Abstract
Aphaenogaster carolinensis Wheeler produces substrate vibrations by striking a substrate with its mandible and dragging its mandible across the surface. We have described this behavior and conducted laboratory trials to isolate some of the conditions under which it may occur. Individual strikes were consistent in pattern and duration, but there was great variation in the duration of, and the number of strikes associated with, a single substrate vibration generating event. In laboratory trials, this behavior occurred most frequently in response to confrontation with non-nest mate con-specifics and did not require the presence of a food source or colony territory to be initiated.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Substrate vibration generating behavior (SVGB) has been observed in some ant species. Past descriptions have generally focused on stridulation behavior, where a scraper on the petiole is rubbed against a file-like structure on the gaster. This behavior has been found to be associated with the performance of mechanical tasks in Solenopsis invicta (Buren) (Rauth and Vinson 2006), and for recruitment to food sources by leaf cutter ants (Roces and Hoelldobler 1996) and ants of the genera Messor (Grasso et al. 1999) and Aphaenogaster (Markl and Holldobler 1978; Partan and Marler 1999). Similar stridulation behavior has also been reported for another hymenopteran, the velvet ant (Mutillidae), where it has been demonstrated to be an effective method of defense against predators (Masters 1979). We have discovered a substrate vibration generating behavior, by the ant Aphaenogaster carolinensis Wheeler, that does not involve stridulation. This behavior was first observed at a baited ant collecting station that was fitted with an accelerometer to detect substrate vibrations by foraging ants. It was observed that A. carolinensis workers, after a confrontation with members of the same species, presumably from another nest, would sometimes scrape or strike with their mandibles at the substrate they were standing on, causing a vibration that was detectable by the accelerometer. Our goal with this project was to induce A. carolinensis to perform this behavior in a laboratory so that the behavior could be recorded and described, and to perform laboratory trials to isolate some of the conditions under which it may occur.
Methods
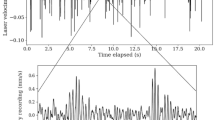
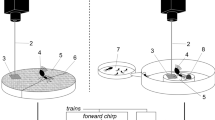
Workers of A. carolinensis, Aphaenogaster lamelidens Mayr, and Pheidole bicarinata Mayr were collected at baits from spatially separated locations (at least 30 m apart) within a hardwood forest on the campus of The University of Mississippi in the fall of 2006. Workers of the same species that were collected at a single location were treated as nest mates while those from separate locations were treated as non-nest mates. This treatment did allow for the possibility that individuals collected at the same location were from different nests, as was likely the case in our first observation of the SVGB, but it was far less likely that individuals collected from different locations would be nestmates, which was our primary concern. Individuals were brought into the laboratory the day of capture and placed in two way combinations (Table 1) in a 3-in. diameter circular metal container with a two-inch high wall that was coated with Fluon (Northern Products, Inc.) to prevent ant escape. The container was fitted from below with an accelerometer (1 V/G PCB 352B) attached by a magnet to monitor substrate vibration (Fig. 1). Forty trials were conducted involving two A. carolinensis workers from separate locations (non-nest mates). Half of these trials (n = 20) were conducted with, and the other half without, a food source (peanut butter cracker) present. Aphaenogaster carolinensis workers were also placed in the container with conspecifics from the same location (nest mates), with A. lamelidens workers, and with P. bicarinata workers in separate trials (Table 1). All trials were allowed to continue for up to either 30 min or until an SVGB was detected. No single ant was used in more than one trial. Ten trials during which substrate vibration generating behavior occurred were recorded using the accelerometer and a PCB Model 480M167 signal conditioner connected to a laptop through the microphone input (Fig. 1). Recording and analysis were facilitated by using RAVEN (Raven Pro 1.3, Cornell Lab of Ornithology 2006). A substrate vibration generating event was considered to be a collection of repeated strikes by an individual worker. From the recorded trials we were able to measure the duration of strikes, the number of strikes per event and the duration of events.
Visual documentation of substrate vibration generating behavior was achieved by recording two events using a high-speed video camera (Photron FASTCAM-ultima APX). One event was recorded from above in the aforementioned metal container at 4,000 frames/s (Fig. 2). The other was recorded in a glass beaker from a lateral vantage point at 1,000 frames/s (Fig. 3). Neither of these events were included in the trials described above because of the potential adverse effects of the intense lighting required for high speed video.
Results
Substrate vibration generating behavior was more likely to occur in trials involving two non-nest mate A. carolinensis workers (57.5%), than in trials where A. carolinensis was paired with other ant species (25%) (Yates corrected χ 2 = 4.43, p = 0.035) (Table 1). The presence of a food source did not alter the frequency of substrate vibration generating events. All encounters began with an initial period of antennae touching that could last for several minutes. When presumed nest mates were paired, except in one case where a SVGB occurred, this behavior was not followed by aggressive confrontation or posturing. In contrast, during numerous other trials involving non-nest mates or inter-specific pairs, there was great deal of aggressive behavior that followed, including chasing and the pinning of one ant by the other. The time between the initiation of trials and an SVGB event ranged from 38 s to almost 23 min, with only six of 29 events occurring within the first 3 min. There was substantial variation in the duration of, and the number of strikes associated with, single events (Table 2). The strikes themselves were less variable in duration (Table 2) and followed a typical pattern of a high power strike of the mandible, followed by a drag of the mandible across the substrate surface (Figs. 2, 3, 4).
A faint signal believed to be stridulation was detected during eight of the 70 trials. It only occurred during trials between non-nest mate conspecifics, and six of the eight occurrences were in the presence of food. Three of these stridulation events occurred during trials where the SVGB on which this paper is focused also occurred. The signal from the stridulation was very faint compared to the signal from the SVGB of interest, was inseparable from background noise visually in the wave form, and is not believed to have interfered with the signal we’ve described.
Discussion
The manner in which we have collected this data is not perfect, and further research should be conducted in a more natural setting, where the ants can interact freely and within the appropriate social context, rather than in pairs within a metal cup. That being said, there are some advantages to an experiment like the one we have performed, where the behavior has been generated in isolation from potentially important influences. The substrate vibration generating behavior detected here occurred independently from competition for food, and because workers were removed from the territory surrounding their nests for these trials, we can also say that it occurred independently from competition between colonies for territory. These two results, along with the predictability of the pattern and duration of strikes, lead us to the conclusion that this behavior is a direct response by A. carolinensis to the presence of non-nest mate con-specifics and, to a lesser degree, other ant species. It is similar to warning behaviors such as darting, forward jerking or thrusting that have been described for other social insects (Hermann 1984), but could also be related to a rapping on the substrate with the mandibles or gaster performed by some ant species of the genus Camponotus (Burschinger and Maschwitz 1984). In Camponotus, the rapping behavior was found to amplify the effect of other stimuli on nest-mates and is thought to be associated with attack and alarm situations (Burschinger and Maschwitz 1984).
Previous investigations have described stridulation behavior as occurring in response to the discovery of a food source (Markl and Holldobler 1978; Roces and Hoelldobler 1996; Grasso et al. 1999; Partan and Marler 1999). Our own results with regard to stridulation, although not sufficient in number on their own to be the basis of any conclusions, support this role for stridulation, because the majority of those events occurred in the presence of food (75%). This suggests that the striking behavior, which occurred with or without a food source equally, and stridulation, may serve different, if somewhat overlapping, functions for A. carolinensis.
References
Buschinger A, Maschwitz U (1984) Defensive behavior and mechanisms in ants. In: Hermann HR (ed) Defense mechanisms in social insect. Praeger, New York, pp 95–150
Grasso DA, Mori A, Le Moli F (1999) Recruitment and trail communication in two species of Messor ants (Hymenoptera: Formicidae). Ital J Zool 66:373–378
Hermann HR (1984) Defense mechanisms: general considerations. In: Hermann HR (ed) Defense mechanisms in social insects. Praeger, New York, pp 1–31
Markl H, Hoelldobler B (1978) Recruitment and food retrieval behavior in Novomessor (Formicidae: Hymenoptera). II. Vibrational signals. Behav Ecol Sociobiol 4:183–216
Masters WM (1979) Insect disturbance stridulation: its defensive role. Behav Ecol Sociobiol 5:187–200
Partan S, Marler P (1999) Communication goes multimodal. Science 283:1272–1273
Rauth SJ, Vinson SB (2006) Colony-wide behavioral contexts of stridulation in imported fire ants (Solenopsis invicta). J Insect Behav 19:293–304
Roces F, Hoelldobler B (1996) Use of stridulation in foraging leaf-cutting ants: mechanical support during cutting or short-range recruitment signal? Behav Ecol Sociobiol 39:293–299
Acknowledgements
The following individuals contributed to this project through their advice and assistance: Dr. Tom Fink and Vijay Ramalingam of the National Center for Physical Acoustics at the University of Mississippi, and Dr. James Anderson of the University of Mississippi Field Station. Equipment used for this project was provided by the Insect Acoustics lab at the National Center for Physical Acoustics, under the direction Dr. John Seiner.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Menzel, T.O., Marquess, J.R. The Substrate Vibration Generating Behavior of Aphaenogaster carolinensis (Hymenoptera: Formicidae). J Insect Behav 21, 82–88 (2008). https://doi.org/10.1007/s10905-007-9109-9
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10905-007-9109-9