healthy goats, using methyl green pyronin stain and cytocentrifugation. Arquivos do Instituto Biológico 77:343–347
Maisi P, Riipinen I (1991) Pathogenicity of different species of staphylococci in caprine udder. Br Vet J 147:126–132
Martínez B (2000) El recuento de células somáticas en la leche de cabra, factores de variación y efecto sobre la producción y composición de la leche. Universidad Politécnica de Valencia, Spain, Tesis doctoral, p 307
Matthews JG (2009) Diseases of the goat, 3rd edn. Wiley-Blackwell, pp 213–235
McInnis EA, Kalanetra KM, Mills DA et al (2015) Analysis of raw goat milk microbiota: impact of stage of lactation and lysozyme on microbial diversity. Food Microbiol 46:121–131
Merin U, Silanikove N, Shapiro F et al (2004) Changes in milk composition as affected by subclinical mastitis in sheep and goats. S Afr J Anım Scı 34(5):188–191
Merz A, Stephan R, Johler S (2016) Staphylococcus aureus isolates from goat and sheep milk seem to be closely related and differ from isolates detected from bovine milk. Front Microbiol 7:319. https://doi.org/10.3389/fmicb.2016.00319
Monks J, Henson PM (2009) Differentiation of the mammary epithelial cell during involution: implications for breast cancer. J Mammary Gland Biol 14:159–170
Monks J, Geske FJ, Lehman L et al (2002) Do inflammatory cells participate in mammary gland involution? J Mammary Gland Biol 7:163–176
Muehlherr JE, Zweifel C, Corti S et al (2003) Microbiological quality of raw goat’s and ewe’s bulk-tank milk in Switzerland. J Dairy Sci 86(12):3849–3856
Nord K, Adnøy T (1997). Effects of infection by caprine arthritis-encephalitis virus on milk production of goats. J Dairy Sci 80(10):2391–2397
Nowicka D, Czopowicz M, Bagnicka E et al (2015) Influence of small ruminant lentivirus infection on cheese yield in goats. J Dairy Res 82(1):102–106
Oliver S, Boor K, Murphy SC, Murinda SE (2009) Food safety hazards associated with consumption of raw milk. Foodborne Pathog. Dis. 6:793–806
Oviedo-Boyso J, Valdez-Alarcón JJ, Cajero-Juárez M et al (2006) Innate immune response of bovine mammary gland to pathogenic bacteria responsible for mastitis. J Infect 54:399–409
Paape MJ, Capuco AV (1997) Cellular defense mechanisms in the udder and lactation of goats. J Anim Sci 75(2):556–565
Paape MJ, Wergin WP (1977) The leukocyte as a defense mechanism. J Am Vet Med Assoc 170(10 Pt 2):1214–1223
Paape MJ, Bannerman DD, Zhao X et al (2003) The bovine neutrophil: structure and function in blood and milk. Vet Res 34:597–627
Paape MJ, Mehrzad J, Zhao X et al (2002) Defense of the bovine mammary gland by polymorphonuclear neutrophil leukocytes. J Mammary Gland Biol 7:109–121
Paape MJ, Poutrel B, Contreras A, Marco JC, Capuco AV (2001) Milk somatic cells and lactation in small ruminants. J Dairy Sci 84:237–244
Paape MJ, Shafer-Weaver K, Capuco AV, et al. (2000) Immune surveillance of mammary gland secretion during lactation. Adv Exp Med Biol 480:259–277
Paape MJ, Wiggans GR, Bannerman DD, Thomas DL, Sanders AH, Contreras A, Moroni P, Miller RH (2007) Monitoring goat and sheep milk somatic cell counts. Small Ruminant Res 68(1–2):114–125
Amores J, Gómez-Martín A, Paterna, A, et al (2012a) Evaluation of PCR and culture for Mycoplasma agalactiae detection in fresh mastitic goat samples. In: Proceedings of 19th Congress of the International Organization for Mycoplasmology, Toulouse, 15–20 July
Persson Y, Järnberg A, Humblot P et al (2015) Associations between Staphylococcus aureus intramammary infections and somatic cell counts in dairy goat herds. Small Rum Res 133:62–66
Plummer P, Plummer C (2012) Diseases of the mammary gland. In: Pugh D, Baird A (eds) Sheep and Goat Medicine. Elsevier Saunders, Missouri, pp 442–465
Poutrel B (1983) La sensibilité aux mammites: revue des facteurs liés à la vache. Ann Rech Vét 14(1):89–104
Poutrel B (1984) Udder infection of goats by coagulase-negative staphylococci. Vet Microbiol 9(2):131–137
Poutrel B, Lerondelle C (1983) Cell content of goat milk: California mastitis test, coulter counter, fossomatic for predicting half infection. J Dairy Sci 66:2575–2579
Radostits OM, Gay CC, Blood DC, et al (2007) Veterinary medicine—a textbook of the diseases of cattle, sheep, pigs, goats and horses, 10th edn. W. B. Saunders, pp. 603–700
Rainard P, Riollet C (2006) Innate immunity of the bovine mammary gland. Vet Res 37(3):369–400
Rinaldi M, Moroni P, Paape MJ et al (2007) Evaluation of assays for the measurement of bovine neutrophil ROS. Vet Immunol Immunopathol 115(1–2):107–125
Rovai M, Caja G, Salama A et al (2014) Identifying the major bacteria causing intramammary infections in individual milk samples of sheep and goats using traditional bacteria culturing and real-time polymerase chain reaction. J Dairy Sci 97:5393–5400
Ryan DP, Greenwood PL, Nicholls PJ (1993) Effect of caprine arthritis-encephalitis virus infection on milk cell count and N-acetyl-beta-glucosaminidase activity in dairy goats. J Dairy Res 60(3):299–306
Sánchez A, Corrales JC, Marco J et al (1998) Aplicacion del recuento de células somáticas para el control de las mastitis caprinas. Ovis (Mamitis caprina II) 54:37–52
Sánchez A, Contreras A, Corrales JC et al (2001) Relationships between infection with caprine arthritis encephalitis virus, intramammary bacterial infection and somatic cell counts in dairy goats. Vet Rec 148(23):711–714
Scaccabarozzi L, Leoni L, Ballarini A et al (2015) Pseudomonas aeruginosa in dairy goats: genotypic and phenotypic comparison of intramammary and environmental isolates. PLoS ONE 10(11):e0142973. https://doi.org/10.1371/journal.pone.0142973
Sladek Z, Rysanek D (2006) The role of CD14 during resolution of experimentally induced Staphylococcus aureus and Streptococcus uberis mastitis. Comp Immunol Microbiol Infect Dis 29(4):243–262
Sladek Z, Rysanek D (2010) Apoptosis of resident and inflammatory macrophages before and during the inflammatory response of the virgin bovine mammary gland. Acta Vet Scand 52:12. https://doi.org/10.1186/1751-0147-52-12
Smith MC, Sherman DM (2009) Mammary gland and milk production. In: Goat Medicine, 2nd edn. Wiley-Blackwell, pp 647–679
Sordillo LM, Streicher KL (2002) Mammary gland immunity and mastitis susceptibility. J Mammary Gland Biol Neoplasia 7(2):135–146
Stehling R, Vargas O, Santos E et al (1986) Evolution of caprine mastitis induced with staphylococcal and steptococcal enterotoxin. Arq Bras Med Vet Zootec 38(5):701–717
Sudhan N, Sharma NG (2010) Mastitis—an important production disease of dairy animals. In: Sarva Manav Vikash Samiti, Gurgoan, pp 72–88
Tariba B, Kostelić A, Roić B et al (2017) Caprine arthritis encephalitis virus infection and milk production. Mljekarstvo 67(1):42–48
Tian SZ, Chang CJ, Chiang CC et al (2005) Comparison of morphology, viability, and function between blood and milk neutrophils from peak lactating goats. Can J Vet Res 69(1):39–45
Tormo H, Ali Haimoud-Lekhal D, Laithier C (2006) Les microflores utiles des laits crus de vache et de chèvre: principaux réservoirs et impact de certaines pratiques d’élevage. Renc Rech Rum 13:305–308
Tormo H, Ali Haimoud-Lekhal D, Lopez C (2007) Flore microbienne des laits crus de chèvre destinés à la transformation fromagère et pratiques des producteurs. Renc Rech Rum 14:87–90
Turin L, Pisoni G, Giannino ML et al (2005) Correlation between milk parameters en CAEV seropositive and negative primiparous goats during an eradication program in italian farm. Small Rum Res 57:73–79
Vega S, Martínez López B, Orden JA et al (2004) Prevalencia y etiología de las mamitis subclínicas en el ganado caprino lechero de la Comunidad Valenciana. Laborarorio Avedila 30:2–11
Vesterinen HM, Corfe IJ, Sinkkonen V et al (2015) Teat morphology characterization with 3D imaging. Anat Rec (Hoboken) 298(7):1359–1366
Wahba NM, Elnisr NAG, Saad MN, et al (2011) Incidence of Nocardia species in raw milk collected from different localities of Assiut City of Egypt. Vet World 4(5):201–204
White LJ, Schukken YH, Lam TJG et al (2001) A multispecies model for the transmission and control of mastitis in dairy cows. Epidemiol Infect 127:567–576
Zecconi A, Hamann J, Bronzo V et al (2000) Relationship between teat tissue immune defences and intramammary infections. Adv Exp Med Biol 480:287–293
Zhao Y, Liu H, Zhao X et al (2015) Prevalence and pathogens of subclinical mastitis in dairy goats in China. Trop Anim Health Prod 47(2):429–435
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Quintas, H., Margatho, G., Rodríguez-Estévez, V., Simões, J. (2017). Understanding Mastitis in Goats (I): Etiopathophysiological Particularities. In: Simões, J., Gutiérrez, C. (eds) Sustainable Goat Production in Adverse Environments: Volume I. Springer, Cham. https://doi.org/10.1007/978-3-319-71855-2_18
Download citation
DOI: https://doi.org/10.1007/978-3-319-71855-2_18
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-71854-5
Online ISBN: 978-3-319-71855-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)