Abstract
Polymer nanocomposites are a promising area of research due to quite superior to the conventional composites. However, obtaining a homogeneous distribution of the nanoparticles in the matrix has been a great challenge. Standard processing techniques of nanocomposites are non-practical, requiring longer periods and can affect both mechanical and thermal properties of the final product. The thermokinectic mixer is an interesting alternative due to its high-speed rotation leading to a better dispersion of the nanoparticle without compromising the polymer properties. This paper reports for the first time a nanocomposite of high-density polyethylene (HDPE)/Al2O3 processed by the thermokinetic mixer. The addition of Al2O3 nanoparticle (0 to 4% wt) to the HDPE led to an increase in both the melting and crystallization temperature. It was also observed an improvement of the mechanical properties due to the increase in the crystallinity degree, which is a consequence of the multiple nucleation sites of Al2O3 nanoparticles. An optimal composition was obtained at 4% wt of Al2O3. Thus, the nanocomposites processed by the thermokinetic mixer demonstrated a significant enhancement of the mechanical and thermal properties.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
High-density polyethylene (HDPE) is classified as a commodity and a thermoplastic widely consumed due to the low cost and its physical, mechanical, thermal, and chemical properties [1]. However, the HDPE application has been limited due to its low thermal and mechanical properties. To improve these properties the addition of rigid particles is usually applied [2]. Aluminum oxide or alumina (Al2O3) is an inorganic and ceramic material used in industries due to its advantages, such as high thermal properties, hardness, chemical inertness, and good electrical insulation [3, 4]. Although alumina presents low-fracture strength and low-fracture toughness [5], such disadvantages can be compensated by the incorporation of Al2O3 to a polymer matrix [3]. Therefore, the advantages of alumina nanoparticle and HDPE could be combined by developing an organic–inorganic nanocomposite.
Organic–inorganic composites have attracted interest in the past years. The main idea of this approach is to open a new and unexplored realm of material [6,7,8,9,10]. Several reinforcements in macro, micro, and nanoscale have been studied to improve the mechanical and thermal properties of polymers [11, 12]. The properties of organic–inorganic composites using particulate are strongly affected by many parameters, such as particle shape and size, the reinforcement content, type of matrix, microstructure, and interfacial interaction between particles and matrix [2, 13, 14]. The mixing capacity depends on several factors, including material properties of the pristine polymer, particle properties, processing conditions, and material formulation [15]. There are several methods for dispersing reinforcements into a polymer matrix, including in situ polymerization and melting or solution mixing [16]. The incorporation of particles into a polymer matrix by melt mixing has attracted interest due to its industrial importance [17, 18]. The particles are dispersed in the matrix by mechanical shearing action in the molten state of the polymer matrix.
The preparation of nanocomposites by melt mixing is carried either with a batch mixer or an extruder and also requires dispersal of the particles throughout the polymer matrix [19]. In an industrial scale, extrusion is the most popular technique for melt processing polymers. The sequential steps of extrusion can be summarized as the following: melting, mixing, and compressing polymers by screw action. However, those steps can face problems during optimization. The nanocomposite processing time can take several minutes, being harmful to the thermal behavior of the material [20]. As an alternative solution to these issues, thermokinectic mixing is a compelling technique.
Thermokinectic mixers do not need external heating and a homogeneous composite can be obtained in a short period, i.e. few minutes, decreasing the thermal story of the polymer and limiting its thermal degradation. The operation process of the thermokinectic method can be seen in Fig. 1a. The mixer internal blades rotate on a high-speed shaft, and the high kinetic energy is converted into thermal energy when the accelerated material particle hit the chamber wall of the mixer [21]. This method promotes the dispersion of nanoparticles in a solution containing the solubilized polymer matrix and can improve properties of the composite material, such as conductivity, strength, wear resistance, and optical properties [22]. Therefore, it is believed that a homogeneous dispersion of the alumina nanoparticle in the HDPE matrix could be obtained by the thermokinectic technique in this work.
Mixtures of polymer reinforced with inorganic materials can enhance the polymer thermal conductivity conserving its electrical insulation. In organic–inorganic composites, increasing the reinforcement content generally enhances thermal conductivity. Therefore, high reinforcement loading is often used to obtain high thermal conductivity. However, the higher the reinforcement content, the more likely it is the formation of agglomerates, that induce stress concentration, and thereby decrease the tensile strength, modulus, and ductility of the material [13].
Brandenburg et al. studied the influence of mixing methods on the properties of HDPE nanocomposites with different types of carbon nanoparticles (graphene nanosheets and carbon nanotubes). The processing techniques explored were the solution method and the melt mixing method. While the melt mixing gives a better result for dispersion for the nanocomposite reinforced with carbon nanotubes, the solution mixing best reduced the agglomerate amount in HDPE reinforced with graphene. The crystallinity indices of both nanocomposites were not affected by the processing techniques [16].
Pelto et al. prepared nanocomposites of HDPE reinforced with different types of nanopowders in the presence of vinyltrimethoxysilane (VTMS) coupling agent by melt mixing and verified that the degree of crystallinity of the nanocomposites was consistently lower compared to the pristine HDPE [23].
Nabhan et al. evaluated tribological and mechanical properties of HDPE reinforced by 0.1–0.5 wt% of alumina nanoparticles and observed improvement to HDPE/Al2O3 matrix [24].
Chen et al. fabricated HDPE/alumina composites by melt mixing with direct incorporation of poly(ethylene-co-methacrylic)-based ionomer (EMAA-Na) as an interfacial compatibilizer [25].
In this paper, Al2O3/HDPE nanocomposites prepared by a thermokinectic mixer, with five different alumina contents (0.5, 1, 2, 3, and 4 wt%) were investigated, and their effects on mechanical and thermal properties were studied.
2 Materials and Methods
2.1 Materials
The materials used in this work were: Al2O3 nanoparticles (CAS Number 1344-28-1) with an average particle size of 13 nm; purity ~ 99.83% (Sigma-Aldrich); and HDPE (IE59U3) supplied by BRASKEN with a density of 0.959 g/cm3.
2.1.1 Nanocomposites Processing
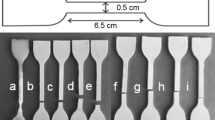
The Al2O3 nanoparticles were mixed with HDPE in a thermokinetic mixer model MH-50H (Fig. 1a), with a frequency of rotation of 5250 rpm, for 60 s. The Al2O3 nanoparticle content varied from 0.5 to 4 wt% of the composition. The nanocomposites were then milled in a knife mill and dried at 50 ºC for 2 h. After that, the nanocomposites were injected directly in a mold with specific dimensions for tensile specimens (Fig. 1b).
2.2 Characterization
2.2.1 Thermogravimetric Analysis (TGA/DTG)
Thermal analyses (TGA) were performed to determine the stability of pristine HDPE and nanocomposites. Thermogravimetric curves were performed by a SII Nanotechnology INC equipment, model Exstar 6000, TG/DTA 6200 series. Experiments were carried out under constant nitrogen flow, in a temperature range from 25 to 600 °C, a 10 °C/min heating rate, and a specimen weight of 10 mg.
2.2.2 Differential Scanning Calorimetry (DSC)
The melting and crystallization temperature and crystallinity degree of pristine HDPE and nanocomposites were obtained by differential scanning calorimetry (DSC). The equipment (SII Nanotechnology INC equipment, model Exstar 6000, TG/DTA 6200 series) was programmed to work at the temperature range between 25 and 200 °C, under a constant nitrogen flow of 40 mL/min. The heating and cooling rates were 10 °C/min. The percent of crystallinity (Xc) was determined from the enthalpy of crystallization of HDPE, Eq. 1,
where Xc is the crystallinity index, \({w}_{t}\) is the weight fraction of reinforcement, and ΔH and ΔH0 are the enthalpy of fusion of the sample and enthalpy of fusion of the 100% crystalline polymer, respectively. ΔH0 was defined for 100% crystalline polyethylene as 293 J/g.
2.2.3 Mechanical Tests
The tensile tests were performed in an EMIC testing machine (model DL2000), equipped with pneumatic claws with a load cell of 5 kN. The load was applied to the specimen at 1.5 m/min crosshead motion rate. Five specimens were analyzed with dimensions in agreement with the respective standard ASTM D 638-10.
3 Results
3.1 Nanocomposites Processing
The properties of the nanocomposites are affected by the content of the nanoparticles dispersed in the polymeric matrix [26]. In the injected nanocomposite tensile specimens, as the amount of alumina powder increased, the grayish color was more intense (Fig. 2). Similarities were seen in the literature. Alsayed et al. [27] prepared tensile specimens of HDPE with zinc oxide as reinforcement with a grayish color similar to the nanocomposite tensile specimens. Umar et al. [28] also reported HDPE composites reinforced with kenaf showing tensile specimens with similar shape and color to the pristine HDPE sample.
3.2 Thermogravimetric Analysis (TGA/DTG)
The thermal properties of the material such as degradation temperature must be well known to determine the manufacturing conditions. The addition of Al2O3 nanoparticles to the HDPE resulted in a change in the color of the nanocomposites, indicating a change in the bandgap due to alumina content. It was also observed that the presence of Al2O3 nanoparticles led to an alteration in the thermal behavior of the nanocomposites when comparing to the HDPE. TGA and DTG curves for pristine HDPE, and nanocomposites are shown in Fig. 3 and thermal parameters are presented in Table 1.
The TGA technique confirmed that the addition of Al2O3 nanoparticles to the HDPE caused a slight increase in the thermal stability of nanocomposites compared to the pristine HDPE, probably due to the joint action of the low permeability of reinforcement leading to a more tortuous path of diffused species, and reduced movement of polymer chains due to the interactions between Al2O3 nanoparticles and HDPE [7]. This result corroborates other studies of the literature and can be attributed to the thermal properties of alumina, as seen by Silva et al. [29] in alumina membranes and by Pratnaik [30], where the Al2O3 melting point was 2072 °C. Viratyaporn and Lehman [31] cited that the incorporation of nanoparticles into polymer systems can enhance the thermal stability of the polymer, which might be the case of HDPE with alumina nanoparticles. At a nanoscale dimension, the high surface/volume ratio of the inorganic material can interfere not only with the thermal properties but also the electrical, optical, mechanical, and dielectric properties of the polymer [32].
Figure 3a shows the thermogravimetric curves (TGA) and Fig. 3b is the derivative (DTG) of materials, in which the weight loss that initiated slowly increases gradually at 400 °C followed by the main weight loss zone for all samples. Table 1 shows the values of the percentages of weight loss in the respective temperature range of materials.
Analyzing Fig. 3 it is possible to observe differences in the decomposition temperatures of the materials. The decomposition temperature of the pristine HDPE and nanocomposites occurred between a range of 400 and 500 °C, in good agreement with other polyethylene composites reported in the literature [7, 33].
As Table 1 indicates, no significant weight change was observed in the samples at 100 °C. The weight loss at 400 °C was due to the pristine HDPE decomposition. The thermal degradation of the nanocomposites increased when compared to the pristine HDPE. Therefore, the thermal stability of nanocomposites (HDPE/Al2O3% wt/wt) is higher compared to the pristine HDPE. This result evidences the interaction between Al2O3 powder and HDPE, which increases the decomposition temperature.
TGA curves of the pristine HDPE presented a higher weight loss compared to the nanocomposites, which is thermally stable in the temperature range between 400 and 500 °C due to the presence of Al2O3 nanoparticles [30]. Both the addition and the increase in Al2O3 nanoparticles content to the HDPE influenced the residual percentage (the pristine HDPE had a 0.5% residue, while HDPE/Al2O3 (4.0%) had a 6.2% residue). Alsayed [27] found similar behavior when studying composites of HDPE reinforced with zinc oxide.
This result was expected, since the specific heat of Al2O3 is lower compared to the HDPE, equal to 775 and 1850 J/K/kg respectively, and the thermal conductivity of alumina (39 W/m/K) is greater compared to the HDPE (0.46–0.50 W/m/K). Thus, the thermal energy was absorbed by the nanocomposites and as a result, the polyethylene chains degraded at higher temperatures, causing an increase in the degree of crystallinity of the nanocomposites compared to the pristine HDPE [10].
3.3 Differential Scanning Calorimetry (DSC)
The values of melting temperature (Tm) and crystallinity index of the second heating and crystallization temperature (Tc) from the cooling cycle were obtained from the DSC curves (Fig. 4), as summarized in Table 2. The melting temperature of the nanocomposites was similar to the pristine HDPE, but the initial solidification temperature of the nanocomposite was higher compared to the HDPE.
The crystallization temperature increased due to the presence of Al2O3 nanoparticles in the polymer matrix, which could act as nucleation sites.
The crystallinity values for nanocomposites using the crystallinity of pristine HDPE as a parameter, considering the proportion of HDPE in the nanocomposites, were calculated. The crystallinity values obtained did not show a correlation with Al2O3 nanoparticle content. All nanocomposites showed a higher crystallinity degree than expected. This behavior can be explained by the fact that the inorganic component act as a nucleating agent [16], and due to the transcrystallinity effect causing an increase in the polymer crystallization [33]. According to Suai et al. [34] crystallinity can also affect mechanical properties.
3.4 Mechanical Properties (Tensile Test)
The results of the tensile tests performed on the pristine HDPE and HDPE/Al2O3 nanocomposites are shown in Table 3. It was observed that the addition of Al2O3 nanoparticles to HDPE caused an increase of elongation at break compared to the pristine HDPE; an optimal result was achieved in the HDPE/Al2O3 (1.0% wt) specimen. This result implies that among all the samples developed in this work the HDPE/Al2O3 (1.0% wt) specimen is the most deformable and has the greatest strength. The mechanical behavior was not positively correlated with the reinforcement amount of HDPE nanocomposite through the experimental tests. The reinforcement also influences the nanocomposite stiffness. Since the matrix is the same for all the nanocomposites, the different behaviors may be attributed to the difference in reinforcement content and more efficient load transfers between the reinforcement and polymer. Typically, stiffer reinforcement inhibits the elongation of a highly ductile matrix due to efficient load transfer, ultimately decreasing the total elongation.
Lins et al. [10] obtained similar mechanical properties in HDPE/alumina (5% wt) composites when investigating the mechanical and thermal properties of HDPE/alumina/glass fiber hybrid composites.
Zhang et al. [13] observed similar behavior when evaluating the effects of particle size and content on the thermal conductivity and mechanical properties of HDPE/ Al2O3 composites processed by a two-roll mill and extrusion. However, the superior performance was observed in the samples in this work. It is believed that this enhancement is attributed to the processing technique explored in this work. The extrusion method has played a very important role in preparing nanocomposites because of its simple and versatile processing method. On the other hand, this method can potentially cause thermal decomposition of the particles used, and consequently, the degradation of the polymer matrix [19,20,21].
Therefore, in the thermokinetic mixer process used to obtain the nanocomposites, the high-speed rotation blades with high kinetic energy (converted to thermal energy) accelerate HDPE and Al2O3 nanoparticles, melting the polymer matrix and embracing the alumina nanoparticles. This process is fast enough to avoid degrading the samples or compromising the thermal stability of the material [21].
Therefore, the thermokinetic mixer system improved the thermal stability of nanocomposites and absorbed energy, causing an increase in the crystallinity and, consequently, an improvement in mechanical properties. The effect of crystallinity on mechanical properties can be observed in Fig. 5.
The addition of Al2O3 nanoparticles to HDPE processed by thermokinetic mixer results in nanocomposites with improved thermal and mechanical properties compared to the pristine HDPE, due to the increase in the degree of crystallinity of the nanocomposites.
4 Conclusion
In this study, HDPE/Al2O3 nanocomposites prepared by thermokinetic mixer, and the effect of different contents of Al2O3 nanoparticles without any surface treatment on the thermal and mechanical properties were investigated. The addition of Al2O3 nanoparticles to the HDPE caused an alteration in the thermal behavior of the nanocomposites. It was observed a slight increase in the melting and crystallization temperatures of nanocomposites compared to the pristine HDPE. The alumina nanoparticles acted as nucleation sites leading to an increase in the crystallization degree and, consequently, an enhancement on the nanocomposite mechanical properties. HDPE/Al2O3 nanocomposite at alumina content of 4 wt% exhibited the best combination of thermal and mechanical properties.
References
K.A.T., Int. J. Res. Eng. Technol. 4, 92 (2015)
M.T.H. Mosavian, A. Bakhtiari, S. Sahebian, Polym. Plast. Technol. Eng. 51, 214 (2012)
I. Ahmad, H. Cao, H. Chen, H. Zhao, A. Kennedy, Y. Qiu, J. Eur. Ceram. Soc. 30, 865–873 (2009)
G.C. da Cunha, C.M. Abreu, J.A. Peixoto, L.P.C. Romão, Z.S. Macedo, J. Inorg. Organomet. Polym. Mater. 27, 674 (2017)
O.L. Ighodaro, O.I. Okoli, Int. J. Appl. Ceram. Technol. 5, 312 (2008)
N. Singh, R. Singh, R. Kumar, Eng. Res. Express 1, 015007 (2019)
A. Roberto, S. Patricia, B. A. Marinkovic, J. Mater. Sci. 49, 7870 (2014)
B. Basu, D. Jain, N. Kumar, P. Choudhury, A. Bose, S. Bose, J. Appl. Polym. 121, 2500 (2011)
X. Wang, R. Song, Y. Chen, Y. Zhao, K. Zhu, X. Yuan, Compos. Sci. Technol. 164, 103 (2018)
S.A.B. Lins, M.C.G. Rocha, J. Thermoplast. Compos. Mater. 32, 1566 (2018)
S. Dabees, B. Ahmed, Bioengineered 11, 679 (2018)
S. Sathees Kumar, G. Kanagaraj, J. Inorg. Organomet. Polym. Mater. 26, 788 (2016)
S. Zhang, X.Y. Cao, Y.M. Ma, Y.C. Ke, J.K. Zhang, F.S. Wang, Express Polym. Lett. 5, 581–590 (2011)
D.R. Mulinari, H.J.C. Voorwald, M.O.H. Cioffi, M.L.C.P. Silva, J. Compos. Mater. 51, 1807 (2016)
M. Šupová, G.S. Martynková, K. Barabaszová, Sci. Adv. Mater. 3, 1 (2011)
R.F. Brandenburg, C.M. Lepienski, D. Becker, L. Antonio, F. Coelho, Materia (2017). https://doi.org/10.1590/s1517-707620170004.0222
A.D. de Oliveira, C.A.G. Beatrice, Nanocompos-Recent Evol. (2018). https://doi.org/10.5772/intechopen.81329
K. Müller, E. Bugnicourt, M. Latorre, M. Jorda, Y.E. Sanz, J.M. Lagaron, O. Miesbauer, A. Bianchin, S. Hankin, U. Bölz, G. Pérez, M. Jesdinszki, M. Lindner, Z. Scheuerer, S. Castelló, M. Schmid, Nanomaterials 7, 74 (2017)
T.G. Gopakumar, D.J.Y.S. Pagé, Polym. Eng. Sci. 44, 1162 (2004)
T.G. Gopakumar, D.J.Y.S. Page, J. Appl. Polym. Sci. 96, 1557 (2005)
J. Ozen, F. Inceoglu, K. Acatay, Y.Z. Manceloglu, Polym. Eng. Sci. 52, 1537 (2012)
M.C. Kissinger-Kane (Thesis, 2007). https://www.researchgate.net/publication/252873193_Investigation_and_characterization_of_the_dispersion_of_nanoparticles_in_a_polymer_matrix_by_scattering_techniques. Accessed 10 Apr 2020
J. Pelto, T. Verho, H. Ronkainen, K. Kaunisto, J. Metsäjoki, J. Seitsonen, M. Karttunen, Polym. Test. 77, 105897 (2019)
A. Nabhan, A.K. Ameer, A. Rashed, Int. J. Adv. Sci. Technol. 28, 481–489 (2019)
X. Chen, J. Sha, T. Chen, H. Zhao, H. Ji, L. Xie, Y. Ma, Compos. Sci. Technol. 170, 7–14 (2018)
M.F. Uddin, C.T. Sun, Compos. Sci. Technol. 70, 223 (2010)
Z. Alsayed, R. Awad, M. Salem, Iran. Polym. J. (2020). https://doi.org/10.1007/s13726-020-00796-7
A. Umar, E.S. Zainudin, S.M. Sapuan, J. Mech. Eng. Sci. 2, 198 (2012)
K. Silva, C.A. Paskocimas, F.R. Oliveira, J. Therm. Anal. Calorim. 103, 267 (2015)
P. Patnaik, Handbook of preparative inorganic chemistry (McGraw-Hill, New York, 2002), p. 1125
W. Viratyaporn, R.L. Lehman, J. Therm. Anal. Calorim. 103, 267 (2011)
A. Bekhoukh, M. Mekhloufi, R. Berenguer, A. Benyoucef, Colloid Polym. Sci. 294, 1877 (2016)
W.R. Waldman, M.A. De Paoli, J.R. Arau, Polym. Degrad. Stab. 93, 1770 (2008)
C. Shuai, X. Yuan, W. Yang, S. Peng, C. He, P. Feng, F. Qi, G. Wang, Polym. Test. 85, 106458 (2020)
Acknowledgements
This research was funded by FAPERJ (E-26/260.026/2018 and E-26/010.001800/2015).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Costa, I.L.M., Zanini, N.C. & Mulinari, D.R. Thermal and Mechanical Properties of HDPE Reinforced with Al2O3 Nanoparticles Processed by Thermokinectic Mixer. J Inorg Organomet Polym 31, 220–228 (2021). https://doi.org/10.1007/s10904-020-01709-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10904-020-01709-0