Abstract
Pit-building antlions capture their prey by digging funnel-shaped pits in loose sand and then laying in wait for prey to fall inside the trap. Behavioral experiments studying predator–prey interactions and measurements of vibrations propagated in sandy substrates revealed that antlions are extremely sensitive to substrate vibrations produced by prey crawling on the sand surface. Prey produce low-frequency sand-borne vibrations, and to locate a source of vibration, antlions rely on time differences of waveforms arriving at their receptors—tufts of hairs positioned on lateral parts of the mesothorax and metathorax. In this chapter, the role of physical properties of sand in substrate-borne vibration transmission is discussed.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
It is well known that some insect predators detect their prey according to vibrations produced by the prey during crawling on solid surfaces (Čokl and Virant-Doberlet 2003b; Cocroft and Rodríguez 2005). This chapter describes the role of vibrations in predator–prey interactions in antlion larvae.
Antlions (Myrmeleontidae) are holometabolous insects whose larvae are known to dig conical pitfall traps in sand or loose soil to catch prey at the bottom of the trap. However, only one-tenth of antlion species are pit-builders. The other sand-dwelling antlions lurk, buried in the substrate, without constructing pits. Often, only the jaws and antennae of the buried pit-building larva are visible. This sit-and-wait predator feeds on small arthropods that slide into the trap.
Well documented is the catching behavior of pit-building antlion species of the genera Myrmeleon and Euroleon (for a review see Griffiths 1980; Napolitano 1998; Scharf and Ovadia 2006; Gepp 2010; Scharf et al. 2011). The most intensively studied species is a common European species, Euroleon nostras. An antlion can wait motionless at the bottom of the conical pit for hours and then suddenly moves like a flash to capture its prey, usually an ant or another small soft-bodied arthropod. An antlion waiting for its prey never moves from the center of the pit, but by opening its mandibles, it shows us that it has detected the ant, crawling on the sand surface, at a distance of a few decimeters from the pit. The larva with violent jerks of the head and thorax tosses sand upon the prey in order to disorient it.
If the prey falls into the conical pitfall trap, things go very fast. The antlion immediately tries to grasp the ant sliding into the pit. If the larva does not succeed in capturing the ant during its first attempt, and/or if the prey evades the larva and starts to climb out of the pit, the larva tosses sand with violent movements of its head and the prothorax, thereby causing miniature landslides carrying the prey back to the antlion’s jaws. The subsequent behavioral pattern is prey grasping. Should the ant prove difficult, the predator will start with prey beating (Napolitano 1998), i.e., it will often flick the ant back and forth against the sides of the pit. Prey beating is a behavior that tends to disorient the prey and thereby gives the predator valuable time to insert its mandibles. In so-called submergence, the larva holding the prey moves down and back into the substrate until the entire antlion and at least part of the prey are not visible. Then, poison is injected through the jaws. When the prey becomes motionless, emergence follows, i.e., the antlion with its prey moves up and forward until the entire prey and at least part of the antlion’s head is visible. After the ant is dead, enzymes are introduced and the body contents are digested and extracted. Prey clearing follows, when the jaws are used to position the dead prey on the dorsal head surface of the larva and then the head is flicked rapidly back, expelling the empty carcass from the pit. Finally, to repair the shape of the pit, pit clearing occurs when surplus sand accumulated on the bottom of the pit is expelled by violent flicks of the head.
This complex predatory behavior of antlions is obviously based on a considerable amount of sensory information. A number of questions arose:
-
1.
What signals are important in prey detection?
-
2.
How are the vibrations transmitted from prey to predator?
-
3.
What kinds of receptors are involved in the vibration detection?
-
4.
Over what range can the antlion detect its prey?
-
5.
Does the antlion detect the direction from which the stimulus is coming? Does the predator orient itself toward the prey?
2 Substrate Vibrations and Topography of the Receptors
Sensory receptors on the body surface of antlion larvae (Fig. 16.1) are involved in predator–prey interactions. The first study of the morphology and histology of antlion hair sensilla and eyes was done one hundred years ago when Doflein (1916) published his fundamental book on the biology of antlion larvae. Existing information on the presence and topography of sensilla using scanning electron microscopy is available for only a few antlion species (Nicoli Aldini 2007; Eisenbeis and Wichard 1987; Lipovšek Delakorda et al. 2009; Cesaroni et al. 2010; Devetak et al. 2010a, b, 2013).
Sensory equipment of a pit-building antlion larva (Euroleon nostras). a Sensilla coeloconica on the mandibles. b Sensilla basiconica on the tip of the antenna. c Sensilla basiconica (asterisk) on the tip of the labial palp. d Tuft of the mechanoreceptive bristles (sensilla chaetica). e Two campaniform sensilla (asterisk) on the tarsus of hind leg. f Campaniform sensillum and plumose hair on head. g Plumose hairs on mesothorax. h Larval eyes (consisting of six stemmata in each eye) positioned on eye tubercle, close to the antenna (a). Electron micrographs courtesy of M. A. Pabst
Chemoreceptors, involved in tasting prey, are sensilla coeloconica on the jaws and sensilla basiconica on the labial palps (Fig. 16.1). Any role of the larval eyes in predatory behavior has not yet been clarified sufficiently, although their structure is well known (Jockusch 1967). Antlions catch their prey even (or especially) during the night when their prey, small arthropods, is active. So vision does not play a major role. Campaniform sensilla (Fig. 16.1) serve to detect deformation of the cuticle, produced during movement of the legs, locomotion of the antlion and very probably also during direct contact with prey, for example, when the antlion holds prey or during prey beating.
The antlion detects its prey by sensing the vibrations that prey generate during crawling on the sand surface (Devetak 1985; Devetak et al. 2007; Fertin and Casas 2007). Mencinger (1998) demonstrated that the predator detects its prey even when vision is excluded. A larva with eyes covered with opaque paint still detects the source of vibration. Intact antlions react with sand tossing even if an observer with gentle movements of a twig or a pencil elicits substrate vibrations on the sand surface. Furthermore, antlion larva responded with violent sand tossing behavior to play back of vibrations recorded during locomotion of prey (Mencinger 2003; Fertin and Casas 2007).
Which receptors are the candidates for detecting substrate vibrations? Le Faucheux (1972) demonstrated that tufts of the mechanoreceptive bristles, sensilla chaetica, positioned on the thorax, play a certain role in detection of substrate vibrations. On the mesothorax and the metathorax, tufts of bristles occur in pairs, one tuft pair on each lateral side of the body segment (Fig. 16.1d). In intact larvae, the prey capture angle was 280°–290°. Both the ability to catch an ant and prey capture angle were diminished when certain groups of bristles were cut off. When the tufts of the sensilla were excluded unilaterally, the ability to catch prey was then limited only to the prey approaching the antlion from the contralateral side (Le Faucheux 1972). When one pair of the mesothoracic tufts was suppressed, the prey capture angle was reduced to 240°–280°. When two pairs of the mesothoracic tufts were eliminated, the angle changed to 200°–210°. When both mesothoracic pairs and one metathoracic pair of bristles were cut off, the prey capture angle was then reduced to 50°–60°. When all thoracic tufts were eliminated, larvae did not react to the presence of prey at all.
3 Vibrations Produced by the Prey
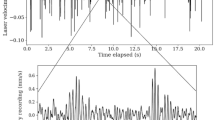
Prey animals—ants and similar small arthropods—produce low-frequency disturbances during walking or crawling on the sand surface. The frequency range of the vibrations produced during locomotion of four arthropod species (mealworm Tenebrio molitor, firebug Pyrrhocoris apterus, ant Formica sp. and woodlouse Trachelipus rathkii) is 0.1–4.5 kHz (Fig. 16.2), and acceleration values (peak level) of the vibrations, recorded at a distance 2–5 cm from the accelerometer, vary from 400 to 600 μm s−2 (ant, firebug) to 1–1.5 mm s−2 (mealworm, woodlouse) (Devetak et al. 2007) (Fig. 16.2).
3.1 How are Vibrations Transmitted from Prey to Predator?
The biological significance of substrate vibrations and mechanisms of signal transmission in plant-dwelling insects is well explored (Michelsen et al. 1982; Gogala 1985; Čokl and Virant-Doberlet 2003a, b). Vibration transmission in a sandy medium, however, has been studied only in desert scorpions (Paruroctonus), fiddler crabs (Uca), and antlions (Euroleon) (Brownell 1977; Brownell and Farley 1979a, b, c; Aicher and Tautz 1990; Devetak et al. 2007).
Transmission depends on a variety of factors, especially on frequency characteristics of the vibrations and physical properties of the sand. Natural sand occupied by antlions is a non-homogenous medium, containing small particles with different sizes and usually with larger stones or plant particles, like twigs and leaves, on the sand surface (Devetak 2000). The vibrations traveling from prey to predator are attenuated by twigs or small stones and reflected from solid objects (larger stones, rocks) (Fig. 16.3).
Reflections and attenuation of an artificial vibrational signal (250 Hz) produced by a Brüel & Kjaer 4810 vibration exciter and recorded with B and K 4381 accelerometer placed on the sand surface 20 cm apart; fine dry sand (particle size ≤0.5 mm) in a layer of 5-cm depth was placed in a plastic container. a Pure artificial signal with 250 Hz; b signal distortion by the reflection (asterisk) from the bottom of the container; c signal attenuation by an obstacle (a stone 5 × 4 × 0.5 cm) placed between the sender and the receiver; and d signal distortion by the reflection from the bottom of the container (asterisk) and by the reflection from a stone positioned 4 cm behind the receiver (double asterisk)
In the field, antlions are capable of discriminating between areas of sand differing in particle size, constructing pits in fine sand and avoiding coarser sand. This was confirmed in laboratory conditions when pit-building decisions and pit diameters depended on sand particle size (Botz et al. 2003; Devetak et al. 2005). When four fractions of sands differing according to particle size were offered to antlions, Euroleon nostras , the larvae preferred to build pits in the finer sand fraction, with a particle size of 0.23–0.54 mm (Devetak et al. 2005).
Sand strongly attenuates vibrations. The frequency spectra of vibrations of crawling prey differed when propagated in sands with different particle sizes (Devetak et al. 2007). The greater the sand particle size, the broader was the frequency range of the vibrations. The same was confirmed for artificial vibrations. Finer sand (particle size ≤0.23 mm) attenuated vibrations highly, and those recognized by antlion larvae traveled only a short distance. Five sands differing in particle size were tested. The damping coefficient (α10) of vibrations at a frequency of 300 Hz for the finest sand fraction (≤0.23 mm) was 2.61 dB cm−1; for two median sand fractions (0.23–0.54 mm, 0.54–1 mm), it was 0.74 and 0.45 dB cm−1, and for two coarser sand fractions (1–1.54 mm, 1.54–2.2 mm), it was 0.29 and 0.26 dB cm−1 (Devetak et al. 2007). In natural habitats, antlions usually occupy fine sands or sands with median particle size, so in those substrates moderate damping occurs, and due to low propagation velocities, the sand enables prey localization.
3.2 Over What Range Can the Antlion Detect Its Prey?
Antlions react to prey at maximal distances of a few decimeters (20–30 cm). Reaction distance is correlated positively with mean particle size (Devetak et al. 2007). In the finest sand fraction, the mean reaction distance was 3.3 cm; in two medium sand fractions, mean reaction distances were 5.5–9.1 cm, and in coarser sand, it was 12 cm.
4 Vibrations Produced by the Antlion
During pit construction, pit clearing and sand tossing toward the prey, jerking movements of the head and prothorax, both parts of the body serving as a shovel, are involved, thereby generating substrate vibrations (Fig. 16.4a). The head is moved left and right to collect sand, also with the help of foreleg movement, and then, the sand is tossed with a violent jerk of the head and prothorax in a dorsal direction. One vibrational “pulse” is composed of the head movements (left and right) and the dorsally oriented jerk. Pulse duration is about 150–300 ms, and the pause between two consecutive pulses lasts for about 200–400 ms. Vibrations produced thereby are very probably important also as an alerting mechanism for conspecifics to maintain a certain distance from the nearest neighbor.
a Signals of sand tossing in Euroleon: a oscillogram and b sonogram of jerking the head left and right c and throwing the sand toward the prey (d). b Oscillogram (a) and sonogram (b) of the signals produced during grasping the prey (asterisk) and during rhythmic movements of the larva burying the prey in the sand (submergence) (double asterisk). For details see text
It is presumed that the abdominal campaniform sensilla have a role in the control of backward digging in sand. During digging, rhythmic jerking movements of the abdomen occur, and the digging bristles on the tip of the abdomen are involved in these jerks (Fig. 16.5a, b). Vibrations with similar frequencies (up to 4–5 kHz) are produced also during grasping an arthropod prey (Fig. 16.4b) and prey beating (Fig. 16.5c). It seems that the time pattern of a vibrational pulse series produced during predatory activity carries more specific information than its frequency spectrum structure. It is possible that these vibrations are important as signals in conspecific communication. As Barkae et al. (2010) supposed, sand tossing may play a role in disturbance of neighboring conspecifics or even heterospecifics.
5 Prey Localization
A wide range of insects can locate a source of vibrations (Cocroft et al. 2000; Čokl and Virant-Doberlet 2003b; Virant-Doberlet et al. 2006). The exploitation of prey-generated vibrations is known in many predatory arthropods, such as spiders, scorpions, stinkbugs, and parasitoid wasps (Brownell and Farley 1979a, b, c; Pfannenstiel et al. 1995; Casas et al. 1998; Barth 2002).
5.1 Does the Antlion Detect the Direction from Which the Stimulus is Coming?
To answer this question, the accuracy of the sand-tossing behavior of Euroleon as a response to the presence of prey was measured using a video recording method (Mencinger 1998; Mencinger-Vračko and Devetak 2008). Sand tossing was elicited most frequently by prey behind the antlion; in contrast, there was no response when prey was in front of the antlion, in the so-called dead angle zone. The sand-tossing angle was highly positively correlated with the prey angle. The response was unaffected even when vision was excluded. When the antlion’s eyes were covered, the sand-tossing angle was still highly positively correlated with the prey angle, in response to mealworm beetles (Mencinger-Vračko and Devetak 2008) and woodlice (Fig. 16.6).
Sand-tossing angle as a function of prey angle. a The antlion is positioned in the center of the pit and its prey—the woodlouse Trachelipus—crawls outside the pit. The predator and the prey are not shown to scale. b and c Accuracy of sand-tossing response of antlions in the presence of the woodlouse: b unimpeded antlions; c antlions with eyes covered with opaque paint. Each dot represents a single response
Propagation velocities of surface vibrations (R-waves) in dry loose sand with particles ≤0.5 mm amount to 25–35 m/s and depend on the frequency (Mencinger-Vračko and Devetak 2008). Due to the low propagation velocities, the time and phase differences of the vibrations at the receptors—tufts of hairs on both lateral sides of the mesothorax and metathorax—may be expected to determine the prey angle. Time differences between vibrations originating in the lateral side of the sand were in the range of 0.2–0.5 ms.
6 Vibrations and Associative Learning in the Antlion
The learning ability of antlions in a context of detecting substrate vibrations has been proven recently (Guillette et al. 2009; Guillette and Hollis 2010; Hollis et al. 2011). In experimental conditions, individual antlions ( Myrmeleon crudelis ) received, once per day, either a vibrational cue presented immediately before feeding (the learning group) or the same cue presented independently of feeding (the control group). Vibrations simulating prey arrival not only produced an anticipatory learned response but also conferred a fitness advantage: Associative learning enabled antlions to extract food more efficiently, construct larger pits, and decrease the amount of time spent in the larval stage. The finding is important because the antlions do not fit the “learning profile” of active approach and avoidance behavior, and thus, they are unlike all other insect species studied to date (Guilette and Hollis 2010).
References
Aicher B, Tautz J (1990) Vibrational communication in the fiddler crab, Uca pugilator. I. Signal transmission through the substratum. J Comp Physiol A 166:345–353
Barkae ED, Scharf I, Subach A, Ovadia O (2010) The involvement of sand disturbance, cannibalism and intra-guild predation in competitive interactions among pit-building antlion larvae. Zoology 113:308–315
Barth FG (2002) A spider’s world: senses and behavior. Springer, New York
Botz JT, Loudon C, Barger JB, Olafsen JS, Steeples DW (2003) Effects of slope and particle size on ant locomotion: implications for choice of substrate by antlions. J Kans Entomol Soc 76:426–435
Brownell P, Farley RD (1979a) Detection of vibrations in sand by tarsal sense organs of the nocturnal scorpion, Paruroctonus mesaensis. J Comp Physiol 131:23–30
Brownell P, Farley RD (1979b) Orientation to vibrations in sand by the nocturnal scorpion Paruroctonus mesaensis: mechanism of target localization. J Comp Physiol 131:31–38
Brownell P, Farley RD (1979c) Prey-localizing behaviour of the nocturnal desert scorpion, Paruroctonus mesaensis: orientation to substrate vibrations. Animal Behav 27:185–193
Brownell PH (1977) Compressional and surface waves in sand: used by desert scorpions to locate prey. Science 197:479–482
Casas J, Bacher S, Tautz J, Meyhofer R, Pierre D (1998) Leaf vibrations and air movements in a leafminer—parasitoid system. Biol Control 11:147–153
Cesaroni C, Nicoli Aldini R, Pantaleoni RA (2010) The larvae of Gymnocnemia variegata (Schneider, 1845) and Megistopus flavicornis (Rossi, 1790) (Neuroptera: Myrmeleontidae): a comparative description. In: Devetak D, Lipovšek S, Arnett AE (eds) Proceedings of the tenth international symposium on neuropterology, Piran, Slovenia, 2008. FNM, Maribor, pp 135–144
Cocroft RB, Rodríguez RL (2005) The behavioral ecology of insect vibrational communication. Bioscience 55:323–334
Cocroft RB, Tieu T, Hoy RR, Miles R (2000) Mechanical directionality in the response to substrate vibration in a treehopper. J Comp Physiol A 186:695–705
Čokl A, Virant-Doberlet M (2003a) Communication with substrate-borne signals in small plant-dwelling insects. Ann Rev Entomol 48:29–50
Čokl A, Virant-Doberlet M (2003b) Vibrational communication. In: Resh VH, Carde RT (eds) Encyclopedia of insects. Academic Press, San Diego, pp 1167–1171
Devetak D (1985) Detection of substrate vibrations in the antlion larva, Myrmeleon formicarius (Neuroptera: Myrmeleonidae). Biol Vestn 33(2):11–22
Devetak D (2000) Competition in larvae of two European ant-lion species (Neuroptera: Myrmeleontidae). J Neuropterol 3:51–60
Devetak D, Špernjak A, Janžekovič F (2005) Substrate particle size affects pit building decision and pit size in the antlion larvae Euroleon nostras (Neuroptera: Myrmeleontidae). Physiol Entomol 30:158–163
Devetak D, Mencinger-Vračko B, Devetak M, Marhl M, Špernjak A (2007) Sand as a medium for transmission of vibratory signals of prey in antlions Euroleon nostras (Neuroptera: Myrmeleontidae). Physiol Entomol 32:268–274
Devetak D, Lipovšek S, Pabst MA (2010a) Larval morphology of the antlion Neuroleon microstenus (McLachlan, 1898) (Neuroptera, Myrmeleontidae), with notes on larval biology. Zootaxa 2428:55–63
Devetak D, Lipovšek S, Pabst MA (2010b) Morphology and biology of the antlion Myrmeleon yemenicus Hölzel, 2002 (Neuroptera, Myrmeleontidae). Zootaxa 2531:48–56
Devetak D, Klokočovnik V, Lipovšek S, Bock E, Leitinger G (2013) Larval morphology of the antlion Myrmecalurus trigrammus (Pallas, 1771) (Neuroptera, Myrmeleontidae), with notes on larval biology. Zootaxa 3641:491–500
Doflein F (1916) Der Ameisenlöwe. Eine biologische, tierpsychologische und reflexbiologische Untersuchung. Fischer, Jena
Eisenbeis G, Wichard R (1987) Ant lions (Myrmeleonidae). In: Eisenbeis G, Wichard (eds) Atlas on the biology of soil arthropods. Springer, Berlin, pp 278–283
Fertin A, Casas J (2007) Orientation towards prey in antlions: efficient use of wave propagation in sand. J Exp Biol 210:3337–3343
Gepp J (2010) Ameisenlöwen und Ameisenjungfern. Myrmeleontidae, Westarp Wissenschaften Hohenwarsleben
Gogala M (1985) Vibrational communication in insects (biophysical and behavioural aspects). In: Kalmring K, Elsner N (eds) Acoustic and vibrational communication in insects. Parey, Hamburg Berlin, pp 117–126
Griffiths D (1980) The feeding biology of ant-lion larvae: prey capture, handling and utilization. J Anim Ecol 49:99–125
Guillette LM, Hollis KL (2010) Learning in insects, with special emphasis on pit-digging larval antlions (Neuroptera: Myrmeleontidae). In: Devetak D, Lipovšek S, Arnett AE (eds) Proceedings of the Tenth International Symposium on Neuropterology, Piran, Slovenia, 2008. FNM, Maribor, pp 159–170
Guillette LM, Hollis KL, Markarian A (2009) Learning in a sedentary insect predator: antlions (Neuroptera: Myrmeleontidae) anticipate a long wait. Behav Processes 80:224–232
Hollis KL, Cogswell H, Snyder K, Guillette LM, Nowbahari E (2011) Specialized learning in antlions (Neuroptera: Myrmeleontidae), pit-digging predators, shortens vulnerable larval stage. PLoS One 6:1–7
Jockusch B (1967) Bau und Funktion eines larvalen Insektenauges. Untersuchungen am Ameisenlöwen (Euroleon nostras Fourcroy, Planip., Myrmel.). Z vergl Physiol 56:171–198
Le Faucheux M (1972) Le role des soies thoraciques dans la capture des proies par la larve d’Euroleon nostras Fourcroy (Névroptère). Rev Comp Animal 6:217–221
Lipovšek Delakorda S, Pabst M-A, Devetak D (2009) Morphology of the eyes and sensilla in the antlion larvae (Neuroptera: Myrmeleontidae). In: Pabst MA, Zellnig G (eds) MC 2009—microscopy conference, Graz, Austria, vol 2, Life Sciences. Verlag der Technischen Universität Graz, pp 403–404
Mencinger B (1998) Prey recognition in larvae of the antlion Euroleon nostras (Neuroptera: Myrmeleontidae). Acta Zool Fenn 209:157–161
Mencinger Vračko B (2003) Substrate vibrations as important stimulus for the prey recognition by the antlion larva Euroleon nostras (Geoffroy in Fourcroy, 1785). University of Ljubljana, Biotechnical Faculty, Ljubljana. M.Sc. Thesis. /in Slovene with English summary/
Mencinger-Vračko B, Devetak D (2008) Orientation of the pit-building antlion larva Euroleon (Neuroptera, Myrmeleontidae) to the direction of substrate vibrations caused by prey. Zoology 111:2–8
Michelsen A, Fink F, Gogala M, Traue D (1982) Plants as transmission channels for insect vibrational songs. Behav Ecol Sociobiol 11:269–281
Napolitano JF (1998) Predatory behavior of a pit-making antlion, Myrmeleon mobilis (Neuroptera, Myrmeleontidae). Fla Entomol 81:562–566
Nicoli Aldini R (2007) Observations on the larval morphology of the antlion Myrmeleon bore (Tjeder, 1941) (Neuroptera Myrmeleontidae) and its life cycle in the Po Valley (northern Italy). Ann Mus civ Storia nat Ferrara 8:59–66
Pfannenstiel RS, Hunt RE, Yeargan KV (1995) Orientation of a hemipteran predator to vibrations produced by feeding caterpillars. J Insect Behav 8:1–9
Scharf I, Ovadia O (2006) Factors influencing site abandonment and site selection in a sit-and-wait predator: a review of pit-building antlion larvae. J Insect Behav 19:197–218
Scharf I, Lubin Y, Ovadia O (2011) Foraging decisions and behavioural flexibility in trap-building predators: a review. Biol Rev 86:626–639
Virant-Doberlet M, Čokl A, Zorović M (2006) Use of substrate vibrations for orientation: from behaviour to physiology. In: Drosopoulos S, Claridge MF (eds) Insect sounds and communication: physiology, behaviour, ecology, and evolution. Taylor and Francis, New York, pp 81–97
Acknowledgments
I thank Professor A. Čokl for helpful suggestions on an early version of the manuscript. This project was supported by the Slovene Ministry of Higher Education, Science and Technology within the Biodiversity Research Programme (Grant No. P1-0078).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Devetak, D. (2014). Sand-Borne Vibrations in Prey Detection and Orientation of Antlions. In: Cocroft, R., Gogala, M., Hill, P., Wessel, A. (eds) Studying Vibrational Communication. Animal Signals and Communication, vol 3. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-662-43607-3_16
Download citation
DOI: https://doi.org/10.1007/978-3-662-43607-3_16
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-662-43606-6
Online ISBN: 978-3-662-43607-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)