Abstract
Males of the solitary bee Amegilla (Asarapoda) paracalva employ two mate-locating tactics: aggressive defense of sites from which virgin females are emerging and patrolling flower patches that are visited by conspecific females. At one study site, a single male was able to control an entire emergence area for one or more days. Multiple males patrolled one flower patch, interacting aggressively on occasion but no one individual was able to monopolize this resource. Territorial males at the emergence site secured mates by waiting by tunnels for receptive virgin females to emerge after metamorphosis. Males patrolling the flower patch pounced upon flower visiting conspecifics and mated with receptive females there. Territorial males at the emergence site were larger than average individuals, probably because of the advantage larger males have when grappling with opponents. Flower patrolling males were smaller than territorial males at the emergence sites, perhaps because of the advantages gained by these males from rapid, agile flight.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Studies of solitary bees have contributed to an understanding of the role of sexual selection in the evolution of male mating tactics (Alcock et al. 1978; Paxton 2005). This work has helped confirm the value of Emlen and Oring’s (1977) conceptual framework of mating system evolution. Emlen and Oring argued that the diversity in male mating behaviour was a function of ecological differences among species that affect the distribution of potential mates. So, for example, when emerging receptive females are clustered spatially, as they are in some species of solitary bees that nest independently in large aggregations, males are expected to evolve a female defense strategy.
One bee species with such a mating system is Amegilla dawsoni (Rayment), an apid whose females often nest in large, dense aggregations, producing daughters that mate once soon after emerging; as predicted, males search for and find emerging females, which they guard in order to mate with as soon as they leave their emergence burrow (Houston 1991; Alcock 1996a, b). Once mated, females become unreceptive, which places a premium on finding and controlling emerging females. This factor in turn favors males capable of winning fights for females, which occur regularly within the emergence area (Alcock 1997). The intense wrestling combat associated with battles for emerging females has apparently led to the evolution of an unusually large male size class such that some males are larger than the largest females (Alcock 1996b). In the Hymenoptera, females are typically considerably larger than males (Stubblefield and Seger 1994), a pattern that applies to insects generally, and especially to those species (such as A. dawsoni) that are relatively large (Teder and Tammaru 2005). Yet despite the apparent reproductive advantage secured by the larger males of Dawson’s burrowing bees, nesting females produce two size classes of sons and the smaller (minor) sons greatly outnumber those in the larger (major) size class (Tomkins et al. 2001).
This puzzling phenomenon has yet to be fully explained, although female provisioning tactics appear to be affected by declining foraging success as the nesting season progresses. Over time, females require longer and longer foraging trips to secure full loads of nectar and pollen, and this change is correlated with an increasing probability that females will switch from making large offspring to the production of small sons (Alcock et al. 2005). Similar shifts in provisioning behaviour occur in other solitary bees (Seidelmann 2006).
Another species of Amegilla, A. (Asarapoda) paracalva Brooks, occurs in the same regions of Western Australia as A. dawsoni and indeed sometimes nests in the same places as its congener at more or less the same time of the year (Houston 1991). A. paracalva shares a number of other features with A. dawsoni: the species is univoltine with winter emergence and nesting in Western Australia; females form compact nesting aggregations; the bee utilizes pollen and nectar from a variety of flowering plants (Houston 1991). Our preliminary observations have, however, indicated that the male size dimorphism found in A. dawsoni is absent in A. paracalva. Because a study of A. paracalva could provide comparative data relevant to the puzzles associated with the unusual features of morphology and behaviour in A. dawsoni, we present information here on the natural history of A. paracalva, which expands a brief account (Houston 1991) on the nesting behaviour of the bee.
Methods
The study was conducted on 12 days from 10 to 22 July 2008 at the Kennedy Range National Park (latitude: 24° 34′ 45″ S; longitude: 115° 02′ 59″ E; altitude: 289 m), roughly 150 km east of Carnarvon, W.A. In previous years, females of A. paracalva have nested on the edge of a large clay pan just to the right of the access road to the camping area in the park, and about 1 km from the eastern edge of the cliffs that are the dominant feature of the park. In 2008, males and females emerged from nesting sites used the preceding year (Fig. 1). At the time the study was initiated in 2008, a substantial number of bees had already emerged, judging from the several dozen emergence holes observed there on 10 July. Subsequently, the site was monitored with the goal of capturing and weighing additional emerging bees, which were found by scanning the area at regular intervals in a search for individuals appearing near the surface in either new or previously constructed emergence tunnels. When an about-to-emerge bee was detected by virtue of the small, irregular opening that the emerging bee made as it began to gnaw a circular opening in the soil, a vial was placed over the burrow exit. When the bee crawled out, it was captured in the vial and taken to a portable Ohaus scale. There the vial and bee were weighed with the weight of the vial subtracted to yield the weight of the bee, which was subsequently marked on the dorsum of the thorax with a DecoColor paint pen (if it was a male) and released. Females were released unmarked after being weighed. The weights of bees gained in this manner provide evidence on the nature of any size dimorphisms within and between the sexes. (In A. dawsoni, body weights are highly correlated with head-widths, a standard measure of body size (Alcock 1996a)).
In addition, some males of A. paracalva seen at both emergence areas, and at patches of rough bluebells (Trichodesma zeylanicum (Burm. f.) R. Br.; Boraginaceae), were captured in an insect net, weighed, given distinctive paint marks and released. The behaviour of known males was monitored with respect to interactions with emerging or flower-visiting females as well as interactions with conspecific males. In addition, the duration of mate-searching activity by identifiable males was recorded while monitoring the emergence site from the early morning before bees began to exit until the early afternoon when no emerging bees had been seen for 30 min or more. On 21 July at the bluebells site, a period that was centered around midday was divided into 52 five-minute observation blocks. During each block, a record was made of the identity of any marked males seen at this location. Subsequently, each marked male’s presence at the flowers was measured in terms of the total number of five-minute blocks during which that individual was seen on that day.
All means are presented ±1 S.D.
Results
Adult Emergence in A. paracalva
As the first adult bees emerge from their underground brood pots, they gnaw their way up to the surface creating an exit burrow; as the season progresses, other bees make their way into existing tunnels before crawling out onto the surface. Two emergence areas on the southern edge of the large clay pan contained more than 200 and 100 exit burrows, respectively, by the end of the study. The two sites were approximately 100 m apart.
On any given day, males tended to emerge in the late morning whereas females tended to emerge in the early afternoon. Over the course of the study, only 25 of 96 males for which time of emergence was recorded came out after 12:30 PM; in contrast, 55 of 76 emerging females left their exit tunnels after 12:30 PM (Chi square = 36.6; P < 0.001). The delay in emergence of the females cannot be explained in terms of an increase in developmental time required for body growth by the larger sex because both males and females completed development months before their emergence, given the univoltine life cycle of this species.
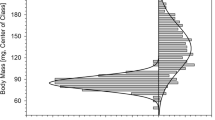
Emerging males weighed less on average than emerging females (mean weights = 0.17 ± 0.03 g vs. 0.25 ± 0.03 g; t = 16.9, df = 163; P < 0.001). In addition, the largest males (up to 0.25 g) were smaller than the largest females (up to 0.32 g). Moreover, there was no indication that males exist as two size classes (Fig. 2).
Male Behaviour at Emergence Sites
Some adult males patrolled the emergence areas during the middle of the day. Patrolling behaviour consisted of low flights over the emergence area with many short stops at emergence holes. The bees often followed much the same circular route roughly 4 to 8 m in length over a portion of the site (or all of it). Typically one male dominated a given site in terms of being seen there for a much longer total time per day than any other individual. Of the eight detailed daily records of patrolling activity made at sites 1 and 2 over the course of the study, the total patrolling time recorded for the most consistent patroller on a given day ranged from 51 to 172 min (mean = 99 ± 46 min, n = records from five different males). No other male was seen at a site during these days for more than 27 min (mean = 10 ± 7 min). All territorial residents returned to their patrolling sites over 2 or more days with one individual seen on 10 days of the study; some displaced residents continued to visit one or both of the emergence sites after they had lost their territory to a newcomer.
The rarity of joint patrolling of an emergence area stemmed from the aggressive response of the main patroller to any visitors, which were quickly detected and rapidly pursued. Fifty-two such chases were recorded over the course of the study; in all but two instances, the male that returned to patrol a site was the previous resident patroller. On four occasions, a resident struck a visitor in midair with such force that they both fell to the ground where they grappled vigorously for a few seconds before the visitor broke free and flew off. Thus, the main patroller appeared to be highly territorial, a conclusion supported by frequent observations of the bees chasing passing wasps and butterflies as well as conspecific males. Furthermore, as is true for many insects with territorial males, resident patrollers were larger on average (mean = 0.20 ± 0.03 g) than males in the general population as judged from the sample of emerging males (mean = 0.17 ± 0.03 g) (t = 3.90, df = 108, P < 0.001).
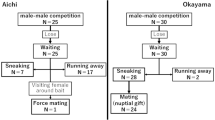
The males patrolling emergence sites were searching for emerging bees, as shown by their repeated inspection of exit burrows. When a male found a tunnel that contained a conspecific, whether male or female, he ceased patrolling for a time and waited by the opening (Fig. 3). If the emerging bee was a male (n = 11), the waiting resident sometimes permitted the emerging male to leave at once (n = 4) but more often the resident mounted this individual (n = 7) before quickly releasing him. In one case, however, a resident male briefly “courted” the emergent male by stroking the underside of its abdomen with his hindlegs.
Of the 15 records of males waiting by an exit burrow and then mounting a female as she emerged, apparent copulation ensued in 11 cases (Fig. 4), with the female quickly breaking free from the resident male in the other four interactions. Mounted males appeared to make or attempt to make genital contact immediately after mounting the female. Once contact was established, the male vigorously stroked the underside of the female’s abdomen with his large hindlegs; each movement of the hindlegs produced a brief audible “zip.” These rubbing leg movements continued even after the male had broken genital contact, although at intervals males again bent the tip of their abdomen around to touch or probe the tip of their partner’s abdomen. The female was released by the male after mounts that lasted from about 1.5 to 18 min (mean = 6.5 ± 5.4 min, n = 11).
Therefore, territorial males at the Kennedy Range emergence sites secured newly emergent females as mates. Once, a resident patroller also captured and mated a female of uncertain reproductive status as she inspected exit tunnels in the manner of a female propecting for a nest site.
The total time spent daily in sporadic episodes of patrolling by resident males was only a part of the interval between first and last sighting of males on any given day (Table 1). In fact, on average, residents were present for anywhere from 40% to 71% of the daily period that spanned the first and last sighting of males patrolling a site. (Note that male “white blue” patrolled both sites on July 19; his cumulative patrolling total consisted of 40% of the time males engaged in mate searching at the two emergence sites on that day.)
Moreover, patrolling residents generally appeared well before the first female to emerge, and they often disappeared for the day well before the last female to emerge. As a result, the overlap between the patrolling time and female emergence period was only partial (Table 2). Therefore, although patrolling males did occasionally find and mate with emerging females, some virgin females left the emergence area without copulating because no male was present when they emerged from the ground. Indeed in the period from 12 to 18 July, when a total of 53 females were observed emerging from site A, 26 exited from their emergence tunnel after the last patrolling male had left the site for the day. We have no explanation for this puzzling behaviour. Other unmated females included those that emerged while males were copulating with another female (on 1 day, five females emerged and departed while the resident male was engaged in a prolonged copulation at site A).
Alternative Mating Tactics in A. paracalva
It appears that a substantial proportion (on the order of 50%) of the females emerging at site A were not mated by the patrolling residents. Females that were not detected, captured and mated immediately upon emergence probably constituted a source of mates for the males patrolling small stands of flowering rough bluebells, which were visited by both sexes for nectar. On seven occasions, males grasped, mounted and stroked females on flowers (Fig. 5). Once a male captured a female perched on a shrub adjacent to some bluebells; the pair was in turn netted and when the marked female was released she was the target of five additional brief mountings.
In addition, over the course of 10 hr of observation on 3 days at one small cluster of bluebells, we saw six cases of foraging males being hit and mounted by presumptive mate-searching males. Another five records were made of aerial captures of fellow males; these may have been instances of mistaken sexual identity or they may have been aggressive encounters designed to drive competitors away. Apparent aggressive interactions among males at bluebells were not uncommon around midday on July 21, when a total of 17 male-male chases occurred, some involving 3 males simultaneously. These pursuits often led rivals to fly upward in a spiral pattern before flying off in different directions.
Despite the evident aggressive nature of male-male interactions near flowers, the mean weight (0.16 ± 0.03 g) of males (n = 15) found patrolling flower patches was significantly less than that of the males (n = 13) exhibiting emergence site territoriality (0.20 ± 0.03; t = 3.90, df = 26; P < 0.001). As was true for emergence site males, those bees patrolling flowers often returned to their patrolling site after leaving it temporarily on any given day. Five marked males that returned to the bluebell site on July 21 after having also been there on July 20 were seen during 5 (10%) to 23 (44%) of the 52 five-min censuses made during the period from 10:25 to 14:45 (mean = 13.2 of the 52 samples).
Discussion
Males of A. paracalva are smaller than conspecific females on average, as is typical for Hymenoptera, where selection for fecundity usually favors larger females more than selection for fighting ability favors larger males (Stubblefield and Seger 1994). Moreover, the size distribution of males follows the typical bell-shaped curve for organisms generally. In both respects, A. paracalva differs from A. dawsoni, a species in which male size distribution is bimodal with some males in the larger size class weighing more than any female (Alcock 1999). The larger males of A. dawsoni emerge in the latter part of the male flight season; if the same were true for A. paracalva, they would have been detected during our study, which took place after the initial phase of adult emergence.
Despite this morphological difference between the species, males of A. paracalva and A. dawsoni behave similarly in many respects. In both species, males tend to emerge before females on any given day. After becoming active adults, some males search aggressively for emerging females, attacking and driving rivals away from productive sites. In both species, some males avoid the physical combat associated with mate-acquiring at emergence sites and instead patrol flower patches in search of potential mates there (Alcock 1997). In both locations, some males exhibit site fidelity, returning to their searching area over a period of days. The alternative flower-based mating tactics of both species are a reflection of the fact that not all emerging females are immediately captured and mated, the apparent stimulus for the initiation of unreceptivity by females. Within the emergence area, males of both species detect emerging conspecifics before they have left the emergence tunnel; by waiting at the exit, they are in position to grasp and mate with the newly emerged female as soon as she leaves the tunnel. In both species, copulation follows quickly in cases in which the male captures the female firmly, followed by a period of several minutes during which the male engages in post-copulatory courtship (Simmons et al. 2000). This behaviour involves repeated stroking of the sides of the abdomen of the female with the male’s legs, producing audible rhythmic sounds.
But there are behavioural differences as well. A single male of A. paracalva is capable of keeping its competitors away from an entire emergence area whereas in A. dawsoni, dozens, hundreds, or even thousands of males often cruise over much larger emergence areas without being aggressive—until an emerging female has been discovered. When a male finds such a female, he then attempts to guard the very small area around her, and is prepared to grapple with other males if they try to gain control of this mini-territory (Alcock 1996b). The large territories of A. paracalva males almost certainly reflect the fact that would-be territory holders are confronted with a much smaller number of competitors than are mate-guarding male A. dawsoni, thanks to the much smaller number of nests in the aggregations of A. paracalva.
The nature of male aggressiveness in the two species varies in accordance with the size of the defended territory and the mobility of rival males. In A. paracalva, territorial males spot intruders when they are several meters distant and chase after them, with the newcomer usually leading his opponent on a chase of many meters. On occasion, however, the territorial male catches the intruder in flight in which case he may knock his opponent to the ground where the two bees grapple and bite one another. Rapid pursuits and midair captures of other males do not occur within the emergence area in Dawson’s burrowing bee.
The absence of a large male size class in A. paracalva may be related to the kind of physical aggression it exhibits. In this species, the importance of rapid flight, aerial agility, and the ability to strike opponents in the air may act against unusually large males both in the emergence area and at flowers where a premium applies to maneuverability. Relatively small body size has been associated with greater aerial agility in a variety of animals (Blanckenhorn 2000) ranging from sandpipers (Blomqvist et al. 1997; Székely et al. 2004) to certain dragonflies (Serrano-Meneses et al. 2008) and midges (Neems et al. 1990; Crompton et al. 2003). Therefore, females of A. paracalva that produced costly sons of exceptional size might not be rewarded for their relatively large investment in brood provisions per offspring. In contrast, the large sons of female A. dawsoni do not engage in rapid pursuits of rivals but instead use their large size to block access to an emergence hole containing a female, and also to win wrestling matches with smaller rivals.
Finally, does the natural history of A. paracalva cast light on why females of A. dawsoni produce so many minor sons when the relatively rare major males in the population seemingly have extremely high fitness? The size distribution of minor males in this species matches that of all males in A. paracalva and A. murrayensis (K. Hogendoorn, personal communication). Phylogenetic information is required to determine whether making “minor” sons is the ancestral trait while the production of major sons by A. dawsoni is a derived trait. Even if this proves to be the case, it will remain unclear why females of A. dawsoni continue to produce many more agile, rapid flying minors than large fighting majors despite the fact that minor males of this species appear to have very low reproductive success.
References
Alcock J (1996a) Provisional rejection of three alternative hypotheses on the maintenance of a size dichotomy in Dawson’s burrowing bee (Amegilla dawsoni) (Apidae, Apinae, Anthophorini). Behav Ecol Sociobiol 39:181–188
Alcock J (1996b) The relation between body size, fighting, and mating success in Dawson’s burrowing bee, Amegilla dawsoni (Apidae, Apinae, Anthophorini). J Zool 239:663–674
Alcock J (1997) Competition from large males and the alternative mating tactics of small males of Dawson’s burrowing bee (Amegilla dawsoni) (Apidae, Apinae, Anthophorini). J Insect Behav 10:99–113
Alcock J (1999) The nesting behavior of Dawson’s burrowing bee, Amegilla dawsoni (Hymenoptera: Anthophorini), and the production of offspring of different sizes. J Insect Behav 12:363–384
Alcock J, Barrows EM, Gordh G, Hubbard LJ, Kirkendall L, Pyle DW, Ponder TL, Zalom FG (1978) The ecology and evolution of male reproductive behavior in the bees and wasps. Zool J Linn Soc Lond 64:293–326
Alcock J, Simmons LW, Beveridge M (2005) Seasonal change in offspring sex and size in Dawson’s burrowing bees (Amegilla dawsoni) (Hymenoptera: Anthophorini). Ecol Entomol 30:247–254
Blanckenhorn WU (2000) The evolution of body size: what keeps organisms small? Q Rev Biol 75:385–407
Blomqvist D, Johansson OC, Unger U, Larsson M, Flodin LA (1997) Male aerial display and reversed sexual size dimorphism in the dunlin. Anim Beh 54:1291–1299
Crompton B, Thomason JC, Mclachlan AJ (2003) Mating in a viscous universe: the race is to the agile, not to the swift. Proc Roy Soc Lond B 270:1991–1995
Emlen ST, Oring LW (1977) Ecology, sexual selection, and the evolution of mating systems. Science 197:215–223
Houston TF (1991) Ecology and behaviour of the bee Amegilla (Asaropoda) dawsoni (Rayment) with notes on a related species (Hymenoptera: Anthophoridae). Rec West Aus Mus 15:91–109
Neems RM, McLachlan AJ, Chambers R (1990) Body size and lifetime mating success of male midges (Diptera: Chironomidae). Anim Behav 40:648–652
Paxton RJ (2005) Male mating behaviour and mating systems of bees: an overview. Apidologie 36:145–156
Seidelmann K (2006) Open-cell parasitism shapes maternal investment patterns in the Red Mason bee Osmia rufa. Behav Ecol 17:839–846
Serrano-Meneses MA, Córdoba-Aguilar A, Azpilicueta-Amorin M, González-Soriano A, Székely T (2008) Sexual selection, sexual size dimorphism and Rensch’s rule in Odonata. J Evol Biol 21:1259–1273
Simmons LW, Tomkins JL, Alcock J (2000) Can minor males of Dawson’s burrowing bee, Amegilla dawsoni (Hymenoptera: Anthophorini) compensate for reduced access to virgin females through sperm competition? Behav Ecol 11:319–325
Stubblefield JW, Seger J (1994) Sexual dimorphism in the Hymenoptera. In: Short R, Balaban E (eds) The differences between the sexes. Cambridge University Press, Cambridge, pp 71–103
Székely T, Freckleton RP, Reynolds JD (2004) Sexual selection explains Rensch’s rule of size dimorphism in shorebirds. Proc Natl Acad Sci 101:12224–12227
Teder T, Tammaru T (2005) Sexual size dimorphism within species increases with body size in insects. Oikos 108:321–334
Tomkins JL, Simmons LW, Alcock J (2001) Brood provisioning strategies in Dawson’s burrowing bee, Amegilla dawsoni (Hymenoptera: Anthophorini). Behav Ecol Sociobiol 50:81–89
Acknowledgements
Thanks to Sue Alcock, Carol Simmons and Freddy Simmons for their contributions to the bee project.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Alcock, J., Bailey, W.J. & Simmons, L.W. The Mating System of Amegilla (Asarapoda) paracalva Brooks (Hymenoptera: Apidae). J Insect Behav 23, 69–79 (2010). https://doi.org/10.1007/s10905-009-9196-x
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10905-009-9196-x