Abstract
Textile fabrics with functional properties have a huge interest in many applications, including protective clothing, packaging materials, and healthcare, etc. This study aims to modify textile fabric’s surface and investigate the potentiality of Ag nanoparticles for the preparation of value-added and improved functional textiles. It demonstrates the mussel-inspired in-situ deposition of Ag nanoparticles on cotton fabric pre-modified by dopamine molecules. Surface characterization of nano-Ag deposited fabric is done by SEM (Scanning Electron Microscopy) and EDS (Energy Dispersive Spectroscopy) by which the well deposition of Ag nanoparticles is confirmed. The crystalline size of the Ag nanoparticles has been determined by SEM and X-ray diffraction spectroscopy. As functional properties, antimicrobial activity, UV protection, and crease resistance are investigated. The results reveal that the nano-Ag deposition introduces the excellent antibacterial property to cotton fabric against S. aureus (gram-positive) and E. coli (gram-negative). The fabric shows good UV protection power and significant crease resistance. The fabric has also been dyed with reactive dyestuff, and improved dyeing performance is found. Importantly, no significant changes in mechanical properties of the textile cotton fabric are found by surface modification and deposition of Ag nanoparticles.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The use of nanoscience and nanotechnology is state of the art in the recent era. Nanotechnology is a versatile and growing field that deals with nanoscale materials [1]. Since the properties and characteristics of nanoparticles are active at the nanostructure level, they are essential components of nanotechnology [2]. Nanoparticle formation alters the chemical and physical properties of bulk materials, and its higher reactivity is due to its high surface area to volume ratio [3] and molecular level activities.
The textile industry is benefited by using the attributes of nanoparticles in different ways. For textile product advancement, the enormous contribution of the nanoparticle is appreciably noticeable [4]. Incidentally, the role of nanotechnology in textile has been fascinatingly outlined by Vigneshwaran [5]. The author stated the surface modification of textile using nanotechnology, and imparting desirable quality like antimicrobial activity, UV absorption/blocking, self-decontamination, etc., on-base fabric by using metallic nanoparticles. The textile sector has been developed enormously by using different types of nanoparticles in various ways. The notable instances are developing textile characteristics, modifying the physical, chemical, and biological properties of textile, engineered, synthesized, and altered the conventional materials and processes to create future generation of materials, devices, systems, and structures [6]. One of the significant ways to achieve the described property is through efficient and relevant nanoparticle deposition on the textile surface. Some nanoparticles have felicitated their contribution to growing functional textiles wonderfully. Silver nanoparticles are traditional and superior for their huge benefits as antimicrobial and antiviral perspective [7,8,9]. In addition, the silver nanoparticle is widely used and continuously drawing remarkable attention in research and commercial fields due to its intense action towards a broad range of microorganisms, lower toxicity, chemical stability, good conductivity, antibacterial activity, high selectivity, durability, and biocompatibility [10,11,12].
Besides, silver nanoparticles have been commonly used in pharmaceutical and biomedical applications such as anti-microbe, anti-parasite, and anticancer [13, 14]. Silver nanoparticles from a natural source like the plant, fruit, seed, and leaf extract also provide some promising properties, particularly antimicrobial activity [15, 16]. However, textile researchers have also reported the outstanding achievement of antimicrobial textiles using silver nanoparticles [17,18,19]. Direct coating or in-situ immobilizations are the two main ways of nanoparticle deposition on fabrics surface. The direct coating might have discontinuity problems, less durable, aggregation of additives, and low effectivity [20]. Conversely, in-situ deposition overcomes those problems and even ensures intensive affixation, reduces wastage of particles, and improved durability. Our previous work asserted that nanoparticles form directly within the textile surface while using the in-situ process. This method is beneficial since the nanoparticle receives the textile material as a deposition medium almost immediately after creation. So, nanoparticle wastages are lower, and durability is higher for this method. Furthermore, since the particle distribution is uniform, this approach does not require a stabilizer [21].
However, the silver nanoparticle has not sufficient attraction, deposition, and affixation power towards fabric, so an additional adhesive or fixing agent is required to immobilize the particle into the fabric. So the adhesive is primarily used in the direct coating by pad dry and cure method. The improper selection of adhesive material may lead to the problem of durability and effectivity of nano-treated fabric. Instead of using adhesive coating material, a good option is fiber surface modification to enhance the attraction and improve the fixation of nanoparticles. Different ways are available to modify the textile surface for better performance of nanoparticle deposition. Fiber modification in the case of in-situ nano deposition offers a significant opportunity for instant fixation [22, 23]. In this research, the fabric is modified chemically by using dopamine hydrochloride. It is an agent that mimics mussel adhesion explored by the researcher by introducing the compound found in mussels responsible for their universal adhesive property. In literature, Dopamine is expressed as an excellent substance capable of being self-polymerized and makes a film on the material surface, and creates covalent or non-covalent bonds with organic or inorganic materials [24]. Further, the ability of Dopamine's catechol groups to make noble metallic salts into metallic nanoparticles [25] and immobilize the nanoparticles onto the substrate without aggregation and leaching has forwarded it as a ubiquitous surface modification agent [26]. It has substantial adhesive property to improve surface performance, and firmly affix the nanometal on the textile surface.
Cotton is the most popular natural fiber in the world. Its use is higher than that of the other natural fibers because of its comfortability, biodegradability, dyeability, and other important characteristics. Nevertheless, the fibers are more prone to bacterial growth and have tendency to crease formation. Therefore, nanoparticle treatment can overcome the problem and make the cotton more attractive and functional in use. Hamad and Khashan [27] depicted the functionality of silver in textile by cotton fabric. Thus, the present study focuses on the deposition of silver nanoparticles on cotton fabric to make it a more valuable textile to satisfy the modern market.
Here, the nano-Ag is deposited on dopamine modified cotton fabric to obtain multifunctional property. Silver nano formation and deposition have been made to get benefit from the self-polymerization and reduction power of dopamine hydrochloride. Lui et al. [28] made silver plating on cotton fiber by dopamine modification, analyzed the surface resistivity, and found a high electric conductive cotton surface. Dopamine is also used to form sericin-based composite film to incorporate the silver in nano form [29]. The man-made fibers are also modified by using Dopamine. Kuang et al. used polydopamine to develop hydrophilic and antistatic surfaces through surface functionalization [30]. Li et al. functionalized aramid fiber with Dopamine to improve surface properties and develop UV resistance [31]. Further, it is also found to be used in polyester and silk fiber for in-situ deposition of Ag nanoparticles [32, 33]. Dopamine modification for in-situ immobilization of Ag-nanoparticle on cellulosic paper was studied recently by one of our authors [26]. Those works are the inspiration and encouragement of the present study. Furthermore, there is very little research on in-situ nano-Ag deposition on dopamine-modified cotton fabrics, where the functional, mechanical, and dyeing properties are studied. Here a convenient and easy way is followed to make a dopamine solution and fabric modification. Also, the dyeing efficiency study will speed up the pre-modification of Dopamine in dyed fabric and expose its other characteristics. Therefore, the current study will reveal all mentioned functional properties and the investigation into mechanical performances and finally bring one step ahead to invent advanced value-added textiles.
2 Experimental Section
2.1 Materials
A scoured and bleached 100% cotton fabric of plain weave structure is used in this investigation. The woven fabric consists of 80 ends per inch (EPI), 75 picks per inch (PPI), and 168 GSM (gram per square meter). The chemicals: dopamine hydrochloride, silver nitrate, hydroxymethyl aminomethane, and HCl are purchased as the analytical grade from Sigma Aldrich, Germany. Two dyestuffs are used for dyeing, namely Reactobond Red 2GX (CI: Red 194) and Reactobond Blue BRX (CI: Blue 221), are collected from Meghmani Dyes and Intermediates Ltd., Ahmedabad, India. Deionized water is used throughout the experiment.
2.2 Mussel-Inspired Deposition of Nano-Ag
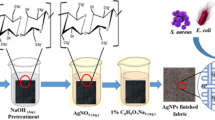
Mussel-inspired deposition of silver nanoparticles on the cellulose fabric with the aid of dopamine hydrochloride is shown in Fig. 1. The figure demonstrates the dopamine-cellulose formation with a chemical structure that refers to the pre-functionalization of cotton fabric. Firstly, the buffer solution is prepared by hydroxyethyl ammonium base. A 10 mM (millimole) of dopamine solution is dissolved in the tris buffer. The pH of the solution is maintained at 8.5 with diluted hydrochloric acid. The immersion and continuous stirring for 24 h make a coated fabric named dopamine-cellulose of blackish shade, as shown in Fig. 2b. For a better understanding of color differentiation, the photographic image of untreated fabric is shown in Fig. 2a. Subsequently, the dopamine functionalized cotton is kept for eight hours in silver nitrate salt. Due to the redox activity and adhesive property of the dopamine molecules, Ag nanoparticles are formed from AgNO3 and eventually glued to the fabric surface. One of the present authors [26] described this mechanism in detail where Ag nanoparticles were allowed to deposit on the cellulose-based materials.The Ag nanoparticles are tethered strongly to the fabric because the particles are strongly coordinated by the catechol functional groups of dopamine molecules, which are covalently bonded to the cellulosic surface. Finally, the fabric is placed in a vacuum oven for twelve hours at 400C. Figure 2c shows the nano-Ag deposited, while Fig. 2a shows the untreated fabric.The color difference of untreated, dopamine-modified, and nano-Ag deposited fabric (D.NanoAg) are shown in Table 1. The color coordinates L*, a*, and b* and color difference (DE) in Table 1 indicate that the Ag nanoparticles deposition makes the fabric darker than the untreated fabric. The color strength (K/S) and reflectance value (R%) specify that the treated fabric is blackish in the shade.
2.3 Measurement and Analysis
2.3.1 Characterizations
The surface morphology of nano-Ag deposited fabric is examined using high-resolution scanning electron microscopy. SEM analysis involves bombarding the surface of a sample with an intensely focused scanning electron beam generating a large number of secondary electrons whose strength is influenced by surface topography. SEM analysis requires sampling, which is also a focus of other researchers’ work [26]. Rajkumar et al. [34] prepared their glass samples by giving a thin layer of gold film using the sputtering technique. Herein the thoroughly washed and fully air-dried samples are glued on a conducting carbon strip. The sample-loaded strip is then mounted to a chamber for a very thin layer of platinum to ensure conductivity of the surface. The sputtering unit (Jeol JFC-1600 auto fine coater, Japan) makes a 75 nm thin platinum layer on our nonconductive cotton fabric in a vacuum environment at about 45 s. The sample is then examined by the Field Emission Scanning Electron Microscope (model no: JEOL JSM-6700F, serial no: SM1761001050105), which operates 5 kV accelerating voltage and is equipped with an energy dispersive X-ray force. The same instrument is used to measure EDS (energy dispersive spectroscopy) to get the compositional map of the sample [35]. XRD (x-ray diffraction) analysis is performed using an X-Ray Diffractometer from Phillips, Expert Pro, Holland. This approach is used to determine whether a substance is crystalline or amorphous at the atomic level [16, 36]. The XRD study is performed using a Cu K radiation (λ = 1.5406 Å), X-ray source with a diffraction angle of 2θ = 20º–80º. The standard XRD database (JCPDS database) is used for phase identification, as used by the other researchers [37, 38]. Fourier transform infrared spectroscopy (FTIR) is used to monitor chemical changes and cotton fabric bonding with nano-silver. ATR/FTIR spectrometer characterizes untreated and nano-treated fabrics (Model: Frontier, PerkinElmer, USA). The scanning range is 1000–450 cm−1 [39]. For XRD and FTIR data computation, Igor Pro software is used.
2.3.2 Functional Property Analysis
The antimicrobial properties are determined following standard test methods ASTM E2149-01, and results are expressed by bacterial reduction percentage (R%). The quantitative antimicrobial test method is developed to evaluate the resistance of non-leaching antimicrobial treated specimens to the growth of microbes under dynamic contact conditions [40]. Antimicrobial activity is examined against Staphylococcus aureus (gram-positive) and Escherichia coli (gram-negative) bacteria. Each culture is suspended in a small amount of nutrient broth, spread on the nutrient agar plate, and incubated at 37 ºC for 24 h. Two single colonies are picked off with an inoculating loop from the agar plate, suspended in a 5 mL nutrient broth, and incubated for 18 h at 100 rpm and 37 ºC. A final concentration of 1.45 × 105–6.44 × 105 colony-forming units per milliliter (CFU/mL) is prepared by appropriately diluting each culture with a sterile buffer solution (0.3 mM phosphate buffer, pH 7.2), which is used as a diluent in all experiments. These dilute culture solutions are used for the antimicrobial test. The number of viable species in suspension is estimated, and the percentage reduction is measured based on recoveries from the suitable untreated sample. This approach is intended for those surfaces with a 50–100% reduction capacity for the required contact time [41]. The following equation (Eq. 1) is used to calculate the bacterial reduction percentage.
No = the number of colonies in untreated and Nt = number of colonies after 1-h of introducing samples. This calculation above is repeated for 24-h of contact time again to see the prolonged action of the nano-Ag deposited fabric against two bacteria. For evaluating the durability of nano-Ag treated cotton fabric toward repeated laundering, the treated sample is submerged in a washing solution containing non-ionic detergent (2 g/L). The samples were stirred for 15 min at 50 °C. Then, it is rinsed with tap water and air-dried. This procedure is repeated 5, 10, 15, and 20 times. Later, the antimicrobial property of the washed sample is determined [42].
PerkinElmer UV visible machine from the USA is used to measure UV protection of the treated and untreated fabric. The results are expressed by the transmittance percentage of incident solar light of wavelength 220–700 nm [42]. The transmittance curve of incident light has been analyzed by dividing the following three regions, namely A, B, and C of the solar spectrum, (i) UV-A of wavelength: 320–400 nm, (ii) UV-B of 280–320 nm, and (iii) UV-C of 200–280 nm.
Fabric crease resistance is measured by the recovery angle of fabric after creasing. The more angle of recovery indicates more crease resistance. It is a quantitative measurement of the easy-care property of the fabric. In the current research, the crease recovery angle of untreated and treated fabric is measured by the AATCC standard test method [43].
2.3.3 Dyeing Performance Analysis
The samples are dyed with red and blue reactive dyes (1% shade) to investigate the dyeing performances described by Simu et al. [17]. The color strength, dye exhaustion, fixation, and colorfastness have been calculated and evaluated to reflect dyeing performances. Those performances are determined by the spectrophotometer of the data color series-600 + . The kubelka Munk theory expressed by Eq. 2 is followed to calculate color strength (K/S) [18]. Equation 3 determines the percentage of dye exhaustion, while Eq. 4 determines the rate of fixation. Standard ISO105CO3 assesses colorfastness for washing, ISO105X12 for rubbing, and ISO105B02 is for light.
where, K is the absorbance coefficient, S is the scattering coefficient, and R is the reflectance.
Co = Initial concentration of dye in the dye bath, Cs = Concentration during the process
2.3.4 Mechanical Property Analysis
For analysis of the mechanical property, tensile strength, elongation, tearing strength, bending length, frictional coefficient are considered. The tensile strength test is measured by method ISO13934-1:2013, and tearing strength is measured by ISO13937-2:2000. Titan universal strength tester of Jeams Heals is used for strength measurement. Fabric stiffness expressed by bending length is determined by ASTM D1388 using Shirley stiffness tester. Martindale abrasion and pilling tester of SDL International, England, determines abrasion resistances followed by ISO 12947 and pilling resistance followed by ISO 12945-2.
All experimental samples are conditioned in the standard atmosphere at 20 ± 5 °C and 65 ± 2% relative humidity for 12 h in a conditioned laboratory before every test.
3 Results and Discussion
3.1 Characterization of Fabric
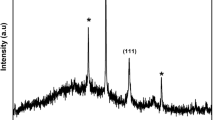
The surface morphology of untreated and Ag deposited fabric is characterized by SEM analysis, shown in Fig. 3a–e. The figure shows the enormous deposition of nano-Ag on Dopamine modified fabric. Figure 3a shows the untreated fabric where no Ag nanoparticle exists. It can be noted that this method offers more deposition of Ag nanoparticles than that of other in-situ deposition methods of our previous work [22, 39]. The SEM image demonstrates the effective immobilization of Ag nanoparticles into the cotton fabric surface. It can be mentioned that dopamine modification is a better choice than alkali modification [22] for nano-Ag deposition as it can deposit more nano-Ag. Hence the treated fabric surface shows a uniform coverage of nano-Ag as shown in Fig. 3b, which is the image of 5000 magnification and size around 90 nm found from a size measuring software as shown in Fig. 3c. Figure 3d represents the higher magnification of fabric surface where the smaller nanoparticle (33–43 nm) is explored by size measuring software shown in Fig. 3e. So the synthesized nanoparticle is in size ranges from (33–90 nm). The EDS curve shows the elemental mass composition of fabrics. As in Fig. 3f, the untreated fabric shows carbon (C) and oxygen (O), i.e., the fundamental element of cellulose. Metallic silver nanoparticles exhibit an absorption peak at approximately 3 keV due to surface plasmon resonance [44]. Our EDS study of the nano-Ag deposited fabric also reflects a peak at 3 keV (Fig. 3g), indicating the Ag nanoparticle's presence. Ag immobilized fabric yields 2.1% Ag nanoparticles with carbon, oxygen, nitrogen, and chlorine, as shown in Table 2. Dopamine hydrochloride (C8H12ClNO2) is the source of nitrogen and chlorine particles. Another important characterization is XRD, as shown in Fig. 4. The peaks of the XRD pattern indicate the evidentiary formation of Ag nanoparticles. We found the peak 2θ values at 38.8°, 44.4°, 64.9°, 78.07° that can be recognized by the reflection of (111), (200), (220), (311) planes of the face-centered at cubic silver according to standard XRD file of Ag nanoparticle (JCPDS file no. 04-0783). A similar peak of silver nanoparticles is found by other researchers [45, 46]. Chen et al. [47] found the peak of Ag nanoparticle at 2θ = 38°, 44°, 64.3°, and 77.3° on their one-pot synthesis of Ag nanoparticles. The others peak found in the current work at 2θ = 45.5°, 50.98° and 72.49° are responsible for cellulosic structure; similar peaks of cotton fabric were also observed in our previous study [22]. Analyzing the corresponding peaks of Ag and using Bragg's equation (Eq. 4), the size of nano-Ag has found around 60 nm.
where, λ = 1.54060 Å, 0.9 × λ = 1.38654, θ = 2θ/2, d = the full width at the half maximum intensity of the peak.
FTIR analysis is shown in Fig. 5. The peaks of untreated fabric are found at around a wavenumber of 3424 cm−1 due to the O–H bond. The peak 2913 cm−1 is for C-H stretching. The peaks 2170, 1647, 1445, 1375 cm−1 are respectively for O–H, C=C, C–O, and C=O stretching. The above peaks are the main characteristic peaks of a cellulose structure of cotton fabric [48]. According to the IR table, the peaks (3500–3200) cm−1 corresponding to O–H stretching are present in both cellulose and Dopamine. All other peaks obtained for nano-Ag immobilized fabric (D.nanoAg) are also similar to untreated fabric. Since cellulose's broad –OH absorption suppresses both –NH (amide) and catechol –OH of the dopamine moiety, no major changes are observed after dopamine adjustment. Further, silver does not respond well to infrared radiation, but residual stabilizers and particular synthesis reagents might respond [49]. FTIR analysis revealed no major changes on cotton fiber surfaces, indicating that no new functional groups were formed during the fabrication process with nano-Ag deposition into cotton surfaces[50].
3.2 Functional Property Analysis
3.2.1 Antibacterial Activity
The antimicrobial activity expressed by bacterial growth reduction percentages obtained for both 1 and 24 h of bacterial contact time is shown in Fig. 6a–h. The figure represents the number of bacteria existing in untreated and nano-Ag immobilized fabric. After an hour, it is discovered that a significant amount of bacteria is reduced due to the action of Ag deposited fabric. Compared to the untreated fabric in Fig. 6a, c, the bacterial growth on the disk plate of nano-Ag treated fabric is lower, as shown in Fig. 6b, d. The bacterial reduction percentage for untreated and treated fabric for both bacteria is quantitatively demonstrated in Table 3. The table shows that the nano-Ag deposited fabric has good antimicrobial activity, around 86% for S. aureus and 93% for E. coli.
Moreover, for 24-h contact, no bacterial growth is found on the disk, as shown in Fig. 6f, h. Excellent antibacterial activity (R% = 100) against S. aureus and E. coli also retains ~ 98% after 20 washes (Table 3). It seems to give complete protection to the cotton fabric from microorganisms, and in that respect, it can be considered as the noble outcome of this work. Silver nanoparticles incorporated by polydopamine modification on polyester and silk fabric show appreciable antimicrobial activity measures by the zone of inhibition method [33, 51]. Polydopamine surface coating along with Ag nanoparticle on other material also showed excellent antimicrobial activity [23]. Our present effort is consistent with other reports of the antimicrobial activity of silver nanoparticles. Thus the treated fabric could be a good choice for medical textile and other applications to ensure complete antibacterial protection with excellent wash durability.
3.2.2 UV Blocking Property (Ultraviolet Protection)
The obtained results of the transmittance value of untreated and treated fabrics are placed in Fig. 7. The average transmittance for different wavelengths is also shown in Table 4. The figure and table show that the nano deposited fabric imparts excellent UV protection ability of the fabric as the sample D.NanoAg shows12% transmittance in the UV-A region and 11–12% in the UV-B region. The less transmittance percentage means more blockage of UV rays and higher UV protection. It indicates that due to silver nanoparticle deposition, the fabric blocks around 90% UV rays.
Thus the treated fabric produced by nano-Ag deposition can protect the user from harmful UV rays and reduce the sub burns and other skin diseases. It can be pointed out that silver nanoparticles itself have no UV protection power based on literature review. The reason for UV blockage might be the blackish color of the nano-Ag immobilized fabric. It can be observed from images in Fig. 2c that the dopamine pre-functionalization and further nano deposition make the fabric more blackish. Rajaboopathi and Thambiduraial [52] showed the UV transmittance of cotton fabric coated with colloidal seaweed extract functionalized silver nanoparticles. They found a 20% UV transmission by coated fabric, i.e., 80% UV protection and 40% protection for uncoated fabric. It resembles to ultraviolet protection factor (UPF) ~ 6.7 and moderate UV protection. Mahmud et al. [53] reported high UV protection of silk fabric by obtaining 80% UV blockage. It is well known that black has a higher propensity to absorb solar light and cannot relay the entire incident light. High UV absorption due to the dark brown color of the nano-Ag coated fabric could explain the high UV blocking [54]. The present treated fabric shows around 90% UV block equivalent to UPF ~ 10. It indicates moderate to good protection [55]. Therefore, the UV protection power of the dopamine-modified nano-Ag deposited cotton fabric is much appreciable.
3.2.3 Crease Resistance
The crease resistance of untreated and the nano-Ag deposited fabrics (D.NanoAg) are determined by crease recovery angle in degrees, as shown in Fig. 8. The figure demonstrates that the nano-Ag deposition increases the crease resistance power of fabric at around 5%. The crease resistance is the expression of the easy-care property of the fabric. It is also related to the serviceability of fabric. The obtained results confirm that the nano deposition in this way imparts an additional quality of improving crease resistance of cotton fabric. The crease formation happens on the cotton fabric due to the re-bonding of the free hydroxyl group present in the cellulose structure [56]. When the cotton fiber is modified with dopamine hydrochloride, some of its free hydroxyl groups participate in chemical bonding. Besides, the nano-Ag also enters into the fiber interior and lessens the fabric-free area or amorphousness. The fabric does not get enough space for re-bonding, and the free –OH group participates in bonding. In that case, the fabric will be more prone to come back to its original position after distortion by the action of external forces, and consequently, the crease resistance improves.
3.3 Dyeing Performance
The dyeing performance of the treated and untreated fabric is expressed in terms of color strength, dye exhaustion, fixation, and colorfastness measurement. Here two reactive dyestuffs of red and blue are used for dyeing, and the obtained results of the mentioned points are presented in Tables 5 and 6. The tables represent that the overall dyeing performance of treated fabric increases due to nano-Ag deposition. In the performance study, the Ag modification brings around 12% improvement of color strength, 3% dye exhaustion, and 2.4% of dye fixation for red dyestuff. The blue color also shows an improvement of color strength, dye exhaustion, and dye fixation. The overall colorfastness of treated fabric is also satisfactory for both red and blue dyestuff. The improvement of dyeing performance is due to the mordanting power of Ag nanoparticles that ensures to grip more dyestuffs on the fabric surface. Our previous work also revealed the improvement of color performance of cotton fabric where Ag nano was deposited by in-situ method on alkali pre-modified fabric [39]. Since color performance increases, this treatment also positively influences the dyeing performance of the cotton fabric. It can be noted that this treatment has no adverse effect on shade stability. Eventually, the study indicates an optimistic impact of dopamine polymer on the reactive dyed cotton fabric.
3.4 Mechanical Properties
The important mechanical properties of nano-Ag immobilized fabric compared with untreated fabric are shown in Table 7. Minimal changes are found in tensile strength, tearing strength, and elongation for nano-Ag treated fabric. The bending length is slightly higher because the metallic nanoparticle goes to the fiber interior and imparts stiffness. The frictional resistance is quite similar for treated and untreated fabric. However, the analysis of the mechanical properties of nano-Ag deposited cotton fabric by the in-situ method is less dealt with by previous researchers [57]. Even mechanical properties of Dopamine pre-modified and nano-Ag immobilized fabric are also found unclear. So we can only equate this method to a few available in-situ nano-Ag deposition systems. Rehan et al. [57] revealed an ~ 18% reduction in the mechanical strength of cellulose fabric after in-situ nano-Ag deposition via UV radiation.
Additionally, our previous work of in-situ immobilized Ag reported much reduction of strength (~ 14%), where the fabric was pre-functionalized by alkali (NaOH) [22]. In that case, the alkaline modification harmed the main cellulose structure. Due to the cellulosic polymer degradation, a decrease in strength was found. Nevertheless, this work deals with dopamine pre-functionalized nano-Ag deposited cotton. Yet, the results appear to be superior to those obtained using other in-situ deposition techniques. Therefore, dopamine functionalization has no detrimental impact on cellulose structure since the current study finds a negligible reduction of mechanical strength (Tensile strength, warp—0.3%, weft—1.19%).
4 Conclusions
The current study investigates the functional, mechanical, and dyeing properties of nano-Ag deposited fabric that has been pre-functionalized with dopamine hydrochloride. The SEM images confirm the formation and deposition of nanoparticles. The elemental mass composition of the untreated and nano-Ag treated fabric is investigated using EDS. The particle size and crystalline form of silver nanoparticles are determined using XRD. The findings show 100% bacterial reduction for both S. aureus and E. coli bacteria after a 24-h contact period in unwashed condition. The wash durability of this bacterial reduction power is also found excellent as the fabric retains ~ 98% reduction after 20 washes. A single hour of bacterial contact also shows promising results as ~ 86% reduction against S. aureus and ~ 93% against E. coli. The UV protection of Ag-deposited fabric is also very appreciable, with 90% blockage. Moreover, nano-Ag deposition by the current process improves crease-resistance and dyeing efficiency over the untreated fabric. Insignificant changes of the mechanical properties of nano-Ag deposited fabric are found in the present investigation. However, this change can be considered minor compared with other appreciable functional properties. The present findings will be beneficial to the advancement of innovative textile products.
Abbreviations
- SEM:
-
Scanning electron microscopy
- EDS:
-
Energy dispersive spectroscopy
- XRD:
-
X-ray dispersive spectroscopy
- FTIR:
-
Fourier transform infrared spectroscopy
- WI:
-
Whiteness Index
- K/S:
-
Color strength
- UV:
-
Ultraviolet
- AATCC:
-
The American Association of Textile Chemists and Colorists
References
K. Chand, D. Cao, D. Eldin Fouad, A. Hussain Shah, A. Qadeer Dayo, K. Zhu, M. Nazim Lakhan, G. Mehdi, S. Dong, Green synthesis, characterization and photocatalytic application of silver nanoparticles synthesized by various plant extracts. Arab. J. Chem. 13, 8248–8261 (2020). https://doi.org/10.1016/j.arabjc.2020.01.009
M.C. Roco, Nanoparticles and nanotechnology research. J. Nanoparticle Res. 1, 1–6 (1999). https://doi.org/10.1023/A:1010093308079
N. Nagar, V. Devra, Textile dyes degradation from activated peroxomonosulphate by green synthesize silver nanoparticles: a kinetic study. J. Inorg. Organomet. Polym. Mater. 29, 1645–1657 (2019). https://doi.org/10.1007/s10904-019-01127-x
P.J. Rivero, A. Urrutia, J. Goicoechea, F.J. Arregui, Nanomaterials for functional textiles and fibers. Nanoscale Res. Lett. (2015). https://doi.org/10.1186/s11671-015-1195-6
N. Vigneshwaran, Modification of textile surfaces using nanoparticles. Surf. Modif. Text. (2009). https://doi.org/10.1533/9781845696689.164
K. Patra, Application of nanotechnology in textile engineering: an overview. J. Eng. Technol. Res. (2013). https://doi.org/10.5897/jetr2013.0309
M. Asadullah Jahangir, S. Sarim Imam, A. Muheem, K. Yamini, Silver nanoparticles: the good, the bad and the future research and reviews. J. Pharm. Nanotechnol. 4, 1–10 (2016)
K.K. Bamzai, G. Kour, B. Kaur, S.D. Kulkarni, Synthesis of silver nanoparticles through chemical reduction and its antibacterial effect. J. Magn. Magn. Mater. 327, 159–166 (2013). https://doi.org/10.1016/j.jmmm.2012.09.013
A. Harada, H. Ichimaru, T. Kawagoe, M. Tsushida, Y. Niidome, H. Tsutsuki, T. Sawa, T. Niidome, Gold-treated silver nanoparticles have enhanced antimicrobial activity. Bull. Chem. Soc. Jpn. 92, 297–301 (2019). https://doi.org/10.1246/bcsj.20180232
E. Sánchez-López, D. Gomes, G. Esteruelas, L. Bonilla, A.L. Lopez-Machado, R. Galindo, A. Cano, M. Espina, M. Ettcheto, A. Camins, A.M. Silva, A. Durazzo, A. Santini, M.L. Garcia, E.B. Souto, Metal-based nanoparticles as antimicrobial agents: an overview. Nanomaterials 10, 1–43 (2020). https://doi.org/10.3390/nano10020292
Shahid-Ul-Islam, B.S. Butola, F. Mohammad, Silver nanomaterials as future colorants and potential antimicrobial agents for natural and synthetic textile materials. RSC Adv. (2016). https://doi.org/10.1039/c6ra05799c
M. Radetić, Functionalization of textile materials with silver nanoparticles. J. Mater. Sci. (2013). https://doi.org/10.1007/s10853-012-6677-7
A.C. Gomathi, S.R. Xavier Rajarathinam, A. Mohammed Sadiq, S. Rajeshkumar, Anticancer activity of silver nanoparticles synthesized using aqueous fruit shell extract of Tamarindus indica on MCF-7 human breast cancer cell line. J. Drug Deliv. Sci. Technol. 55, 101376 (2020). https://doi.org/10.1016/j.jddst.2019.101376
Y. Junejo, M. Safdar, M.A. Akhtar, M. Saravanan, H. Anwar, M. Babar, R. Bibi, M.T. Pervez, T. Hussain, M.E. Babar, Synthesis of tobramycin stabilized silver nanoparticles and its catalytic and antibacterial activity against pathogenic bacteria. J. Inorg. Organomet. Polym. Mater. 29, 111–120 (2019). https://doi.org/10.1007/s10904-018-0971-z
A.K. Mishra, K.N. Tiwari, R. Saini, P. Kumar, S.K. Mishra, V.B. Yadav, G. Nath, Green synthesis of silver nanoparticles from leaf extract of Nyctanthes arbortristis L. and assessment of its antioxidant, antimicrobial response. J. Inorg. Organomet. Polym. Mater. 30, 2266–2278 (2020). https://doi.org/10.1007/s10904-019-01392-w
A.G. Rama Krishna, C.S. Espenti, Y.V. Rami Reddy, A. Obbu, M.V. Satyanarayana, Green synthesis of silver nanoparticles by using sansevieria roxburghiana, their characterization and antibacterial activity. J. Inorg. Organomet. Polym. Mater. 30, 4155–4159 (2020). https://doi.org/10.1007/s10904-020-01567-w
F. Zhang, X. Wu, Y. Chen, H. Lin, Application of silver nanoparticles to cotton fabric as an antibacterial textile finish. Fibers Polym. (2009). https://doi.org/10.1007/s12221-009-0496-8
S. Ghosh, S. Yadav, N. Reynolds, Antibacterial properties of cotton fabric treated with silver nanoparticles. J. Text. Inst. 101, 917–924 (2010). https://doi.org/10.1080/00405000903031053
I.S. Tania, M. Ali, Z. Islam, Solaiman, Development of antimicrobial activity and mechanical performances of cotton fabric treated with silver nano particles (AgNPs), in AIP Conf. Proc. 2121, 2019, p. 15003. https://doi.org/10.1063/1.5115968.
T. Jiang, L. Liu, J. Yao, In situ deposition of silver nanoparticles on the cotton fabrics. Fibers Polym. 12, 620–625 (2011). https://doi.org/10.1007/s12221-011-0620-4
I.S. Tania, M. Ali, M.T. Arafat, 6—Processing techniques of antimicrobial textiles, in Text. Inst. B. Ser., ed. by M.I.H.B.T.-A.T. from N.R. Mondal (Woodhead Publishing, 2021), pp. 189–215. https://doi.org/10.1016/B978-0-12-821485-5.00002-0.
I.S. Tania, M. Ali, M.S. Azam, In-situ synthesis and characterization of silver nanoparticle decorated cotton knitted fabric for antibacterial activity and improved dyeing performance. SN Appl. Sci. 1, 1–9 (2019). https://doi.org/10.1007/s42452-018-0068-x
I.I. Niyonshuti, V.R. Krishnamurthi, D. Okyere, L. Song, M. Benamara, X. Tong, Y. Wang, J. Chen, Polydopamine surface coating synergizes the antimicrobial activity of silver nanoparticles. ACS Appl. Mater. Interfaces. 12, 40067–40077 (2020). https://doi.org/10.1021/acsami.0c10517
W. Chen, S. Zhang, L. Sun, M. Zhang, X. Chen, Surface modification of polypropylene nonwoven fabrics by grafting of polydopamine. Adv. Polym. Technol. 37, 3519–3528 (2018)
R. Liu, Y.L. Guo, G. Odusote, F.L. Qu, R.D. Priestley, C. Fe, O 4 polydopamine nanoparticles serve multipurpose as drug carrier, catalyst support and carbon adsorbent. ACS Appl Mater. Int. 5, 9167–9171 (2013)
M.S. Islam, N. Akter, M.M. Rahman, C. Shi, M.T. Islam, H. Zeng, M.S. Azam, Mussel-inspired immobilization of silver nanoparticles toward antimicrobial cellulose paper. ACS Sustain. Chem. Eng. 6, 9178–9188 (2018). https://doi.org/10.1021/acssuschemeng.8b01523
A. Hamad, K.S. Khashan, A. Hadi, Silver nanoparticles and silver ions as potential antibacterial agents. J. Inorg. Organomet. Polym. Mater. 30, 4811–4828 (2020). https://doi.org/10.1007/s10904-020-01744-x
H. Liu, L. Zhu, J. Xue, L. Hao, J. Li, Y. He, B. Cheng, A novel two-step method for fabricating silver plating cotton fabrics. J. Nanomater. (2016). https://doi.org/10.1155/2016/2375836
Y. Cai, R. Tao, G. He, H. Song, K. Zuo, H. Jiang, W. Wang, One-step synthesis of silver nanoparticles on polydopamine-coated sericin/polyvinyl alcohol composite films for potential antimicrobial applications. Molecules 22, 721 (2017)
X.-H. Kuang, J.-P. Guan, R.-C. Tang, G.-Q. Chen, Surface functionalization of polyamide fiber via dopamine polymerization. Mater. Res. Express. 4, 95302 (2017). https://doi.org/10.1088/2053-1591/aa7fc6
M. Li, K. Cheng, C. Wang, S. Lu, Functionalize aramid fibers with polydopamine to possess UV resistance. J. Inorg. Organomet. Polym. Mater. (2021). https://doi.org/10.1007/s10904-021-01910-9
G.Q. Kuang, X.H. Guan, J.P. Chen, W. Tang, R.C. Cheng, Coloration and functional modification of silk fiber via dopamine polymerization, in 2017 2nd International Conference on Materials Science (Atlantis Press, 2017), pp. 795–798.
Z.A. Raza, A. Rehman, F. Anwar, A. Usman, Development and antibacterial performance of silver nanoparticles incorporated polydopamine–polyester-knitted fabric. Bull. Mater. Sci. 39, 391–396 (2016)
G. Rajkumar, M. Rajkumar, V. Rajendran, S. Aravindan, Influence of Ag2O in physico-chemical properties and HAp precipitation on phosphate-based glasses. J. Am. Ceram. Soc. 94, 2918–2925 (2011). https://doi.org/10.1111/j.1551-2916.2011.04725.x
N. Erdman, D.C. Bell, R. Reichelt, in Scanning Electron Microscopy BT - Springer Handbook of Microscopy, ed. By P.W. Hawkes, J.C.H. Spence (Springer International Publishing, Cham, 2019), pp. 229–318. https://doi.org/10.1007/978-3-030-00069-1_5.
V. Vishwakarma, S. Uthaman, 9—Environmental impact of sustainable green concrete, in Micro Nano Technol., ed. by M.S. Liew, P. Nguyen-Tri, T.A. Nguyen, S.B.T.-S.N. and C.-B.M. Kakooei (Elsevier, 2020), pp. 241–255. https://doi.org/10.1016/B978-0-12-817854-6.00009-X.
G. Rajkumar, V. Dhivya, S. Mahalaxmi, K. Rajkumar, G.K. Sathishkumar, R. Karpagam, Influence of fluoride for enhancing bioactivity onto phosphate based glasses. J. Non. Cryst. Solids. 493, 108–118 (2018). https://doi.org/10.1016/j.jnoncrysol.2018.04.046
F. Mahnaz, M. Mostafa-Al-Momin, M. Rubel, M. Ferdous, M.S. Azam, Mussel-inspired immobilization of Au on bare and graphene-wrapped Ni nanoparticles toward highly efficient and easily recyclable catalysts. RSC Adv. 9, 30358–30369 (2019). https://doi.org/10.1039/c9ra05736f
I.S. Tania, M. Ali, R.H. Bhuiyan, Experimental study on dyeing performance and antibacterial activity of silver nanoparticle-immobilized cotton woven fabric. Autex Res. J. 21, 45–51 (2021). https://doi.org/10.2478/aut-2019-0074
F. Ferrero, M. Periolatto, Antimicrobial finish of textiles by chitosan UV-curing. J. Nanosci. Nanotechnol. 12, 4803–4810 (2012). https://doi.org/10.1166/jnn.2012.4902
E. Ali, H.-T. El-Zanfaly, Application of antimicrobials in the development of textiles. Asian J. Appl. Sci. 4, 585–595 (2011)
H.M.M. Ibrahim, M.S. Hassan, Characterization and antimicrobial properties of cotton fabric loaded with green synthesized silver nanoparticles. Carbohydr. Polym. 151, 841–850 (2016). https://doi.org/10.1016/j.carbpol.2016.05.041
AATCC Test Method 66–2008, Wrinkle recovery of woven fabrics: recovery angle, AATCC Technical Manual, American Association of Textile Chemists and Colorists, Research Triangle Park, NC, pp. 91–94, (2010).
T. Dutta, A.P. Chattopadhyay, M. Mandal, N.N. Ghosh, V. Mandal, M. Das, Facile green synthesis of silver bionanocomposite with size dependent antibacterial and synergistic effects: a combined experimental and theoretical studies. J. Inorg. Organomet. Polym. Mater. 30, 1839–1851 (2020). https://doi.org/10.1007/s10904-019-01332-8
T. Theivasanthi, M. Alagar, Electrolytic synthesis and characterization of silver nanopowder. Nano Biomed. Eng. (2012). https://doi.org/10.5101/nbe.v4i2.p58-65
N. Agasti, N.K. Kaushik, One pot synthesis of crystalline silver nanoparticles. Am. J. Nanomater. 2, 4–7 (2014)
Q.Y. Chen, S.L. Xiao, S.Q. Shi, L.P. Cai, A one-pot synthesis and characterization of antibacterial silver nanoparticle-cellulose film. Polymers (Basel) (2020). https://doi.org/10.3390/polym12020440
P. Peets, K. Kaupmees, S. Vahur, I. Leito, Reflectance FT-IR spectroscopy as a viable option for textile fiber identification. Herit. Sci. 7, 1–10 (2019). https://doi.org/10.1186/s40494-019-0337-z
K.W. Fan, A.M. Granville, Surface property modification of silver nanoparticles with dopamine-functionalized poly(pentafluorostyrene) via RAFT polymerization. Polymers (Basel). 8, 1–12 (2016). https://doi.org/10.3390/polym8030081
S. Li, T. Zhu, J. Huang, Q. Guo, G. Chen, Y. Lai, Durable antibacterial and UV-protective Ag/TiO2@fabrics for sustainable biomedical application. Int. J. Nanomedicine. 12, 2593–2606 (2017). https://doi.org/10.2147/IJN.S132035
Z. Lu, J. Xiao, Y. Wang, M. Meng, In situ synthesis of silver nanoparticles uniformly distributed on polydopamine-coated silk fibers for antibacterial application. J. Colloid Interface Sci. 452, 8–14 (2015). https://doi.org/10.1016/j.jcis.2015.04.015
S. Rajaboopathi, S. Thambidurai, Evaluation of UPF and antibacterial activity of cotton fabric coated with colloidal seaweed extract functionalized silver nanoparticles. J. Photochem. Photobiol. B Biol. 183, 75–87 (2018). https://doi.org/10.1016/j.jphotobiol.2018.04.028
S. Mahmud, M.Z. Sultana, M.N. Pervez, M.A. Habib, H.H. Liu, Surface functionalization of “Rajshahi silk” using green silver nanoparticles. Fibers. 5, 1–18 (2017). https://doi.org/10.3390/fib5030035
M. Shateri-Khalilabad, M.E. Yazdanshenas, A. Etemadifar, Fabricating multifunctional silver nanoparticles-coated cotton fabric. Arab. J. Chem. 10, S2355–S2362 (2017). https://doi.org/10.1016/j.arabjc.2013.08.013
Z. Bilimis, Measuring the UV protection factor (UPF) of fabrics and clothing, Apl. Note, Agil. Technol. Inc., Aust. (2011) 1–4.
I.S. Tania, S.M.M. Kabir, M.Z. Uddin, Effects of resin treatments on the quality of cotton fabric dyed with reactive dye. Fibres Text. East. Eur. 26, 102–107 (2018). https://doi.org/10.5604/01.3001.0010.7804
M. Rehan, A. Barhoum, G. Van Assche, A. Dufresne, L. Gätjen, R. Wilken, Towards multifunctional cellulosic fabric: UV photo-reduction and in-situ synthesis of silver nanoparticles into cellulose fabrics. Int. J. Biol. Macromol. 98, 877–886 (2017). https://doi.org/10.1016/j.ijbiomac.2017.02.058
Acknowledgements
The authors like to express their cordial thanks and gratitude to the Mechanical engineering department of BUET for supporting this experimental work. We are thankful to Glass and Ceramics Engineering, and the Biomedical Engineering departments of BUET for characterization analysis and antibacterial test. We also appreciate Dr. Mamun Kabir, Associate Professor, BUTEX, collecting UV data.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tania, I.S., Ali, M. & Azam, M.S. Mussel-Inspired Deposition of Ag Nanoparticles on Dopamine-Modified Cotton Fabric and Analysis of its Functional, Mechanical and Dyeing Properties. J Inorg Organomet Polym 31, 4065–4076 (2021). https://doi.org/10.1007/s10904-021-02034-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10904-021-02034-w