Abstract
Some burrower bugs (Heteroptera: Cydnidae) show complex patterns of maternal care, including defense against predators and the provisioning of food to nymphs. Recently, the subsocial cydnid bugs have attracted the interest of researchers as model systems to study the behavioral ecology of parental investment. However, there have been few attempts to quantify the fitness benefits of maternal behavior other than provisioning. Here, we examined the maternal behavior of Adomerus triguttulus and its adaptive significance in terms of offspring survival in the field. A. triguttulus young depend on fallen nutlets of myrmecophorous mints, Lamium spp. Under field conditions, females attend offspring, from eggs to second instar nymphs, in nests on the ground under the litter. When disturbed, the females showed aggressive responses against the source of disturbance. The females often carried spherical clutches of eggs away from the nest when heavily disturbed. Female-removal experiments in the field demonstrated a defensive function of the female behavior; predators, such as ants, attacked egg clutches without females and the clutches often disappeared during the experiment. Egg clutches without females sometimes also suffered from fungal infection. Selective factors on maternal defensive behavior in A. triguttulus are discussed in terms of habitat properties possibly emerging from insect–plant associations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Parental care is a basic form of sociality in animals. In arthropods including insects, maternal (or paternal) behavior is not common, but it is known in different lineages. A variety of environmental factors that select for parental care behavior have been identified (Tallamy and Wood 1986; Costa 2006). Natural enemies, predators and parasitoids, are common agents leading to parental care in insects. It is often suggested that predation and/or parasitism risks depend on oviposition sites and modes (e.g. Tallamy and Schaefer 1997). Thus, differences in oviposition sites and modes may affect subsequent care behavior and its fitness consequences. In addition, offspring behavior after hatching would also be associated with parental behavior.
Heteroptera has been known as one of the rare insect orders that include many species showing diverse forms of parental care behavior (Melber and Schmidt 1977; Tachikawa 1991; Tallamy and Schaefer 1997). In most subsocial heteropterans, females lay eggs in masses that adhere to substrates, plant leaves or stems, and show specific straddling posture on the egg mass and aggressive responses against approaching enemies (e.g., Eberhard 1975; Melber and Schmidt 1975; Kudo 1990). Many studies have demonstrated the adaptive significance of this type of parental care (e.g., Eberhard 1975; Melber et al. 1980; Kudo 2002). Some species extend parental care to broods moving from natal sites; parents settle in a position near feeding broods and show a specific vigilance posture against approaching enemies (e.g., Kudo 1996, 2000).
On the other hand, in a few other subsocial heteropteran taxa, eggs are attached to the parent body (Belostomatidae and Coreidae: Smith 1997; Tallamy 2001) or form an independent spherical mass, which the parent can carry (Cydnidae: Filippi et al. 2001). There have been few studies on such subsocial systems in terms of defensive functions of parental behavior.
Cydnidae and the closely related family Parastrachiidae contain subsocial species (Melber and Schmidt 1977; Tallamy and Schaefer 1997; Filippi et al. 2001). Their maternal care is complex and consists of different behavioral components and functions, including the defense against predation, the provisioning of food such as fruits or seeds and the production of trophic eggs (Nakahira 1994; Filippi-Tsukamoto et al. 1995; Kight 1997; Hironaka et al. 2005). Recently, the subsocial cydnid bugs have attracted the interest of researchers as model systems to study the behavioral ecology of parental investment, over which family members are potentially under a variety of conflicts. For Parastrachia japonensis and Sehirus cinctus, there is an increasing number of studies that explore diverse aspects of maternal provisioning, ranging over proximate mechanisms (e.g., Nomakuchi et al. 1998; Kölliker et al. 2005, 2006), costs and benefits in reproductive success (Filippi-Tsukamoto et al. 1995; Filippi et al. 2000; Agrawal et al. 2005) and genetic background (Agrawal et al. 2001). Internal or external factors affecting maternal behavior have also been addressed (Kight 1997, 1998; Kight and Cseke 1998; Filippi et al. 2002). However, studies on maternal care in other subsocial cydnid species remain largely as anecdotal descriptions. Even for P. japonensis and S. cinctus, there have been few attempts to quantify fitness benefits of maternal behavior other than provisioning under field conditions (Filippi et al. 2001).
Preliminary observations suggest that in the cydnid bug Adomerus triguttulus, females guard their egg clutches against predators (Tachikawa 1991). However, there have been no studies that analyzed such maternal behavior with quantitative data and evaluated its adaptive significance under field conditions. In the present study, we provide a quantitative description and experimental analyses of maternal behavior in A. triguttulus, focusing on its defensive function.
Materials and Methods
Life History of Adomerus triguttulus
Overwintered A. triguttulus females breed from late May to July in Sapporo, northern Japan (Nakahira 1992). The females feed upon developing seeds of the host Lamium spp. Gravid females move from the plant to the ground and lay eggs under the leaf litter; they form a globular egg-mass and then attend it (Tachikawa 1991). During the attendance on eggs, the females continue to produce inviable trophic eggs and add them to the egg-mass (Nakahira 1994; Kudo and Nakahira 2004, 2005; Kudo et al. 2006). Nymphs feed on trophic eggs soon after hatching and the trophic-egg feeding enhances their subsequent growth and survival (Kudo and Nakahira 2004). After hatching, females provision broods with host nutlets (Nakahira 1992; Nakahira and Kudo unpublished data). Nymphs that are recently independent of their mother feed on fallen nutlets on the ground in aggregations, but later instar nymphs are usually found solitary (Tachikawa 1991). Cannibalism is often observed among later instar nymphs (Nakahira 1992).
A. triguttulus is not an obligatory semelparous species; under rearing conditions, females raise more than one brood (Kudo and Nakahira, unpublished data). However, it remains unknown whether or not such iteroparity is accomplished under field conditions.
Observations of Maternal Behavior in the Field
From May to July in 1990 and 1991, we observed reproductive behavior of A. triguttulus in populations that depend on Lamium album and L. purpureum at the Hokkaido University campus. We monitored several Lamium patches and the occurrence of gravid females on plants, which were feeding on developing seeds on inflorescences. When females brooding egg masses on the ground under leaf litter were found, we carefully picked up the females and individually marked their pronotum with opaque paint for discrimination, and then gently put them back on their egg masses. This disturbance sometimes induced females to carry their egg masses to a different site near the original site. When maternal egg-carrying occurred, the distance between the original and the new nesting site was measured.
We examined the duration of maternal attendance in a field population that depends on L. album. When females brooding egg masses were found, we marked the nesting sites with numbered small flags attached to fine, 15 cm long bamboo poles. Then we monitored the females and their offspring at two to four day intervals until broods were dispersed from the nesting sites and the females disappeared. We avoided daily observations, because frequent disturbance at observations, including temporary removal of the litter cover, might have caused dispersal of the broods and the females to desert the broods.
Maternal Behavior: Responses Against a Disturbance
Subsocial heteropteran females that attend their offspring show specific behavior when disturbed (e.g., Eberhard 1975; Melber et al. 1980; Kudo et al. 1989; Kudo 1990; Filippi-Tsukamoto et al. 1995). We tested whether A. triguttulus females shows such specific maternal behavior in the laboratory.
Twenty-four gravid females with visibly swollen abdomens were collected on Lamium plants. The females were individually confined in petri dishes (9 cm diameter) with filter paper under 16 h light: 8 h dark, 20°C ± 0.5 conditions. A small paper shelter for oviposition sites and Lamium nutlets were supplied in each petri-dish.
We approached females that were both pre-ovipositional and post-ovipositional (attending egg masses) from the front with a forceps and gently touched their pronotum five times with the forceps. We recorded the initial responses shown by females during the disturbance. The responses were classified into seven acts of three categories (to avoid having cells with small expected values; see the results) and compared the response between gravid females before oviposition and females attending clutches beneath paper shelters. For the latter females, we temporarily removed the paper shelter and conducted the disturbance experiments. Furthermore, we introduced potential predators, Lasius and Formica ants, in petri dishes with females brooding egg masses and observed the females’ responses.
Adaptive Significance of Maternal Care of Eggs: Female Removal Experiments in the Field
We conducted female removal experiments in an A. triguttulus population that depends on L. purpureum, in June 1992.
A. triguttulus females lay eggs in a spherical mass and thus it is impossible to count the number of eggs in intact egg masses. To estimate the initial number of viable eggs contained in each clutch at the beginning of female removal experiments (see below), we constructed a linear regression model between the clutch weight and the number of viable eggs contained in a clutch. We allowed females, which were different from those used in the removal experiments, to lay eggs for 24 h after the start of oviposition in the laboratory. These clutches (N = 29) contain almost all viable eggs (Nakahira 1994; Kudo and Nakahira 2004). We weighed the mass of clutches, counted the number of eggs contained and consequently, obtained the following regression model: Y = 9,717.459X + 6.977, Y: the number of viable eggs, X: clutch weight (g) (R2 = 0.919, F1,27 = 307.86, P < 0.0001).
The females were marked with paint for individual discrimination. The females and their egg clutches were then released on the ground within L. purpureum patches in the field, where they had been collected. After release, the females carried their egg masses (see “Results”) under leaf litter where they attended them. We divided the females into the following two experimental groups: Female removal group, females were removed from the egg masses (N = 37); Control group, females were left with eggs (N = 47). We covered egg masses in the female removal group with litter to maintain conditions that were analogous to those with females. The position of the nests (clutches) on the ground was marked with small numbered flags. At the time the egg masses were released, there was no significant difference between the two groups in the number of viable eggs, which was estimated using the regression model described above (female removal, mean ± SD, 71.3 ± 12.8; control, 69.2 ± 15.2; t 82 = 0.69, P = 0.49).
Ten days after release, we collected the egg masses (and females) in both experimental groups. Some egg masses (and/or females) had disappeared during the experiments (see the results). The egg masses were kept in petri dishes with moistened filter paper under 16 h light/8 h dark, 20 ± 0.5°C conditions. The collected egg masses in the control group, except for the one from which the female had disappeared during the experiment, were allowed to remain with the females. At the time of collection, there was no egg mass that had hatched; they hatched 4.8 ± 0.6 (SD) days (range: 4–6) later. We compared the frequency of missing egg-masses and egg survival in egg masses collected (the number of hatched nymphs/the initial number of viable eggs estimated) between the two groups.
All statistical analyses were conducted using StatView 5.0 (SAS Institute Inc., Cary, NC, USA).
Results
Maternal Behavior
Females found with egg masses under leaf litter, often in a shallow chamber, assumed a posture of holding the egg masses with their legs. During this attendance, the females were often observed to touch the egg surface with their stylets or antennae. When intensely disturbed, e.g., by removing the litter covering the nest, the females often left the nest temporarily, walked around, and then carried the egg mass to a new nesting site under the litter by dragging it using the fore-legs and stylets. The average distance between the original and relocated nesting site (for 37 cases observed) was 4.1 ± 3.3 (SD) cm (max. 15 cm).
In a population that depends on L. album, 12 out of the 29 monitored females attended second instar broods. Early third-instar broods were found at 13 nesting sites, but none of these were with females. The later instar nymphs dispersed from nesting sites.
When disturbed by objects, such as predators, females with eggs showed a variety of behavioral responses: direct movement toward the source of disturbance, lifting and vibrating the abdomen, tilting the body toward the object (situating itself in a position between the clutch and the object), dragging the clutch backward, vibrating the antennae while touching the clutch surface with the stylets (grooming), remaining immobile, and retreating from the source of disturbance behind the egg mass. The former four were categorized into “defensive", the middle two into “neutral” and the latter one into “evasive”. No females released chemical substance perceptible during the experiment. There was a significant difference in responses against the experimental disturbance using forceps between parental and gravid females (Table 1), indicating that the defensive responses were almost restricted to post-ovipositional females.
In the laboratory, when introduced ants approached their clutches, females were sometimes observed to successfully repel the ants from their clutches using the above-mentioned defensive behaviors.
Adaptive Significance of Maternal Egg-care
A. triguttulus eggs were potentially exposed to high predation pressure in the field (Fig. 1A, B). In the female removal group, prominent scars on egg masses that had presumably been caused by predation were observed during the experiment. We sometimes observed predation events directly; Lasius or Formica ants were carrying whole egg masses off or were biting a part of the egg masses. Unidentified predatory mites were also observed attacking eggs on egg masses. No parasitoid wasps were found.
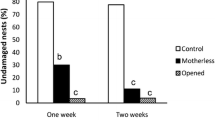
Effects of maternal attendance on offspring survival in Adomerus triguttulus. R The female-removal group in which females were removed from egg-masses (N = 47). C The control group in which egg masses were kept intact (N = 37). A Frequency of missing egg-masses during the experimental period of ten days. B Hatchability in collected egg masses (no. hatchlings/initial no. viable eggs estimated).
The number of egg masses collected ten days after the start of experiments was 14 in the female-removal group (N = 37), whereas it was 40 in the control group (N = 47), i.e., seven egg masses and their mothers had disappeared during the experiment. The seven missing females were never found. The difference in frequencies of missing egg-masses was highly significant (chi-square = 18.14, P < 0.0001; Fig. 1A).
The fourteen egg masses in the female-removal group were heavily damaged. We often observed that unidentified fungi had grown on the damaged eggs in the egg-masses. One such egg mass was completely infected by fungi and no hatchlings emerged from it. This was also the case for one egg mass in the control group, from which the female went missing during the experiment (for an unknown reason). The hatchability of collected egg-masses was much higher in the control group than in the female-removal group (Mann–Whitney U test, z = −5.20, P < 0.0001; Fig. 1B).
Discussion
In a variety of subsocial taxa, ecological factors such as stress from the physical environment, food availability, competition, and natural enemies, are known to be associated with the evolution of parental care (Tallamy and Wood 1986; Costa 2006).
Tallamy and Wood (1986) suggested that food resources constitute a primary selection pressure shaping reproductive modes in insects and consequently lead to convergent evolution of parental behavior among different lineages. In phytophagous insects, the most important agent leading to parental care is other arthropod enemies (e.g., Wood 1976; Windsor 1987; Nafus and Schreiner 1988; Kudo et al. 1992; Kudo et al. 1995). This is also the case in subsocial heteropterans that deposit eggs on host plant leaves; offspring are potentially exposed to predation and/or parasitism pressure and parent females provide effective defense for their offspring (e.g., Eberhard 1975; Melber et al. 1980; Tallamy and Denno 1981; Kudo et al. 1989; Mappes et al. 1997; Kudo 2002).
Plants provide a variety of habitat types, as well as food resources, to herbivores. Predation and parasitism pressure against the herbivores may vary not only among different plant species but also even among different habitat types emerging from a single plant species (Price et al. 1980). In such insect-plant systems, the magnitude of predation and/or parasitism on herbivores is often mediated by plant chemical and physical properties (Marquis 1996; Bottrell et al. 1998). The plant-mediated selection pressure may affect fitness consequences of parental care in phytophagous insects (Hardin and Tallamy 1992; Kudo and Hasegawa 2004). Nesting sites of A. triguttulus are on the ground around Lamium plants. The Lamium plants are myrmecochorous; they disperse seeds using ants attracted by a nutritional material, elaiosome attached to nutlets (Beattie 1985). The nutlets fallen on the ground attract ants and thus the density of foraging ants is high around plants. Other carnivorous or ominivorous insects, such as ground beetles, also feed on elaiosome (e.g., Ohara and Higashi 1987). The attractive nature of Lamium can lead to high predation pressure against herbivores, especially seed-feeders, foraging on the ground. Experimental removal of A. triguttulus brooding females in the field has revealed that predation pressure against offspring is potentially severe and defense by the brooding females is essential to offspring survival under such environments.
The offspring survival may be affected by other parental activities than direct defense against predators (e.g., Kölliker and Vancassel 2007). Filippi et al. (2000) demonstrated that in the subsocial bug P. japonensis, progressive provisioning enhances nymphal survival under high predation-pressure environments through inhibiting nymphal dispersal from safe nesting sites. A. triguttulus also show similar parental-care options other than defense: progressive provisioning of host nutlets (Nakahira 1992; Nakahira and Kudo unpublished data) and trophic egg supply (Nakahira 1994; Kudo and Nakahira 2004). These two types of provisioning show plasticity depending on maternal phenotype, offspring conditions, and environmental conditions (Kudo and Nakahira 2005; Nakahira and Kudo, unpublished data). Interactive effects of different parental-care options on offspring survival would be fascinating subjects for future studies.
Soil habitats are also severe for insects in terms of risks other than predation, because there is increased microbial infection risk; for example, ants use chemical substances against microbial infection (Hölldobler and Wilson 1990) and their grooming behavior also seems to be crucial (Oi and Pereira 1993). Microbial disease has been recognized as an important agent leading to the evolution of social systems in insects (Schmid-Hempel 1998). In pre- or subsocial species nesting in the soil, parental behaviors often functions as countermeasures against micro-organisms (e.g., Lamb 1976; Strohm and Linsenmair 2001; Suzuki 2001).
A. triguttulus egg-masses, in particular those without females, sometimes suffered fungal infection. The fungal infection is presumably promoted by the presence of damaged eggs. In the field, crushed eggs were observed even in clutches attended by females; approximately 20% of viable eggs in such clutches were killed in the control group of the female removal experiments. These losses were probably due to small predators, such as mites. Mites are also recorded as egg predators for the subsocial bug P. japonensis that nests on the ground (Tsukamoto and Tojo 1992; Filippi-Tsukamoto et al. 1995). It may be difficult for females to detect such small predators and thus to repel them completely. Dragging of egg masses by females when heavily disturbed could also damage surface eggs (Hironaka et al. 2005). Trophic eggs that are deposited on the surface of the clutch may protect inner viable eggs from such damages due to predation and dragging (Kudo and Nakahira 2004; Hironaka et al. 2005). However, the damaged eggs, even trophic ones, would induce fungal infection that may spread over whole egg masses. In P. japonensis, when egg masses without females were kept on moistened filter paper, the egg masses, in which some eggs were attacked by mites, were infected by fungi and showed extremely low hatchability (Tsukamoto and Tojo 1992). As suggested in P. japonensis (Tsukamoto and Tojo 1992), it is possible that A. triguttulus females guard eggs against fungal infection as well as predation.
References
Agrawal AF, Brodie ED III, Brown J (2001) Parent-offspring coadaptation and the dual genetic control of maternal care. Science 292:1710–1712
Agrawal AF, Combs N, Brodie ED III (2005) Insights into the costs of complex maternal care behavior in the burrower bug (Sehirus cinctus). Behav Ecol Sociobiol 57:566–574
Beattie AJ (1985) The evolutionary ecology of ant-plant mutualisms. Cambridge University Press, Cambridge
Bottrell DG, Barbosa P, Gould F (1998) Manipulating natural enemies by plant variety selection and modification: a realistic strategy? Annu Rev Entomol 43:347–367
Costa J (2006) The other insect societies. Belknap Press of Harvard University Press, Cambridge, MA
Eberhard WG (1975) The ecology and behavior of a subsocial pentatomid bug and two scelionid wasps: strategy and counterstrategy in a host and its parasites. Smithson Contrib Zool 205:1–39
Filippi L, Hironaka M, Nomakuchi S, Tojo S (2000) Provisioned Parastrachia japonensis (Hemiptera: Cydnidae) nymphs gain access to food and protection from predators. Anim Behav 60:757–763
Filippi L, Hironaka M, Nomakuchi S (2001) A review of the ecological parameters and implications of subsociality in Parastrachia japonensis (Hemiptera: Cydnidae), a semelparous species that specializes on a poor resource. Popul Ecol 43:41–50
Filippi L, Hironaka M, Nomakuchi S (2002) Risk-sensitive decisions during nesting may increase maternal provisioning capacity in the subsocial shield bug Parastrachia japonensis. Ecol Entomol 27:152–162
Filippi-Tsukamoto L, Nomakuchi S, Kuki K, Tojo S (1995) Adaptiveness of parental care in Parastrachia japonensis (Hemiptera: Cydnidae). Ann Entomol Soc Am 88:374–383
Hardin MR, Tallamy DW (1992) Effects of predators and host phenology on the maternal and reproductive behaviors of Gargaphia lace bugs (Hemiptera: Tingidae). J Insect Behav 5:177–192
Hironaka M, Nomakuchi S, Iwakuma S, Filippi L (2005) Trophic egg production in a subsocial shield bug, Parastrachia japonensis Scott (Heteroptera: Parastrachiidae), and its functional value. Ethology 11:1089–1102
Hölldobler B, Wilson EO (1990) The ants. Belknap Press of Harvard University Press, Massachusetts
Kight SL (1997) Factors influencing maternal behaviour in a burrower bug, Sehirus cinctus (Heteroptera: Cydnidae). Anim Behav 53:105–112
Kight SL (1998) Precocene II modifies maternal responsiveness in the burrower bug, Sehirus cinctus (Heteroptera). Physiol Entomol 23:38–42
Kight SL, Cseke JJ (1998) The effects of ambient temperature on the duration of maternal care in a burrower bug (Heteroptera: Cydnidae). J Kans Entomol Soc 71:185–187
Kölliker M, Vancassel M (2007) Maternal attendance and the maintenance of family groups in common earwigs (Forficula auricularia): a field experiment. Ecol Entomol 32:24–27
Kölliker M, Chuckalovcak JP, Brodie ED III (2005) Offspring chemical cues affect maternal food provisioning in burrower bugs, Sehirus cinctus. Anim Behav 69:959–966
Kölliker M, Chuckalovcak JP, Haynes KF, Brodie ED III (2006) Maternal food provisioning in relation to condition-dependent offspring odours in burrower bugs (Sehirus cinctus). Proc R Soc Lond B 273:1523–1528
Kudo S (1990) Brooding behavior in Elasmucha putoni (Heteroptera: Acanthosomatidae), and a possible nymphal alarm substance triggering guarding responses. Appl Entomol Zool 25:431–437
Kudo S (1996) Ineffective maternal care of a subsocial bug against a nymphal parasitoid: a possible consequence of specialization to predators. Ethology 102:227–235
Kudo S (2000) The guarding posture of females in the subsocial bug Elasmucha dorsalis (Heteroptera: Acanthosomatidae). Eur J Entomol 97:137–139
Kudo S (2002) Phenotypic selection and function of reproductive behavior in the subsocial bug Elasmucha putoni (Heteroptera: Acanthosomatidae). Behav Ecol 13:742–749
Kudo S, Hasegawa E (2004) Diversified reproductive strategies in Gonioctena (Chrysomelinae) leaf beetles. In: Jolivet PH, Santiago-Blay JA, Schmitt M (eds) New developments in the biology of Chrysomelidae. SPB Academic Publishing bv, The Hague, Netherlands, pp 727–738
Kudo S, Nakahira T (2004) Effects of trophic-eggs on offspring performance and rivalry in a sub-social bug. Oikos 107:28–35
Kudo S, Nakahira T (2005) Trophic-egg production in a subsocial bug: adaptive plasticity in response to resource conditions. Oikos 111:459–464
Kudo S, Sato M, Ohara M (1989) Prolonged maternal care in Elasmucha dorsalis (Heteroptera, Acanthosomatidae). J Ethol 7:75–81
Kudo S, Maeto K, Ozaki K (1992) Maternal care in the red-headed web-spinning sawfly, Cephalcia isshikii (Hymenoptera: Pamphiliidae). J Insect Behav 5:783–795
Kudo S, Ishibashi E, Makino S (1995) Reproductive and subsocial behaviour in the ovoviviparous leaf beetle Gonioctena sibirica (Weise) (Coleoptera: Chrysomelidae). Ecol Entomol 20:367–373
Kudo S, Nakahira T, Saito Y (2006) Morphology of trophic eggs and ovarian dynamics in the subsocial bug Adomerus triguttulus (Heteroptera: Cydnidae). Can J Zool 84:723–728
Lamb RJ (1976) Parental behavior in the Dermaptera with special reference to Forficula auricularia (Dermaptera: Forficulidae). Can Entomol 108:609–619
Mappes J, Mappes T, Lappalainen T (1997) Unequal maternal investment in offspring quality in relation to predation risk. Evol Ecol 11:237–243
Marquis RJ (1996) Plant morphology and recruitment of the third trophic level: subtle and little-recognized defenses? Oikos 75:330–334
Melber A, Schmidt GH (1975) Sozialverhalten zweier Elasmucha-Arten (Heteroptera: Insecta). Z Tierpsychol 39:403–414
Melber A, Schmidt GH (1977) Sozialphänomene bei Heteropteren. Zoologica 127:19–53
Melber A, Hölscher L, Schmidt GH (1980) Further studies on the social behaviour and its ecological significance in Elasmucha grisea L. (Hem.-Het.: Acanthosomatidae). Zool Anz 205:27–38
Nafus DM, Schreiner IH (1988) Parental care in a tropical nymphalid butterfly Hypolimnas anomala. Anim Behav 36:1425–1431
Nakahira T (1992) Reproductive history and parental behaviour in the cydnid bug Adomerus triguttulus. M.Sc. Thesis. Hokkaido University, Sapporo (in Japanese)
Nakahira T (1994) Production of trophic eggs in the subsocial burrower bug Admerus (sic) triguttulus. Naturwissenschaften 81:413–414
Nomakuchi S, Filippi L, Tojo S (1998) Selective foraging behavior in nest-provisioning females of Parastrachia japonensis (Hemiptera: Cydnidae): cues for preferred food. J Insect Behav 11:605–619
Ohara M, Higashi S (1987) Interference by ground beetles with the dispersal by ants of seeds of Trillium species (Liliaceae). J Ecol 75:1091–1098
Oi DH, Pereira RM (1993) Ant behavior and microbial pathogens (Hymenoptera: Formicidae). Fla Entomol 76:63–74
Price PW, Burton CE, Gross P, McPherson BA, Thompson JN, Weise AE (1980) Interactions among three trophic levels: influence of plants on interactions between insect herbivores and natural enemies. Annu Rev Ecol Syst 11:41–65
Schmid-Hempel P (1998) Parasites in social insects. Princeton University Press, Princeton, NJ
Smith RL (1997) Evolution of paternal care in the giant water bugs (Heteroptera: Belostomatidae). In: Choe JC, Crespi BJ (eds) The evolution of social behavior in insects and arachnids. Cambridge University Press, Cambridge, pp 116–149
Strohm E, Linsenmair KE (2001) Females of the European beewolf preserve their honeybee prey against competing fungi. Ecol Entomol 26:198–203
Suzuki S (2001) Suppression of fungal development on carcasses by burying beetle Nicrophorus quadripunctatus (Coleoptera: Silphidae). Entomol Sci 4:403–405
Tachikawa S (1991) Studies on subsocialities of heteroptera in Japan. Tokyo Agricultural University Press, Tokyo
Tallamy DW (2001) Evolution of exclusive paternal care in arthropods. Annu Rev Entomol 46:139–165
Tallamy DW, Denno RF (1981) Maternal care in Gargaphia solani (Hemiptera; Tingidae). Anim Behav 29:771–778
Tallamy DW, Schaefer CW (1997) Maternal care in the Hemiptera: ancestry, alternatives, and current adaptive value. In: Choe JC, Crespi BJ (eds) The evolution of social behavior in insects and arachnids. Cambridge University Press, Cambridge, pp 94–115
Tallamy DW, Wood TK (1986) Convergence patterns in subsocial insects. Annu Rev Entomol 31:369–390
Tsukamoto L, Tojo S (1992) A report of progressive provisioning in a stink bug, Parastrachia japonensis (Hemiptera: Cydnidae). J Ethol 10:21–29
Windsor DM (1987) Natural history of a subsocial tortoise beetle, Acromis sparsa Boheman (Chrysomelidae, Cassidinae) in Panama. Psyche 94:127–150
Wood TK (1976) Alarm behavior of brooding female Umbonia crassicornis (Homoptera: Membracidae). Ann Entomol Soc Am 69:340–344
Acknowledgments
This study was supported in part by Grant-in-Aid for Scientific Research (C: 13640628) from the Japan Society for the Promotion of Science.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nakahira, T., Kudo, Si. Maternal Care in the Burrower Bug Adomerus triguttulus: Defensive Behavior. J Insect Behav 21, 306–316 (2008). https://doi.org/10.1007/s10905-008-9129-0
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10905-008-9129-0