Abstract
Perception of the female sex pheromone in Utetheisa ornatrix (Lepidoptera: Arctiidae) is responsible for induction and adjustment of calling by females and the collective phenomenon termed “female pheromonal chorusing”. We found five olfactory-active compounds in the U. ornatrix female gland. When females were exposed to the entire pheromone or to two of its (synthetically prepared) components, (Z,Z,Z)-3,6,9-eicosatriene and (Z,Z,Z)-3,6,9-heneicosatriene, they were more likely to call during a given night, begin calling earlier, and briefly increase signal frequency with which they extrude their abdomen, an observable indication of calling in this species. Some females even initiated calling during photophase when exposed to the pheromone components. In general, female U. ornatrix are more sensitive to the complete blend of pheromone than to its individual compounds. We also tested the hypotheses: 1) that abdominal extrusion per se increases the rate of pheromone release; and 2) that greater abdominal pumping rhythm increases pheromone release rate. Contrary to our expectations: 1) females did not respond more strongly to a pulsed pheromone stimulus than to the constant release of pheromone at the same average release rate; and 2) we did not find a relationship between the frequency of abdominal pumping and pheromone release rate. Possible explanations for these unexpected findings are discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In lepidopteran communication, females are typically regarded as non-interactive advertisers before courtship and pair formation. They are believed to signal their receptivity and location to males via long-distance pheromones. Potential responses to the sex pheromones of neighboring females are generally overlooked. However, some neurophysiological and behavioral evidence suggests that the latter may not be accurate for some moth species, and that communication by females may be more interactive and complex than previously considered. Researchers have found electrophysiological responses of female antennal neurons to conspecific female sex pheromone components in several lepidopteran families: Arctiidae (Panaxia quadripunctaria, Schneider et al. 1998; Utetheisa ornatrix, Grant and O’Connell 2000), Noctuidae (Heliothis virescens and H. subflexa, Groot et al. 2005; Hillier et al. 2006; Spodoptera littoralis, Ljungberg et al. 1993), and Sphingidae (Manduca sexta, Kalinová et al. 2001). Conspecific females and/or their sex pheromone are also known to induce calling in females in Tortricidae (Choristoneura fumiferana, Palaniswamy and Seabrook 1978, 1985; Cydia fagiglandana and C. splendana, Den Otter et al. 1996), and to attract (Tortricidae; C. fagiglandana and C. splendana; Den Otter et al. 1996) or repel other females (Noctuidae; H. armigera and H. zea, Saad and Scott 1981).
At a basic level, pair formation in the arctiid moth U. ornatrix conforms to the general feature of female-emitted pheromones eliciting upwind flight in conspecific males. Female U. ornatrix begin calling at dusk and normally continue for several hours. As in various arctiid moths, calling behavior is highly apparent and entails pumping of the abdomen, i.e., rhythmically extruding the terminal segments that bear the pheromone glands, at a rate of 1.5–3.0 sec−1 (Conner et al. 1980, 1985). Three active components of the U. ornatrix female pheromone have been identified: (Z,Z,Z)-1,3,6,9-heneicosatetraene (1,Z3,Z6,Z9-21Hy), (Z,Z,Z)-3,6,9-heneicosatriene (Z3,Z6,Z9-21Hy), and (Z,Z)-6,9-heneicosadiene (Z6,Z9-21Hy) (Conner et al. 1980; Jain et al. 1983). Importantly, electroantennogram (EAG) responses to one of the components, Z3,Z6,Z9-21Hy, occur in females (Grant and O’Connell 2000) and in males (Conner et al. 1980; Jain et al. 1983).
A recent study reported that female U. ornatrix within olfactory range of neighboring females synchronize the onset of their daily calling periods and exhibit a higher abdominal pumping rate than isolated individuals (Lim and Greenfield 2007). Grouped females were also more likely to call on a given night, and to call without interruption and for a longer duration than females that were olfactorally isolated from neighbors (Lim and Greenfield 2007). These data show that females modify the timing and intensity of calling in the presence of neighboring females, a phenomenon termed “female pheromonal chorusing” (Lim and Greenfield 2007). Evidence that females respond specifically to conspecific sex pheromone is circumstantial, although as pointed out by Grant and O’Connell (2000), “gender-specific body odors” may conceivably play a role in generating the interactive behavior. In the present study, we examine the specific responses in calling of U. ornatrix females to conspecific female sex pheromone, and its separate components, to understand the mechanisms and evolution of this communal sexual display.
In a previous study, Lim and Greenfield (2007) reported that calling females increased abdominal extrusion rates in response to the presence of calling neighbors. We propose that the extrusion rate indicates the “vigor” with which calling is performed, and is related to energetic expenditure during calling, and pheromone release rate. The latter is based on the hypothesis that rhythmic abdominal pumping serves to increase peak pheromone release rate, thereby maximizing potential attraction of males (Schal and Cardé 1985; Dusenbery 1989). Alternative adaptive hypotheses for abdominal pumping include the creation of an additional axis of the pheromone signal, temporal modulation, along which species specificity may be encoded, and the reduction of sensory adaptation in the male receiver (Conner et al. 1980). Therefore, we examined two additional questions concerning the potential response of female U. ornatrix to the conspecific female pheromone: Do females exhibit a heightened response to a pulsed pheromone signal, and does the abdomen extrusion rate relate to the pheromone release rate?
Methods and Materials
Study Population
A laboratory population of U. ornatrix was bred from moths collected in Highlands County, Florida, USA. Larvae were maintained on a standard pinto-bean diet (Shorey and Hale 1965), supplemented with 20% (w/w) of seeds of Crotalaria mucronata, the host plant of U. ornatrix at the collection site (Dussourd et al. 1991), in an environmental chamber at 26°C and 14L:10D photoperiod. Moths from the sixth through ninth generations, from the time of collection from the field, were used for the experiments. Inbreeding was minimized by establishing 10 maternal lines from the original field collection (ca. 300 caterpillars) and out-crossing them. To ensure standard physiological and behavioral states among experimental animals, we only used virgin individuals and tested them once on the second or third day after eclosion, i.e., adult individuals aged 24–48 hr.
Pheromone Gland Extraction
Pheromone gland extracts were prepared from individual U. ornatrix female moths and analyzed at different times of the diel period, by gas chromatography (GC) and gas chromatography-mass spectrometry (GC-MS). For preparation of the extracts, the terminal 3 or 4 abdominal segments (bearing the pheromone gland) of a female were dissected with microscissors, and placed individually in a vial containing 100 μl of hexane and a mixture of 100 ng each of (Z)-5-tridecene and (Z)-7-tetradecene as internal standards. Dissection of the pheromone glands was performed 2 hr before (N = 8 females) and 2 hr after (N = 40 females) the onset of scotophase. These times were chosen because females rarely call during photophase and mostly attain peak abdominal extrusion rate ca. 2 hr after the onset of scotophase (Lim and Greenfield 2007). Of the 40 individuals tested during scotophase, 26 were calling at the time of gland dissection. The glands were removed from the vial after 30 min and the extracts stored at −20°C until GC analysis and behavioral bioassay.
Gas Chromatography and Electroantennographic Detection
A Hewlett-Packard (HP) 5890 Series II gas chromatograph, equipped with either a DB-5 or DB-225 column (30 m × 0.25 mm ID, J & W Scientific, Folsom, CA, USA) and a 50:50 effluent split, was used for gas chromatography-electroantennographic detection (GC-EAD) analysis. Helium was the carrier gas, with a flow rate of ca. 30 ml min−1 for both FID and EAD. Before GC analysis, the gland extracts were concentrated to 10–20 μl, by using a gentle stream of nitrogen, and 1 μl injected into the GC in splitless mode. Initial oven temperature was 60°C, held for 1 min, then increased to 250°C for DB-5 and 200°C for DB-225 at 10°C min−1. The outlet for the EAD had a purified and humidified air stream flowing over the antennal preparation at 600 ml/min.
Gas chromatography-mass spectrometry (GC-MS) analyses of gland extracts were performed with an HP 5890 Series II GC interfaced to an HP 5972 Mass Selective Detector. The GC-MS was equipped with a DB-5 column (identical to that used in the GC-EAD system), which was temperature-programmed as for the GC-EAD analyses. Electron ionization mass spectra were recorded from 30 to 550 amu at 70 eV. Chemicals were identified by comparison of retention indices and mass spectra with those of authentic standards. Two synthetic compounds, (Z,Z,Z)-3,6,9-eicosatriene (Z3,Z6,Z9-20Hy) and Z3,Z6,Z9-21Hy, were provided by Russell Jurenka (Iowa State University, Ames, IA, USA). Isomeric and chemical purity of these two compounds were >99.5%, as determined by GC.
Whole-body preparations were used for EAD experiments. A virgin male or female moth was immobilized on a plasticine block with small U-shaped copper wires, and its antennae were further restrained with thinner copper wires. A glass microelectrode (tip diameter ≈ 1 mm, 0.86 mm I.D., A-M Systems Inc., Washington, USA) filled with 0.1 M KCl solution was introduced through the cuticle of the distal part of the antenna and served as a recording electrode; another glass microelectrode penetrated a compound eye to serve as a reference electrode. Silver–silver chloride junctions were used to maintain electrical contact between the electrodes and amplifier. The EAD signal was first amplified with a high-input impedance (>1012 Ω) headstage preamplifier (Syntech®, Hilversum, The Netherlands), and further processed with a PC-based signal processing system (Syntech®, Hilversum, The Netherlands).
Bioassay of Calling
To test the influences of female pheromone on calling behavior, we presented females with filter paper discs (2 cm diam., Whatman® no. 1) impregnated with U. ornatrix female pheromone gland extract or synthetic compounds, Z3,Z6,Z9-20Hy and Z3,Z6,Z9-21Hy. Z3,Z6,Z9-20 Hy was a newly identified olfactory-active compound for both sexes of U. ornatrix (see “Results”); therefore, we tested it to evaluate its function in female–female pheromonal interaction. We could not test synthetic 1,Z3,Z6,Z9-21Hy because of its chemical instability (Huang et al. 1983), but we tested the responses of females to synthetic Z3,Z6,Z9-21Hy.
One equivalent of gland extract (IE), the mean amount of pheromone extracted from a naturally calling female during scotophase, was used as a standard unit throughout the experiments. Preliminary GC-EAD and behavioral assays showed that U. ornatrix females are sensitive to hexane (personal observation). Therefore, each filter paper disc was dried in an airflow for 30 sec before presentation in our behavioral bioassays.
We monitored the behavior of each of the test females during 15-sec observations made every 5–15 min throughout a 60- to 120-min period during early scotophase at 25 ± 1°C. During these observations, we noted: (1) whether or not the female was calling and, if yes, (2) the number of abdominal extrusions per 15 sec. To avoid observer bias, a double-blinded observer was randomly assigned for each test. From these data, we determined the following three indices of each individual female’s calling: (1) the time each individual was first observed calling, with scotophase onset designated as 0 min; (2) the mean signal frequency, measured in abdominal extrusions per minute, of each individual showing calling behavior during at least one observation; (3) the total time that an individual spent calling, inferred from the number of observations when she was observed calling (Lim and Greenfield 2007). For all data, we listed the time of a reported event as the midpoint of the 5- to 15-min cycle of observations during which it was seen.
Call Induction During Photophase
We evaluated whether pheromone could induce females to call by testing its effect during mid-photophase, when U. ornatrix ordinarily do not call. Single females were placed in individual glass cells (d × l = 1 cm × 5 cm), connected by tubing to a chemical source at one end and to an exhaust at the other (flow rate <10 cm/sec). Test individuals were placed in cells at least 6 hr before the onset of scotophase, and their behavior observed every 15 min over the following 5 hr at 25°C. We initiated observations 6 hr before scotophase onset to avoid the possibility that the circadian rhythms of some females might trigger calling near the end of photophase (Silvegren et al. 2005).
No behavioral responses were observed from females to 1 IE during photophase. Therefore, we repeated the trials using higher quantities of gland extracts (5 IE, N = 50; 10 IE, N = 19; 15 IE, N = 15) and synthetic Z3,Z6,Z9-21Hy (100 ng, N = 16; 200 ng, N = 18; 400 ng, N = 10; Conner et al. 1980). A post hoc analysis indicated that 100 ng of Z3,Z6,Z9-21Hy was approximately equivalent to the mean quantity found in 1 IE (see “Results ”). As a control, we tested 48 individuals with filter paper discs containing hexane only.
Call Stimulation During Scotophase
Using the apparatus described earlier, we tested the influence of both pheromone gland extracts and synthetic pheromone components on calling behavior of female U. ornatrix during scotophase. Ten (N = 17) or 20 ng (N = 15) of Z3,Z6,Z9-20Hy (N = 32 sets of six females), equivalent to the mean quantity in 1 IE and 2 IE, respectively, 50 (N = 24 sets) or 100 ng (N = 10 sets) of Z3,Z6,Z9-21Hy (N = 34 sets), equivalent to the mean quantity in 0.5 IE and 1 IE, respectively, or 1 IE (N = 11 sets), 2 IE (N = 13 sets), 4 IE (N = 15 sets), 6 IE (N = 15 sets), and 10 IE (N = 20 sets) of gland extract (N = 74 sets), were tested. We also tested additional sets (N = 54) of six individuals with the control stimulus. We made 15-sec observations for each individual every 5 min for 1 hr after odor presentation. For a given set of six females tested together in the apparatus, we recorded: (1) time of calling first observed for each individual, (2) proportion of individuals calling, and (3) mean signal frequency, expressed as the average of the maximum signal frequencies of individual moths showing calling behavior.
Calling Acceleration During Scotophase
We tested whether signal frequency of calling females increased after exposure to pheromone. As described in the previous section, we monitored signal frequencies of 42 individual females and recorded the frequency during and after exposure to 0.05 IE of gland extract. We used the low quantity of 0.05 IE to test only the immediate behavioral response to the pheromone. Because signal frequency usually reaches its peak between 50 and 90 min after the onset of calling (Lim and Greenfield 2007), we presented the pheromone 20 min after the onset of calling to avoid coinciding with the natural increase in signal frequency. We observed and recorded the signal frequency of each individual during three consecutive 5-min observation periods: 5 min before and 5 min after pheromone presentation, and 5 min thereafter. During the observation, signal frequencies of individual moths were counted each and every minute. Filter paper discs containing only hexane were tested as control (N = 17).
Calling Stimulation by Pheromone Pulsing
Because calling in U. ornatrix females entails a rhythmic pulsing of pheromone release, we tested whether females responded to temporal modulation of pheromone concentration per se. Specifically, we examined whether calling females were more likely to accelerate their signal frequency to higher values when exposed to pulsed pheromone than to a steady stream of pheromone, the release rate of which was identical to the mean value of the pulsed one. To pulse the pheromone stream, we punctured a small hole in the tube upstream from the chemical stimulus (filter paper disc) and tapped a finger on the hole at a rate of 3 sec−1, the maximum signal frequency observed in U. ornatrix females (Conner et al. 1980, 1985; Lim and Greenfield 2007). We first presented the odor 15 min after scotophase onset and then began tapping 10 min later and continued for 5 min. The 10-min delay between odor presentation and the onset of tapping allowed us to measure the signal frequency after exposure to both continuous and pulsed stimuli. We used 0.05 IE as the stimulus in 18 trials of six individuals each. We confirmed that the finger tapping generated a puff at the location of the test individuals by simulating it with a visible marker (incense smoke). A control volatile (filter paper disc with hexane only) was tested in 15 parallel trials of six individuals each. We recorded the signal frequency of each individual during three 5-min periods as follows: 5 min before the tapping during tapping, and during the 5 min after tapping. During each period, we measured the signal frequency of each individual five times, each measurement being the number of abdominal extrusions in 15 sec. To test whether the pheromone puff per se influenced female calling, we also compared the signal frequencies of the females exposed to the pulsed pheromone with the group tested with a steady stream of pheromone in the previous test.
Signal Frequency and Pheromone Release Rate
We tested the hypothesis that the signal frequency is positively related to pheromone release rate during calling and that grouped females, who generally call at elevated signal frequencies during the beginning of the scotophase, are releasing pheromone at higher rates. We established two different airborne pheromone collection apparatus, one for “solitary” and one for “grouped” females. In each apparatus, we placed females singly in individual cells, observed their calling behavior and signal frequency, and collected and quantified pheromone released. Solitary females were held in individual glass cells such that they were not exposed to the pheromone of neighbors, whereas grouped females were held in individual screen cells that exposed them to other females’ pheromone.
We conducted 12 solitary female trials, in which 10 individual females were held singly within 15-ml glass tubes that were all connected to a Tenax odor trap. To prevent the solitary females from perceiving their neighbors’ pheromone accumulating in the collection apparatus, and to enhance collection efficiency, air was exhausted from the apparatus through the odor trapper at a flow rate of 30 cm/sec. We also conducted 12 grouped female trials, in which eight individual females were held singly within screen cells placed in a 12-cm diam Petri dish connected to a Tenax odor trapper. We used different numbers of test individuals in the solitary (10 females for each collection) and grouped trials (eight females for each collection) so that the total number of calling individuals in a trial would be equivalent; typically, in previous tests, we had observed that 50% and 70% of solitary and grouped individuals, respectively, called (Lim and Greenfield 2007). Each of the screen cages holding the grouped females were separated by at least 0.5 cm to prevent direct physical contact between the moths. As in the solitary female trials, air was exhausted from the apparatus through the odor trap at a rate of 30 cm sec−1. The Tenax odor trap consisted of a glass Pasteur pipette (l × d = 5 × 0.5 cm) packed with 300 mg of pre-cleaned Tenax (35/60 mesh, Alltech®, Deerfield, IL, USA) held in place by glass wool plugs.
In both solitary and grouped female trials, volatile chemicals were collected for 5 hr after onset of scotophase. While collecting pheromone, we observed calling behavior by each female every 10 min. We estimated pheromone release rates of individual females by dividing the total amount of pheromone collected by the total signaling time in a trial (equal to the sum of total signaling durations of each of the 10 solitary or eight grouped females in the trial). Three grouped females and 10 solitary females that either died before or oviposited while in the apparatus for the 5-hr trial were not included in the analyses. After collection, 100 ng each of (Z)-5-tridecene and (Z)-7-tetradecene were added to the Tenax trap as internal standards, and the Tenax rinsed four times with 400 μl of hexane. The solution was concentrated under a gentle stream of nitrogen to a final volume <50 μl and analyzed by GC. Unless otherwise noted, variation in all analyses is reported as mean ± standard deviation.
Results
Pheromone Components
Gas chromatography-electroantennographic detection (GC-EAD) and GC-MS analyses confirmed the presence of the three previously identified pheromone compounds, Z6,Z9-21Hy (Retention time = 8.88 min), Z3,Z6,Z9-21Hy (Rt = 9.25 min), and 1,Z3,Z6,Z9-21Hy (Rt = 9.43 min) in our U. ornatrix population. Both males and females showed strong antennal responses to all three compounds. Besides these compounds, the analyses revealed two additional GC-EAD-active compounds. One was identified as Z3,Z6,Z9-20Hy by comparison of retention time (8.6 min) and mass spectrum with the synthetic compound (Lim, unpublished data). This compound elicited EAD responses from both male and female antennae. The other compound, which had a shorter retention time (8.52 min), elicited responses from male, but not female, antennae. Its identity was not determined in the present study. We also found two distinct profiles of gland extracts from females. In one profile, Z3,Z6,Z9-21Hy was the major component, whereas in the other 1,Z3,Z6,Z9-21Hy was the major component; there was no detectable amount of Z3,Z6,Z9-21Hy in females of the latter profile type. For this study, we did not discriminate these two profiles in our bioassays.
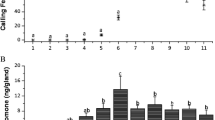
During photophase, only 1,Z3,Z6,Z9-21Hy was found in gland extracts (N = 8, 44.76 ± 54.09 ng/individual). In scotophase, 1,Z3,Z6,Z9-21Hy remained as the major component in general (Fig. 1), although the quantities of all the components varied greatly. The total quantity of the five compounds in gland extracts (354.20 ± 286.47 ng/individual, N = 26 calling individuals) prepared during scotophase was approximately eight times greater than that in photophase females (Mann–Whitney U test, W = 52.0, P < 0.001). There was a general difference in pheromone quantity between calling and non-calling females during scotophase (Fig. 1). The mean quantity of Z3,Z6,Z9-21Hy in calling females (98.72 ± 170.11 ng/individual) was significantly greater than that in non-calling females (1.95 ± 3.14 ng/individual, Mann–Whitney U test, W = 607.0, P = 0.03), as was the mean quantity of the unidentified compound (12.41 ± 12.16 ng/individual in calling, vs. 0.98 ± 1.18 ng per individual in non-calling, females; Mann–Whitney U test, W = 109.0, P = 0.04). The quantity of 1,Z3,Z6,Z9-21Hy in gland extracts did not differ between calling (239.03 ± 288.20 ng/individual; N = 26) and non-calling females (N = 14, 80.71 ± 122.49 g/individual, Mann–Whitney U test, W = 587.5, P = 0.13), although it was the major component. Similarly, the mean quantities of Z3,Z6,Z9-20Hy and Z6,Z9-21Hy in calling females (11.79 ± 22.91 and 1.50 ± 2.17 ng/individual, respectively) were not significantly different than those in non-calling ones (3.85 ± 7.95 and 2.16 ± 1.69 ng/individual, Mann–Whitney U test, W = 588.5, and 75.0, P = 0.09 and 0.37, respectively).
Mean quantity (ng) of each pheromone component extracted from gland extract of individual females that were not calling (NC: open boxes) or calling (C: solid boxes) 2 hr after onset of scotophase. (I) unknown, (II) Z3,Z6,Z9-20Hy, (III) Z6,Z9-21Hy, (IV) Z3,Z6,Z9-21Hy, and (V) 1,Z3,Z6,Z9-21Hy. Box plots show means (X), medians (horizontal line), 25–75% ranges (rectangle), 5–95% ranges (inner horizontal bar), and minimum/maximum values (outer horizontal bar) of quantities
Call Induction During Photophase
Both gland extract and synthetic Z3,Z6,Z9-21Hy induced calling from solitary females during photophase. No females tested began calling in response to the control discs or discs containing 5 IE, but 6 of 19 females responded to 10 IE, a significantly higher proportion than responding to the control (Fisher’s exact test, P < 0.001). Only 1 of 15 females responded to 15 IE (not different from the control, Fisher’s exact test, P = 0.24). Two of three quantities of Z3,Z6,Z9-21Hy elicited a calling response from significantly more test females compared with responses to the control disc: 5 of 16 to 100 ng (Fisher’s exact test, P < 0.001), 4 of 18 to 200 ng (Fisher’s exact test, P = 0.004), and 1 of 10 females to 400 ng (Fisher’s exact test, P = 0.19). Calling induced during photophase was generally brief, lasting only 3.02 ± 2.78 min on average. The latency of call induction during photophase was generally shorter in response to gland extract (30.44 ± 25.24 min; N = 9) than to Z3,Z6,Z9-21Hy (92.5 ± 70.67 min; N = 10; Mann–Whitney U test, W = 124.0, P = 0.05). Mean signal frequencies of calling stimulated by Z3,Z6,Z9-21Hy (75.46 ± 21.42 extrusions · min−1, N = 9) were lower than those stimulated by gland extract (138.89 ± 29.33, N = 3, Mann–Whitney U test, W = 45.0, P = 0.02).
Call Stimulation During Scotophase
The bioassays indicated that both gland extract and individual pheromone components elicited significantly different calling responses during scotophase compared with the control. For each treatment, there was no significant difference in calling pattern in response to different concentrations (Kruskal–Wallis tests for gland extract and Mann–Whitney U tests for individual compounds, ns); therefore, calling responses were pooled. Females presented with gland extract or Z3,Z6,Z9-20Hy (1) were more likely to call (Kruskal–Wallis test, H = 20.80, df = 3, P < 0.001; Dunn’s pairwise comparison, P < 0.01; but this effect was not found in response to Z3,Z6,Z9-21Hy, Dunn’s pair-wise comparison, P > 0.05; Fig. 2a); (2) started calling sooner (Kruskal–Wallis test, H = 19.92, df = 3, P < 0.001; Dunn’s pairwise comparison, P < 0.01 and P < 0.05, respectively; but this effect was not found in response to Z3,Z6,Z9-21Hy, Dunn’s pairwise comparison, P > 0.05; Fig. 2b); and (3) called with a higher signal frequency only in response to Z3,Z6,Z9-21Hy (Kruskal–Wallis test, H = 22.76, df = 3, P < 0.001; Dunn’s pairwise comparison, P < 0.01; Fig. 2c), compared to females presented with the control stimulus.
Behavioral responses and temporal aspects of calling in individual females influenced by three different test volatiles and control. (II) Z3,Z6,Z9-20Hy and (IV) Z3,Z6,Z9-21Hy; 10 and 20 ng of II were equivalent to the quantity of the compound found in one and two female equivalents of gland extract, respectively; 50 ng and 100 ng of IV to 0.5 and 1 equivalents, respectively. (a) Proportion of calling individuals in a trial. (b) Latency (min) of first calling. (c) Signal frequency of calling individuals in each trial. Box plots show means (X), medians (horizontal line), 25–75% ranges (rectangle), 5–95% ranges (inner horizontal bar), and minimum/maximum values (outer horizontal bar) of values
Call Acceleration During Scotophase
Exposure to 0.05 IE of gland extract elicited a brief increase (37%) in signal frequency from 106.43 ± 46.29 to 145.36 ± 31.91 extrusions min−1 during the 5-min presentation of the chemical stimulus. This increase was short-lived and females returned to values equivalent to pre-stimulus frequencies (119.40 ± 47.26 extrusions · min−1) shortly after removal of the stimulus (Repeated Measures ANOVA based on normally distributed data, N = 42, F = 25.24, df = 2, 41, P < 0.001; Fig. 3a). Control females tested in parallel did not show any evidence of change (signal frequencies = 114.06 ± 44.15, 119.88 ± 45.15, and 118.18 ± 40.40 extrusions · min−1; N = 17; Friedman’s test, χ 2 = 1.20, df = 2, P = 0.55; Fig. 3a, open boxes).
(a) Mean signal frequency exhibited by females in response to gland extract (solid boxes) or control stimulus (open boxes). Observation periods: (1) 5-min before the odor presentation, (2) 5-min period after odor presentation, and (3) 5-min period after period 2. Data are taken from 42 test females and 17 control females. (b) Mean signal frequency exhibited by females in response to pulsed pheromone stimulus (solid boxes) or control stimulus (open boxes). Observation periods: (1) 5-min period with steady air stream containing pheromone before pulsing, (2) 5-min period of pulsing, and (3) 5-min period with steady air stream after period 2, respectively. Data are taken from 67 test females and 58 control females. Box plots show means (X), medians (horizontal line), 25–75% ranges (rectangle), 5–95% ranges (inner horizontal bar), and minimum/maximum values (outer horizontal bar) of frequencies
Call Stimulation by Pheromone Pulsing
Females increased their signal frequency from 117.75 ± 30.04 to 147.54 ± 25.06 extrusions · min−1 during the 5-min period when a chemical stimulus (0.05 IE) was pulsed, and then returned to 122.81 ± 28.70 after the cessation of pulsing (Friedman’s test, N = 67, χ 2 = 95.74, df = 2, P < 0.001). There was no change when clean air was pulsed (parallel values 108.55 ± 27.55, 113.28 ± 29.87, and 111.31 ± 28.06 extrusions · min−1; Friedman’s test, N = 58, χ 2 = 3.41 df = 2, P = 0.18).
When we compared peak signal frequencies of females exposed to, pulsed chemical stimulus, continuous chemical stimulus, pulsed clean air, and continuous clean air, we found that groups stimulated by either pulsed or continuous pheromone attained higher signal frequencies than groups stimulated by pulsed clean air (Kruskal–Wallis test, H = 39.83, df = 3, P < 0.001; Dunn’s pairwise comparison, P < 0.01; Fig. 4). However, we found no significant difference between signal frequencies of groups stimulated either by pulsed pheromone and continuous pheromone (Dunn’s pairwise comparison, P > 0.05) or by continuous pheromone and continuous clean air (Dunn’s pair-wise comparison, P > 0.05). Thus, the increase noted above and shown in Fig. 3b probably did not result from pulsed airflow per se, but rather was a short-term response to the onset of the chemical stimulus.
Mean signal frequency exhibited by females in response to pulsed or continuous air with or without pheromone stimulus. Box plots show means (X), medians (horizontal line), 25–75% ranges (rectangle), 5–95% ranges (inner horizontal bar), and minimum/maximum values (outer horizontal bar) of frequencies
Signal Frequency and Pheromone Release Rate
Our 5-hr collections showed that grouped and solitary females released comparable amounts of pheromone. Z3,Z6,Z9-20Hy was the most abundant component released (7.90 ± 7.87 ng/individual for solitary and 7.91 ± 5.27 ng/individual for grouped females; sign test, n+ = 7, n− = 5, ties = 0, P = 0.39). The amounts of Z3,Z6,Z9-21Hy (0.45 ± 0.93 ng/individual for solitary and 0.08 ± 0.27 ng/individual for grouped females; sign test, n+ = 4, n− = 1, ties = 7, P = 0.19) and 1,Z3,Z6,Z9-21Hy (3.87 ± 2.50 ng/individual for solitary and 4.22 ± 4.26 ng/individual for grouped females; sign test, n+ = 8, n− = 4, ties = 0, P = 0.19) also did not differ between solitary and grouped females. The mean pheromone release rates of grouped and solitary females were 0.98 ± 0.92 and 1.03 ± 0.71 ng·individual−1·hr−1, respectively (sign test, n+ = 7, n− = 5, ties = 0, P = 0.39; Fig. 5).
Mean pheromone release rate (ng individual−1 hr−1) of grouped and solitary calling females. Pheromone release rate is the total five compounds. Data are from 78 grouped females (solid box) and 66 solitary females (open box) that called during the collection period. Box plots show means (X), medians (horizontal line), 25–75% ranges (rectangle), 5–95% ranges (inner horizontal bar), and minimum/maximum values (outer horizontal bar) of quantities
Most grouped females in our test called at some time during the pheromone collection periods (mean proportion = 0.85 ± 0.14, N = 12 trials), whereas a lower proportion of solitary females did (0.61 ± 0.19, N = 12 trials; sign test, n+ = 10, n− = 2, ties = 0, P = 0.02). However, grouped and solitary females spent comparable amounts of time calling (129.90 ± 27.47 min vs. 136.40 ± 29.83 min, respectively; sign test, n+ = 6, n− = 6, ties = 0, P = 0.61), and also exhibited comparable mean signal frequencies (117.48 ± 12.75 extrusions·min−1 vs. 116.03 ± 16.66, respectively; sign test, n+ = 7, n− = 5, ties = 0, P = 0.39) as well as peak signal frequencies (162.45 ± 5.28 extrusions·min−1 vs. 156.91 ± 12.97, respectively; sign test, n+ = 6, n− = 6, ties = 0, P = 0.61).
Discussion
The chemical analyses and behavioral assays indicate that perception of the female advertisement pheromone is directly responsible for induction and modification of calling by U. ornatrix females. When U. ornatrix females perceive conspecific female pheromone, they (1) are more likely to call during that night, (2) begin calling earlier, and (3) accelerate their signal frequency to a higher rate during at least a brief interval. Some females even initiate calling during photophase, when such activity normally does not occur, in response to the pheromone or its individual components if presented in high enough concentration. In a previous study (Lim and Greenfield 2007), it was noted that females also increase the total amount of time spent calling during a night in response to the presence of neighboring females. However, the inability to present chemical stimuli in a controlled manner over a prolonged interval prevented us from evaluating the direct effect of sex pheromone on this aspect of calling in U. ornatrix.
Entire Pheromone Bouquet vs. Individual Components
In general, we found that U. ornatrix females are as, or more, sensitive to the entire pheromone blend than to its individual components. During scotophase, both gland extract and Z3,Z6,Z9-20Hy elicited calling from more females and more quickly than did Z3,Z6,Z9-21Hy; the gland extract also elicited calling more quickly during photophase than did Z3,Z6,Z9-21Hy. However, during scotophase, Z3,Z6,Z9-21Hy increased signal frequency in calling females more than either the gland extract or Z3,Z6,Z9-20Hy. The results with Z3,Z6,Z9-20Hy should be viewed with caution, though, as the influence of this compound on orientation of males has yet to be tested either in the laboratory or the field; therefore, its role as a component of the female sex pheromone is not established (see Wyatt 2003).
In many moth species, the male is more sensitive to the entire pheromone bouquet than to its individual components (Cardé and Haynes 2004). In these cases, the sensitivity of males to the specific “blend ratio” of the components may reflect the importance of the ratio in species recognition and in the enhancement of “signal-to-noise ratio” for eliciting a behavioral response from the males. In other species, however, one (major) component may play a more important role throughout the full course of sexual communication and orientation than other (minor) components. Here, males may have an elevated neural sensitivity to the major component (e.g., Baker et al. 2004), and the minor components of the pheromone may function only during limited and specific parts of the pair-forming sequence (e.g., Vickers 2002).
Although many environmental and developmental factors including prior exposure to the female pheromone (Stelinski et al. 2005), prior exposure to host plant volatiles (Ochieng et al. 2002), rearing temperature (Linn et al. 1988), ambient temperature during sexual activity (Ono 1993), age (Greiner et al. 2002), hormonal status (Anton and Gadenne 1999), and circadian rhythm (Silvegren et al. 2005) may affect the sensitivity of pheromone processing in males, males of most moth species generally respond rapidly and selectively to minute changes in blend ratio and quantities of female pheromone components (Cardé and Haynes 2004). This processing is necessary for locating a signaling female before other males do (Greenfield 2002). Female moths, on the other hand, may not be subject to similar selection pressures. Although females may perceive the conspecific female sex pheromone, it is not clear that they would be selected to discern the composition with the same degree of precision and speed as males. That is, females need only detect the presence of conspecific female pheromone within their vicinity and respond appropriately (Lim and Greenfield 2007). This may explain the comparable effects of the gland extract and some of the individual components in our study.
Influence of Pulsed Chemical Stimuli
Contrary to our initial expectation, females did not respond more strongly to the pulsed pheromone stimulus than to the constant release of pheromone at the same average rate. This finding indicates that females may not respond to peak-to-peak changes in pheromone concentration but rather to time-averaged levels. However, the influence of pheromone pulsing on enhancing male responses has yet to be tested.
Signal Frequency and Pheromone Release Rates
Along with a previous study (Lim and Greenfield 2007), our present study indicates increases in female signal frequency, the abdominal extrusion rate, in response to neighboring females and to pheromone. Earlier, we had proposed that this increased signal frequency yields a higher pheromone release rate. Contrary to this prediction, we found no difference between the pheromone release rates of solitary and grouped females. This lack of difference may reflect several factors. First, the low amounts of pheromone released, <1 ng·individual−1·hr−1 (see “Results”), may have prevented us from resolving differences in release rates should they have occurred. Second, the 5-hr collection period (necessary to collect detectable amounts of pheromone; Jia et al. 2001; Lim and Greenfield 2007) may have masked any transitory changes in pheromone release rates caused by the abdominal pumping. Third, total calling times by solitary and grouped females may have also confounded our attempt to examine the relationship between signal frequency and pheromone release rate. Thus, at this time our data neither support nor unequivocally refute the hypothesis that the elevated signal frequency displayed by grouped females causes an increased pheromone release rate and a more intense signal to neighboring female signalers.
References
Anton, S. and Gadenne, C. 1999. Effect of juvenile hormone on the central nervous processing of sex pheromone in an insect. Proc. Natl. Acad. Sci. USA 96:5764–5767.
Baker, T. C., Ochieng, S. A., Cossé, A. A., Lee, S. G., Todd, J. L., Quero, C., and Vickers, N. J. 2004. A comparison of responses from olfactory receptor neurons of Heliothis subflexa and Heliothis virescens to components of their sex pheromone. J. Comp. Physiol. A 190:155–165.
Cardé, R. T. and Haynes, K. F. 2004. Structure of the pheromone communication channel in moths, pp. 283–332, in R. T. Cardé and J. G. Millar (eds.). Advances in Insect Chemical Ecology. Cambridge University Press, Cambridge, UK.
Conner, W. E., Eisner, T., Vander Meer, R. K., Guerrero, A., Ghiringelli, D., and Meinwald, J. 1980. Sex attractant of an arctiid moth (Utetheisa ornatrix): a pulsed chemical signal. Behav. Ecol. Sociobiol. 7:55–63.
Conner, W. E., Webster, R. P., and Itagaki, H. 1985. Calling behaviour in arctiid moths: the effects of temperature and wind speed on the rhythmic exposure of the sex attractant gland. J. Insect Physiol. 31:815–820.
Den Otter, C. J., De Cristofaro, A., Voskamp, K. E., and Rotundo, G. 1996. Electrophysiological and behavioural responses of chestnut moths, Cydia fagiglandana and C. splendana (Lep., Tortricidae), to sex attractants and odours of host plants. J. Appl. Entomol. 120:413–421.
Dusenbery, D. B. 1989. Calculated effect of pulsed pheromone release on range of attraction. J. Chem. Ecol. 15:971–977.
Dussourd, D. E., Harvis, C. A., Meinwald, J., and Eisner, T. 1991. Pheromonal advertisement of a nuptial gift by a male moth (Utetheisa ornatrix). Proc. Natl. Acad. Sci. USA 88:9224–9227.
Grant, A. J. and O’Connell, R. J. 2000. Responses of olfactory receptor neurons in Utetheisa ornatrix to gender-specific odors. J. Comp. Physiol A 186:535–542.
Greenfield, M. D. 2002. Signalers and Receivers. Oxford Press, New York, USA.
Greiner, B., Gadenne, C., and Anton, S. 2002. Central processing of plant volatiles in Agrotis ipsilon males is age-independent in contrast to sex pheromone processing. Chem. Senses 27:45–48.
Groot, A., Gemeno, C., Brownie, C., Gould, F., and Schal, C. 2005. Male and female antennal responses in Heliothis virescens and H. subflexa to consepcific and heterospecific sex pheromone compounds. Environ. Entomol. 34:256–263.
Hillier, N. K., Kleineidam, C., and Vickers, N. J. 2006. Physiology and glomerular projections of olfactory receptor neurons on the antenna of female Heliothis virescens (Lepidoptera: Noctuidae) responsive to behaviorally relevant odors. J. Comp. Physiol. A 192:199–219.
Huang, W., Pulaski, S. P., and Meinwald, J. 1983. Synthesis of highly unsaturated insect pheromones: (Z,Z,Z)-1,3,6,9-heneicosatetraene and (Z,Z,Z)-1,3,6,9-nonadecatetraene. J. Org. Chem. 48:2270–2274.
Jain, S. C., Dussourd, D. E., Conner, W. E., Eisner, T., Guerrero, A., and Meinwald, J. 1983. Polyene pheromone components from an Arctiid Moth (Utetheisa ornatrix): characterization and synthesis. J. Org. Chem. 48:2266–2270.
Jia, F. Y., Greenfield, M. D., and Collins, R. D. 2001. Ultrasonic signal competition between male wax moths. J. Insect Behav. 14:19–33.
Kalinová, B., Hoskovec, M., Liblikas, I., Unelius, C. R., and Hansson, B. S. 2001. Detection of sex pheromone components in Manduca sexta (L.). Chem. Senses 26:1175–1186.
Lim, H. and Greenfield, M. D. 2007. Female pheromonal chorusing in an arctiid moth, Utetheisa ornatrix. Behav. Ecol. 18:165–173.
Linn, C. E., Campbell, M. G., and Roelofs, W. L. 1988. Temperature modulation of behavioural thresholds controlling male moth sex pheromone response specificity. Physiol. Entomol. 13:59–67.
Ljungberg, H., Anderson, P., and Hansson, B. S. 1993. Physiology and morphology of pheromone-specific sensilla on the antennae of male and female Spodoptera littoralis (Lepidoptera: Noctuidae). J. Insect Physiol. 39:253–260.
Ochieng, S., Park, K., and Baker, T. 2002. Host plant volatiles synergize responses of sex pheromone-specific olfactory receptor neurons in male Helicoverpa zea. J. Comp. Physiol. A 188:325–333.
Ono, T. 1993. Effect of rearing temperature on pheromone component ratio in potato tuberworm moth, Phthorimaea operculella, (Lepidoptera: Gelechiidae). J. Chem Ecol. 19:71–81.
Palaniswamy, P. and Seabrook, W. D. 1978. Behavioral responses of the female eastern spruce budworm Choristoneura fumiferana (Lepidoptera, Tortricidae) to the sex pheromone of her own species. J. Chem. Ecol. 4:649–655.
Palaniswamy, P. and Seabrook, W. D. 1985. The alteration of calling behaviour by female Choristoneura fumiferana when exposed to synthetic sex pheromone. Entomol. Exp. Appl. 37:13–16.
Saad, A. D. and Scott, D. R. 1981. Repellency of pheromones released by females of Heliothis armigera and H. zea to females both species. Entomol. Exp. Appl. 30:123–127.
Schal, C. and Cardé, R. T. 1985. Rhythmic extrusion of pheromone gland elevates pheromone release rate. Experientia 41:1617–1619.
Schneider, D., Schulz, S. Priesner, E., Ziesmann, J., and Francke, W. 1998. Autodetection and chemistry of female and male pheromone in both sexes of the tiger moth Panaxia quadripunctaria. J. Comp. Physiol. A 182:153–161.
Shorey, H. H. and Hale, R. L. 1965. Mass-rearing of the larvae of nine noctuid species on a simple artificial medium. J. Econ. Entomol. 58:522–524.
Silvegren, G., Löfstedt, C., and Rosén, W. Q. 2005. Circadian mating activity and effect of pheromone pre-exposure on pheromone response rhythms in the moth Spodoptera littoralis. J. Insect Physiol. 51:277–286.
Stelinski, L. L., Vogel, K. J., Gut, L. J., and Miller, J. R. 2005. Seconds-long preexposures to pheromone from rubber septum or polyethelene tube dispensers alters subsequent behavioral responses of male Grapholita molesta (Lepidoptera: Tortricidae) in a sustained-flight tunnel. Environ. Entomol. 34:696–704.
Vickers, N. J. 2002. Defining a synthetic pheromone blend attractive to male Heliothis subflexa under wind tunnel conditions. J. Chem. Ecol. 28:1255–1267.
Wyatt, T. D. 2003. Pheromones and Animal Behaviour: Communication by Smell and Taste. Cambridge University Press, Cambridge, UK.
Acknowledgments
We thank M. Deyrup of the Archbold Biological Station, Highlands County, Florida, for helping us collect U. ornatrix and its host plants. We also thank M. -Y. Choi, W. E. Conner, T. Eisner, V. Iyengar, and C. W. LaMunyon for providing critical information on U. ornatrix biology and pheromone analyses during various stages of the project and R. Jurenka for providing us with synthetic pheromone components. J. Kelly and two anonymous reviewers offered valuable critiques of earlier versions of the manuscript. K. M. Nus and J. A. Campbell of the Division of Biological Sciences at the University of Kansas took care of the host plants and provided test facilities. A. M. Swatek served as an invaluable laboratory assistant. The Hungerford Fund and the Entomology Graduate Student Summer Scholarship at the University of Kansas, Department of Ecology & Evolutionary Biology provided financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lim, H., Park, K.C., Baker, T.C. et al. Perception of Conspecific Female Pheromone Stimulates Female Calling in an Arctiid Moth, Utetheisa ornatrix . J Chem Ecol 33, 1257–1271 (2007). https://doi.org/10.1007/s10886-007-9291-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-007-9291-4