Abstract
Maternal care in spiders often involves behaviors associated with the protection of eggs and spiderlings against parasitoids and predators (including conspecifics). The females of several species have been documented to move their egg sacs away from natural enemies or to invest in active defense behaviors against web invaders, such as parasitoid wasps or araneophagic spider species, to protect their brood. In this study, we present observations of protective behavior by Uloborus sp. females carrying egg sacs. We also investigated whether brood size and female size influence female aggressive behaviors and response time against an artificial source of disturbance. Females carrying egg sacs almost immediately perceived and reacted aggressively against the artificial stimulus, whereas females without egg sacs moved away or ran to the web margins, avoiding the source of disturbance. The aggressive response was independent of clutch size and female body size, indicating that all females will risk interacting with potential agents of egg mortality. This systematic response by all females with egg sacs may be important for reducing the incidence of attack by the egg predator wasp Bathyzonus sp. (Ichneumonidae).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
According to Trivers (1972), parents should adjust current care in relation to initial investment to minimize the loss of reproductive effort. Thus, in situations where mothers could efficiently prevent egg loss due to agonistic interactions with predators and parasitoids, intensive care is expected, especially after large investment in egg production (e.g., Wallin 1987, Koskela et al. 2000). However, maternal care is costly in cases in which mothers are able to breed again, because females (1) usually reduce foraging activity, limiting the amount of resources available for future reproduction events, and (2) often risk the possibility of producing a new brood by protecting the existing brood in dangerous situations (Clutton-Brock 1991, Buzatto et al. 2007). Therefore, decisions about parental investment must reflect a balance between costs (e.g., energy loss, risk of injuries) and benefits (e.g., probability of brood survivorship) of each behavior and also initial investment in reproductive events (e.g., the value of the protected brood and possibility of new reproductive events).
Maternal care in spiders always involves the construction of a protective silk structure for the eggs, which is usually placed in suitable sites for optimal development (Austin 1985, Hieber 1992, De Vito and Formanowicz 2003, Pike et al. 2012, Yip and Rayor 2014) and/or continuously transported by females (Townley and Tillinghast 2003). The egg sac often operates as a very complex barrier and is sometimes composed of several layered sheets containing fibers from different silk glands (Lepore et al. 2012, Gheysens et al. 2005). It may also contain additional defensive systems (e.g., the incorporation of urticating hairs into the silk, see Marshall and Uetz 1990) or may remain concealed within the detritus columns of stabilimenta (Gonzaga and Vasconcellos-Neto 2005), curled dead leaves (Moya et al. 2010), folded green leaves (Zanatta 2013), under rocks (Pike et al. 2012), or within other types of shelters (see Manicom et al. 2008). The efficiency of the egg sac silk layers at maintaining appropriate temperature and humidity conditions (Opell 1984) and reducing the exposure of eggs to predators and parasitoids (Hieber 1984, Austin 1985, Hieber 1992) are fundamental for female spider reproductive success.
Covering the eggs in silk and placing the egg sac in a suitable site is certainly important; however, many species provide additional protection against predators and parasitoids during egg development (Fink 1987, Whitehouse and Jackson 1998, Gonzaga and Leiner 2013). For example, Gonzaga and Leneir (2013) showed that the survivorship of Helvibis longicauda (Theridiidae) eggs and spiderlings depends on active protection by their mothers against conspecific web invaders. Delena cancerides (Sparassidae) mothers also are effective at chasing and killing potential predators introduced experimentally in their nests (Yip and Rayor 2011).
Within the orb-weaver family Uloboridae, the described protective behaviors of mothers during the egg sac guarding period include (1) the removal of egg sacs to the edge of webs when under threat by Philoponella republicana (Hoffmaster 1982), (2) the placement of the egg sac in a protected area near the orb (usually under a rock or log) by P. oweni (Smith 1997), and (3) the direct attack of parasitoid wasps by Uloborus glomosus (Cushing 1989). During these attacks, U. glomosus females have been observed to jerk the webs and making sweeping motions in response to the parasitoid wasp Arachnopteromalus dasys (Pteromalidae) crawling on the egg sacs (Cushing 1989, Cushing and Opell 1990). Yet, Smith (1997) observed the same parasitoid species attacking the egg sacs of Philoponella arizonica and P. owenii, but these two spider species did not respond aggressively to protect their eggs. The absence of defensive reactions against A. dasys was also observed by Santos and Gonzaga (in prep.) for a new genus of Uloboridae from southeastern Brazil.
It is important to notice that production of additional egg sacs is common in uloborids. Smith (1982), for example, observed that females of Philoponella oweni begin to construct egg-cases in late June and early July and continue to lay eggs through September. After spiderlings emergence the female discards the egg-case, leaves the retreat and spins a new prey capture orb. According to Lubin (1980), Philoponella republicana also guard a single egg sac at a time, but females can produce up to 3 egg sacs during their lifetime (and about 121 eggs per egg sac). In Uloborus glomosus, smaller egg sacs continued to be produced, each being added to the end of a previous egg sac, forming a chain. Peaslee & Peck (1983) also observed the construction of up to 5 egg sacs by Octonoba octonarius (total clutch size varying from 45 to 107 eggs), Grismado (2004) observed multiple egg sacs in Conifaber guarani and Conifaber yasi, and Opell et al. (2007) in Waitkera waitakerensis. Investment in egg defense in cases in which further reproductive events are possible is an indicative of the importance of this behavior to offspring survivorship.
In this study, we evaluated maternal responses to threats in a species of the family Uloboridae, taking previous investment in reproduction (number of eggs in the egg sac) and mother body size into account. In addition to previous reproductive investment, mother body size may affect directly the quality and quantity of maternal care (Steiger 2013) and may be correlated to (1) the risk of injury and/or death during agonist interactions with potential predators and parasitoids, (2) the probability of success during a confrontation, (3) the energetic cost of egg protection, and (4) the amount of energetic resources available for further egg production. Even small mothers, however, may always interact aggressively with potential predators and parasitoids in situations involving large clutches, low injure risk, significant increase in offspring survivorship and/or impossibility to breed again. Thus, females that are able to produce additional offspring and are unable to successfully increase the survivorship of the current brood would be prone to avoid aggressive interactions with predators or parasitoids. On the other hand, an effective reduction in offspring mortality due to female actions and a possible restriction in the opportunities of further reproductive events would result in prompt and consistent responses to stimuli simulating predators. We also described the behavioral repertoire of females during egg sac protection and the frequency of predator wasps attacking the egg sacs.
Material and methods
Study site
Spiders were collected in November 2014 in a Eucalyptus plantation in Fazenda Nova Monte Carmelo, property of Duratex S.A. (18° 45′ 11″ S, 47° 51′ 28″ W), Estrela do Sul, Minas Gerais, Brazil. Areas covered by introduced Eucalyptus are interspersed with fragments of native savanna (Cerrado) in Fazenda Nova Monte Carmelo. Uloborus sp. primarily construct their webs on the lower branches of Eucalyptus, close to the litter layer (0–50 cm).
Study species
The genus Uloborus is currently composed of 80 species, 16 of which occur in South America, with only 4 of these species occurring in Brazil; specifically, Uloborus ater Mello-Leitão 1917, Uloborus minutus Mello-Leitão 1915, Uloborus niger Mello-Leitão 1917, and Uloborus tetramaculatus Mello-Leitão 1940 (World Spider Catalog 2015). All of the Brazilian Uloborus species have been described by Mello-Leitão based on female specimens alone, with three of these species being collected from southeastern Brazil. The descriptions of these species are restricted to color patterns (Mello-Leitão 1915, 1917); thus, it remains unclear as to whether the species studied in Fazenda Nova Monte Carmelo is actually one of these four species. A taxonomic revision of South American uloborids is required to resolve these problems and allow progress on ecological studies on this group.
Procedures
All spiders and egg sacs were collected in the field and on the same day in November 2014. We compared the web construction patterns of females with and without egg sacs by analyzing photographs of webs taken before sampling.
We collected 28 females carrying egg sacs and 18 adult females without egg sacs to conduct behavioral experiments. Each female was maintained in a plastic container (2500 ml) with Eucalyptus sticks to allow web construction. After web construction (48 h after introduction to containers), we performed artificial simulations of wasp attacks by touching the egg sacs gently with a brush. For females without egg sacs, the stimulus was applied on the web close to their abdomen. Artificial stimuli are often used to simulate prey items and potential predators or parasitoids in experiments with spiders (e.g., Tolbert 1975, Coyle 1986, Cushing and Opell, 1990, Mooney and Haloin 2006). However, while this method is inefficient at producing vibrational stimuli similar to those produced by wasps, we assumed that spiders would attack any source of potential risk to their eggs.
We filmed the reactions of all spiders to the stimulus for up to 5 min. When there was no response, we continued recording for at least 10 min. After this procedure, we kept the egg sacs in the containers until the spiderlings emerged. The video recordings were used to document (1) the time at which females first reacted, (2) the time at which females attacked the source of disturbance, and (3) the behavioral repertoire of females throughout the experiment. We also documented the presence or absence of parasitoid and predator larvae already inside eggs after the completion of the behavior response tests, and the number of spiderlings that emerged from each egg sac. The number of eggs and the total body length of spiders were determined using a stereomicroscope (Leica 250C, Wetzlar, Germany) equipped with a SmartTouch controller, a Leica DFC 290 camera, and an LAS Application Suite assembling Interactive Measurement Module (Leica). We assembled an ethogram depicting the behavioral sequences and recorded the number of spiders performing each behavior.
We performed two multiple linear regressions between the dependent variables related to spider aggressiveness, expressed by the time elapsed from the introduction of the stimuli to the first spider reaction (reaction time) and by the time elapsed until the attack against the source of disturbance (attacking time), and the independent variables clutch size and female body length. We also included in the model the interaction between clutch size and female body length. Data normality was verified by the Shapiro-Wilk test (Razali and Wah 2011) and, when necessary, data was transformed to log10. For the purpose of the analysis, the variables clutch size and female body length were centered on their means (i.e., the mean value was subtracted from each observation) to reduce the covariation between linear variables and their interaction terms (Aiken and West 1991). As the maximum reaction time was 32 s and each video lasted 100 s, we used the value of 100 s to represent females that did not respond to stimulation. The same value was used to represent females that did not attack. The response of females carrying egg sacs and adult females collected before egg laying was compared by a t test, based on the reaction time after the stimulus. All statistical analyses were conducted using the software IBM SPSS Statistics (Version 20.0.0).
Results
Before egg laying, Uloborus sp. females remain at the hub of their horizontal orb webs in a typical resting position, extending the first and second pair of long and slender legs to the front of the bodies (Fig. 1a–c). Spiders only abandon this position when capturing prey or copulating. However, the webs of adult females carrying egg sacs differ to those of females without egg sacs. When carrying an egg sac, females build webs with widely spaced threads, lacking spirals and regular radii distribution (Fig. 1d–e). This modified web design is not suitable for intercepting and retaining insects but allows the egg sac to be aligned with the mother and suspended away from potential predators, such as ants (Fig. 2). When resting on these webs, females are positioned with the first two pairs of legs stretched forward and the egg sacs aligned with the abdomen. The overall appearance resembles a suspended twig, especially at a certain distance (Fig. 2a). The Uloborus sp. egg sac is tubular and elongated, containing an average of 89.6 eggs (sd = 37.33, n = 45 egg sacs, range 29–169 eggs). Eggs range from 0.43 to 1.15 mm in diameter (n = 2324 eggs from 20 egg sacs, mean ± sd = 0.70 ± 0.12 mm). After leaving the egg sac, spiderlings remain aggregated around the mother for about 2 days, after which they build their own webs that are initially attached to the web of their mother (Fig. 2b).
The female behavioral repertoire after the initial stimulus included: (1) placing tension on the web threads (the spider used the first and second pairs of legs to extend the web thread connected to the egg sac); (2) vibrating the web (the spider remained in its original position, but shook the web vigorously); (3) attacking the source of disturbance (the spider turned in the direction of the brush and ran toward it); (4) inspecting the egg sac (the spider walked along all of the egg sac extensions several times, touching the sack surface with its legs and chelicerae). Placing tension on the web threads and vibrating the web were most commonly documented behaviors, followed by direct attack toward the source of disturbance and inspection of egg sacs (Fig. 3). From the 28 females analyzed, only one did not respond to stimulation, remaining in the resting position throughout the assay. In the assays of spiders without egg sacs, the females performed the escape behaviors from potential enemies, including moving a few centimeters away or running to the web margins. Again, only one spider showed no reaction to the stimulus.
a, c Behavioral repertoire during egg sac protection by Uloborus sp. females, where 1 represents the resting position; 2 represents the position in which females place tension on the web and vibrate the web; 3–5 represent the attack time, 6–9 represent the inspection of egg sacs after the attack has ended. b Behavioral repertoire in assays of adult females without eggs sacs
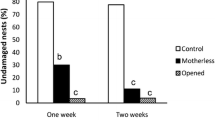
After the experiments, we opened all of the egg sacs and found that the sac belonging to the female that failed to react was occupied by the larva of an egg predator wasp. This larva belonged to a new undescribed species of Bathyzonus (Ichneumonidae) (Fig. 4). After examining 60 egg sacs to evaluate the frequency of parasitism or predation, we found only one other specimen of Bathyzonus sp. The incidence of egg sacs being subject to predation was just 1.67 %.
Reaction time and the time until females initiated attack were not related to female body length, to clutch size or to interaction term between these variables (Tables 1 and 2, Fig. 5). In addition, there were no significant differences between the reaction time of spiders with and without egg sacs (t = −0.656; df = 40; p = 0.5).
Relationship between the independent variables female body length and number of eggs in the egg sac (clutch size), the time elapsed from the introduction of the stimuli to the first spider reaction (reaction time), and the time elapsed until the attack against the source of disturbance (attacking time). Data from females carrying egg sacs
Discussion
Webs constructed by females during the period of maternal care appear to be completely ineffective at trapping and retaining insects. These webs resemble simple “skeleton” webs constructed by other orb-weaver species during molting (Robinson and Robinson 1973, Gonzaga et al. 2010) and some modified cocoon webs spun by spiders attacked by ichneumonid parasitoids (Gonzaga et al. 2015). The absence of cribellate spirals and the presence of fewer radii may enhance web stability and tenacity, reducing damage that occurs when insects become trapped. These webs are also apparently less conspicuous to visually oriented predators. Moreover, less silk is used to build these modified webs compared to that used in normal orb-web construction, which may be crucial for preserving the energy reserves of females when guarding egg sacs. Females, however, are unable to capture prey while carrying egg sacs, allocating all their energy reserves to guarding the eggs. These costs may compromise female survival and, perhaps, future reproductive events.
Changes to web design during the reproductive period are relatively rare in spiders, with only a few cases being reported for orb weavers (Higgins 1990, Sherman 1994). Cases where maternal care is associated with a long period of food abstinence are also uncommon in orb web spiders but have been extensively studied in wolf spiders (e.g., Nyffeler 2000). By analyzing the web construction of many spider species in the genus Miagrammopes, Lubin et al. (1978), reported that during the egg carrying period, Miagrammopes simus (Uloboridae) females do not build webs to capture insects. However, the authors recorded that another species of Miagrammopes maintains a cryptic posture and does not capture prey during the day but then abandons the cryptic posture and spins a cribellate thread at night to forage.
In the current study, Uloborus sp. females carrying egg sacs reacted aggressively toward the source of disturbance, performing a sequence of behaviors very similar to those observed in U. glomosus (Cushing 1989, Cushing and Opell 1990). Cushing and Opell (1990) showed that, when disturbed by the wasp A. dasys (Pteromalidae), U. glomosus responded by jerking their webs and, sometimes, turning around and walking along the egg sacs, sweeping the surface with their front legs. However, the authors also observed that the spiders jerk the webs when spiderlings cause vibrations, suggesting that U. glomosus has a similar response to all stimuli perceived on the egg sac surface. Besides jerking behavior, the responses of Uloborus sp. females also included aggressive attacks against the source of disturbance.
Uloborus sp. females responded to the artificial stimulus in different ways; specifically, females tried to vibrate or place tension on the web threads to drive away the predator/parasitoid, after which they usually attacked the source of disturbance and inspected the egg sacs after the disturbance had stopped. Despite all of these behaviors, enemies may still gain access to the egg sacs and prey on the eggs. In our study, one female showed no response (any reaction or attack) toward the source of disturbance. Offspring desertion is expected, and may represent an adaptive reproductive strategy, in which when parents forgo the costly and/or risky care of an unprofitable current brood to save resources or to increase their own survivorship probability for investing in a future reproductive event (Ward et al. 2009). Another possibility in this particular case is that the egg sac of this female was attacked because she was incapable of expressing the protective behavior. Unlike spiders protecting their broods, females without egg sacs responded to the stimulus by moving away or running to the edge of the web. Thus, the jerking of threads by mothers and attacking the source of disturbance certainly represent actions to protect eggs against predators or parasitoids.
Several studies on other taxonomic groups with extended maternal care have demonstrated a relationship between the intensity and duration of protective behaviors with the body size/condition of a mother, the value of the protected brood (number of individuals, proportion of mother’s total reproductive capacity), and the ability of a mother to produce effective protective actions (Montgomerie and Weatherhead 1988, Buzatto et al. 2007, Ward et al. 2009, Kazama et al. 2010). Our experiment showed that the protective behavior of Uloborus sp. is independent of the body size of a mother and number of eggs in the egg sac. It would be useful to determine whether these spiders are able to produce a new egg sac after the first reproductive event. The large costs and risks of protecting the current egg sac suggest that (1) maternal behavior may be important for brood survivorship during egg phase, (2) females are restricted to one reproductive event, and (3) attacking to prevent egg mortality may enhance female fitness and the benefits are independent of female body size.
Few studies have described the natural enemies of Uloborids in the published literature. Most studies on the subject provide information about attacks by the egg predator A. dasys on the egg sacs of several species. This predator has been documented to consume the eggs of Octonoba sinensis (Gordh 1976), P. arizonica (Smith 1997), P. oweni (Smith 1982, 1997), U. glomosus (Cushing 1989), an unidentified species of Uloboridae (Gordh 1983), a species from a new genus in southeastern Brazil (Santos and Gonzaga, in prep), and O. sinensis (Peaslee and Peck 1983). In the populations of some of these species, the incidence of A. dasys predation is extremely high, reaching 58 % of all collected egg sacs (see Peaslee and Peck 1983). Among egg parasitoids, there is only one record of Idris sp. (Platigastridae) attacking the eggs of a Uloborus species that lives as a commensal in colonies of Stegodyphus sarasinorum (Eresidae) (Bradoo 1972). Here, we presented the first register of predation of an uloborid by a species of Bathyzonus, a genus with an unknown natural history.
In the current study, the proportion of Uloborus sp. egg sacs that had been attacked by the hymenopteran egg predator was very low when compared to the incidence of A. dasys predation observed in other species. The aggressiveness of Uloborus sp. against possible sources of egg mortality may contribute to this low incidence of attack. Alternatively, it may also be explained by a possible low-density Bathyzonus sp. at the study site. Finally, this predator species may attack other uloborids (or even other groups of spiders) and may not be so efficient at locating the cryptic egg sacs of Uloborus sp.
Cushing and Opell (1990) suggested that the egg sac chains of U. glomosus function as cryptic devices making the spiders aligned with them less conspicuous to visual-oriented predators and, consequently, less likely to show active avoidance behaviors when disturbed. The behavior of remaining aligned with the egg sac in a position that may difficult to a predator to identify the contour of the spider body has also been observed in the araneids Cyclosa (Gonzaga and Vasconcellos-Neto 2005) and Allocyclosa (Eberhard 2003), as well as in other uloborids (e.g., Opell 1988). Opell (1980), for example, observed that females of Miagrammopes animotus remain aligned with their egg sacs during the day in a posture that enhances the twig-like appearance of both the female and her egg sac. This behavior, also observed in Uloborus sp., may be considered a component of maternal care, as it possibly reduces the probability of egg sacs being identified by predators, in addition to being an adaptation of mothers to reduce their own risk of predation during a period of immobility (and energy conservation) in their modified webs.
References
Aiken ES, West SG (1991) Multiple regression: testing and interpreting interactions. Sage Publications, London
Austin AD (1985) The functions of spider egg sacs in relation to parasitoids and predators with special reference to the Australian fauna. J Nat Hist 9:359–376
Bradoo NL (1972) Life histories and bionomics of Idris sp. (Scelionidae:Hymenoptera) egg parasite of Uloborus, a commensal on the web of social spider Stegodyphus sarasinorum Karsch. Zool Anz 188:43–52
Buzatto BA, Requena GS, Martins EG, Machado G (2007) Effects of maternal care on the lifetime reproductive success of females in a neotropical harvestman. J Anim Ecol 76:937–945
Clutton-Brock TH (1991) The evolution of parental care. Princeton University Press, Princeton
Coyle FA (1986) The role of silk in prey capture by nonaraneomorph spiders. In: Shear WA (ed) Spiders: webs, behavior, and evolution. Stanford University Press, Stanford, pp. 269–305
Cushing PE (1989) Possible eggsac defense behaviors in the spider Uloborus glomosus (Araneae: Uloboridae). Psyche 96:269–277
Cushing PE, Opell BD (1990) Disturbance behaviors in the spider Uloborus glomosus (Araneae, Uloboridae): possible predator avoidance strategies. Can J Zool 68:1090–1097
De Vito J, Formanowicz Jr DR (2003) The effects of size, sex, and reproductive condition on thermal and desiccation stress in a riparian spider (Pirata sedentarius, Araneae, Lycosidae). J Arachnol 31:278–284
Eberhard WG (2003) Substitution of silk stabilimenta for eggs sacs by Allocyclosa bifurca (Araneae: Araneidae) suggests that silk stabilimenta function as camouflage devices. Behaviour 140:847–868
Fink LS (1987) Green lynx spider egg sacs: sources of mortality and the function of female guarding (Araneae, Oxyopidae). J Arachnol 15:231–239
Gheysens T, Beladjal L, Gellynck K, Van Nimmen E, Van Langenhove L, Maertens J (2005) Egg sac structure of Zygiella x-notata (Arachnida, Araneidae). J Arachnol 33:549–557
Gonzaga MO, Leiner NO (2013) Maternal care and infanticide by males in Helvibis longicauda (Araneae: Theridiidae). Ethology 119:20–28
Gonzaga MO, Vasconcellos-Neto J (2005) Testing the functions of detritus stabilimenta in webs of Cyclosa fililineata and Cyclosa morretes (Araneae: Araneidae): do they attract prey or reduce the risk of predation? Ethology 111:479–491
Gonzaga MO, Sobczak JF, Penteado-Dias AM, Eberhard WG (2010) Modification of Nephila clavipes (Araneae, Nephilidae) webs induced by the parasitoids Hymenoepimecis bicolor and H. robertsae (Hymenoptera, Ichneumonidae). Ethol Ecol Evol 22:1–15
Gonzaga MO, Moura RR, Pêgo PT, Bang DL, Meira FA (2015) Changes to web architecture of Leucauge volupis (Araneae: Tetragnathidae) induced by the parasitoid Hymenoepimecis jordanensis (Hymenoptera: Ichneumonidae). Behaviour 152:181–193
Gordh G (1976) A new genus of Pteromalidae from Missouri, the type species of which parasitizes Uloborus octonarius Muma (Hymenoptera: Chalcidoidea; Araneidae: Uloboridae). J Kans Entomol Soc 49:100–104
Gordh G (1983) New distributional and host records for Arachnopteromalus dasys Gordh (Hymenoptera; Pteromalidae), an egg parasite of uloborid spiders. Proc Entomol Soc Wash 85:181
Grismado CJ (2004) Two new species of the spider genus Conifaber Opell 1982 from Argentina and Paraguay, with notes on their relationships (Araneae, Uloboridae). Rev Iber Aracnol 9:291–306
Hieber CS (1984) The role of the cocoons of orb-weaving spiders. University of Florida, Dissertation
Hieber CS (1992) Spider cocoons and their suspension systems as barriers to generalist and specialist predators. Oecologia 91:530–535
Higgins L (1990) Variation in foraging investment during the intermolt interval and before egg-laying in the spider Nephila clavipes. J Ins Behav 3:773–783
Hoffmaster DK (1982) Predator avoidance behaviors of five species of Panamanian orb-weaving spiders (Araneae; Araneidae, Uloboridae). J Arachnol 10:69–73
Kazama K, Niizuma Y, Watanuki Y (2010) Experimental study of the effect of clutch size on nest defense intensity in black-tailed gulls. Ornithol Sci 9:93–100
Koskela E, Juutistenaho P, Mappes T, Oksanen TA (2000) Offspring defence in relation to litter size and age: experiment in the bank vole Clethrionomys glareolus. Evol Ecol 14:99–109
Lubin YD (1980) Population studies of two colonial orb-weaving spiders. Zool J Linnean Soc 70:265–287
Lubin YD, Eberhard WG, Montgomery GG (1978) Webs of Miagrammopes (Araneae: Uloboridae) in the neotropics. Psyche 85:1–23
Lepore E, Marchioro A, Isaia M, Buehler MJ, Pugno NM (2012) Evidence of the most stretchable egg sac silk stalk, of the European spider of the year Meta menardi. PLoS One 7(2):e30500. doi:10.1371/journal.pone.0030500
Manicom C, Schwarzkopf L, Alford RA, Schoener TW (2008) Self-made shelters protect spiders from predation. PNAS 105:14903–14907
Marshall SD, Uetz GW (1990) Incorporation of urticating hairs into silk: a novel defense mechanism in two neotropical tarantulas (Araneae, Theraphosidae). J Arachnol 18:143–149
Mello-Leitão CF (1915) Alguns generos e especies novas de araneidos do Brasil. Broteria 13:129–142
Mello-Leitão CF (1917) Generos e especies novas de araneidos. Arch da Esc Super Agr e Med Veter 1:3–19
Montgomerie RD, Weatherhead PJ (1988) Risks and rewards of nest defence by parent birds. Quart Rev Biol 63:167–187
Mooney K, Haloin JR (2006) Spider size and guarding of offspring affect Paraphidippus aurantius (Araneae, Salticidae) response to predation threat. J Arachnol 34:98–103
Moya J, Quesada R, Barrantes G, Eberhard WG, Escalante I, Esquivel C, Rojas A, Triana E, Arias A (2010) Egg sac construction by folding dead leaves in Pozonia nigroventris and Micrathena sp. (Araneae: Araneidae). J Arachnol 38:371–373
Nyffeler M (2000) Do adult female lycosids feed during the period of maternal care? Bull Brit Arachnol Soc 11:388–390
Peaslee JE, Peck WB (1983) The biology of Octonoba octonarius (Muma) (Araneae: Uloboridae). J Arachnol 11:51–67
Pike DA, Webb JK, Shine R (2012) Hot mothers, cool eggs: nest-site selection by egg-guarding spiders accommodates conflicting thermal optima. Funct Ecol 26:469–475
Opell BD (1980) Egg sac recognition by female Miagrammopes animotus (Araneae, Uloboridae). J Arachnol 29:244–248
Opell BD (1984) A simple method for measuring desiccation resistance of spider egg sacs. J Arachnol 12:245–247
Opell BD (1988) Do female Miagrammopes animotus (Araneae, Uloboridae) spin color-coordinated egg sacs? J Arachnol 17:108–111
Opell BD, Berger AM, Shaffer RS (2007) The body size of the New Zealand orb-weaving spider Waitkera waitakerensis (Uloboridae) is directly related to temperature and affects fecundity. Invertebr Biol 126:183–190
Razali NM, Wah YB (2011) Power comparisons of Shapiro-Wilk, Kolmogorov-Smirnov, Lillifors and Anderson-Daling tests. J Stat Mod Analyt 2:21–33
Robinson MH, Robinson B (1973) The stabilimenta of Nephila clavipes and the origins of stabilimentum-building in araneids. Psyche 80:277–288
Sherman PM (1994) The orb-web: an energetic and behavioural estimator of a spider’s dynamic foraging and reproductive strategies. Anim Behav 48:19–34
Smith DR (1982) Reproductive success of solitary and communal Philoponella oweni (Araneae: Uloboridae). Behav Ecol Sociobiol 11:149–154
Smith DR (1997) Notes on the reproductive biology and social behavior of two sympatric species of Philoponella (Araneae, Uloboridae). J Arachnol 25:11–19
Steiger S (2013) Bigger mothers are better mothers: disentangling size-related prenatal and postnatal maternal effects. Proc R Soc B 280:20131225. doi:10.1098/rspb.2013.1225
Tolbert WW (1975) Predator avoidance behaviors and web defensive structures in the orb weavers Argiope aurantia and Argiope trifasciata (Araneae, Araneidae). Psyche 82:29–51
Townley MA, Tillinghast EK (2003) On the use of ampullate gland silks by wolf spiders (Araneae, Lycosidae) for attaching the egg sac to the spinnerets and a proposal for defining nubbins and tartipores. J Arachnol 31:209–245
Trivers RL (1972). Parental investment and sexual selection. In: Campbell B (ed.), Sexual selection and the descent of man, 1871–1971). Aldine, Chicago, pp. 136–179
Wallin K (1987) Defence as parental care in tawny owls (Strix aluco). Behaviour 102:213–230
Ward RJS, Cotter SC, Killner RM (2009) Current brood size and residual reproductive value predict offspring desertion in the burying beetle Nicrophorus vespilloides. Behav Ecol 20:1274–1281
Whitehouse MEA, Jackson RR (1998) Predatory behaviour and parental care in Argyrodes flavipes, a social spider from Queensland. J Zool Lond 244:95–105
World Spider Catalog (2015) World Spider Catalog, version 15.5. Natural History Museum Bern. http://wsc.nmbe.ch. Accessed 03 February 2015
Yip EC, Rayor LS (2011) Do social spiders cooperate in predator defense and foraging without a web? Behav Ecol Sociobiol 65:1935–1947
Yip EC, Rayor LS (2014) Maternal care and subsocial behaviour in spiders. Biol Rev 89:427–449
Zanatta MF (2013) História natural, seleção de folhas e locais para nidificação e efeito do cuidado materno em Aysha piassaguera Brescovit, 1992 (Araneae: Anyphaenidae) na Serra do Japi, Jundiaí-SP, Brasil. Dissertation, Universidade Estadual de Campinas
Acknowledgments
We thank Duratex S.A. for providing logistical support and for allowing the study in Fazenda Nova Monte Carmelo, Adalberto J. Santos for identification of Uloborus sp., and Angélica M.P. M. Dias for the identification of Bathyzonus sp. This project was supported by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (research grant to ALN), Fundação de Amparo à Pesquisa do Estado de Minas Gerais (Proc. APQ-02104-14, CRA-30058/12), Instituto Nacional de Ciência e Tecnologia dos Hymenoptera Parasitoides da Região Sudeste (HYMPAR/Sudeste–CNPq/CAPES/Fapesp) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (Proc. 306157/2014-4; 403733/2012-0, 445832/2014-2). Voucher specimens of Uloborus sp. were deposited in the collection of Universidade Federal de Minas Gerais (curator A.J. Santos), Minas Gerais, Brazil. This study complies with the current laws of Brazil.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nascimento, A.L., Gonzaga, M.O. Maternal defensive behaviors of Uloborus sp. (Araneae, Uloboridae): behavioral repertoire and influence of clutch size and female size on female aggressiveness. acta ethol 19, 33–41 (2016). https://doi.org/10.1007/s10211-015-0220-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10211-015-0220-1