Abstract
In this reported article, a highly efficient, stable and low cost CuO/TiO2@MOF-5 composite has been synthesized by in-situ incorporation of pre-synthesized precursors into MOF-5. The formation of synthesized samples has been confirmed by Powder X-ray diffraction (PXRD), Scanning electron microscopy (SEM) images, Energy dispersive X-ray (EDX) images and Ultraviolet visible spectrophotometry. The synthesized samples are found efficient for photoelectrochemical oxygen evolution reaction (OER) and photodegradation of organic dyes (methylene blue/methyl orange). It has been observed that CuO/TiO2@MOF-5 shows lowest onset potential, highest current density and better OER activity as compared to some of previously reported Cu-based working electrodes. It required only 263 mV overpotential to deliver the benchmark 10 mA cm−2 current density. In the same way, CuO/TiO2@MOF-5/NF exhibits maximum ability to degrade methylene blue and methyl orange as compared to all other samples.
Graphic Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
With the increasing demands of energy and rapid consumption of fossil fuels, search of alternate and renewable energy sources has attracted a great research attention. Amongst different alternate energy sources, hydrogen represents itself as a most promising alternate and renewable energy source [1]. The production of hydrogen through photoelectrochemical water splitting has been widely used as one of the most promising way to produce clean and renewable H2 fuel [2]. Water splitting consists of two half reactions: Oxygen evolution reaction (OER) at photoanode and hydrogen evolution reaction (HER) at photocathode. Both these reactions are kinetically sluggish because they required a lot of overpotential [3].
Oxygen evolution reaction (OER) is kinetically more sluggish as compared to HER because of the four-electron coupled reactions [4]. Recently, the most efficient OER catalysts are based on noble metals (Ir or Ru) but due to their scarcity and high cost, they cannot be used at commercial level [5]. So, a lot of research attention has been focused on the development of low cost, efficient and non-noble metal based OER catalysts such as transition metal oxides, phosphides, sulphides and selenides [6,7,8,9].
MOFs (metal-organic frameworks) are a class of organic and inorganic hybrid porous materials, which have been generating a rapid expansion and development in many potential applications such as gas storage, CO2 capture, separation, catalysis etc. This is due to easily tailored, modified and diverse structures of MOFs [10,11,12,13,14,15]. Now a day, MOFs have been emerged as a new class of OER catalysts because of their unique properties [16]. But, efficient MOF as an OER catalyst has not been well explored due to sluggish kinetics and instability. Therefore, different strategies have been proposed to enhance the OER activity of MOFs and one of them is the incorporation of pre-synthesized 3d transition metal oxide nanoparticles into MOFs to make an efficient MOF-based OER catalyst. Out of various MOFs, MOF-5 has been reported as an efficient photocatalyst [17].
Amongst 3d-transition metal oxide nanoparticles, TiO2 and CuO nanoparticles have been widely studied due to their appreciative chemical and physical properties, non-toxicity, chemical stability and low cost [18,19,20]. So, composite formation of CuO with TiO2 as TiO2/CuO nanocomposite has been reported as an efficient photocatalyst for water splitting and photodegradation of many organic pollutants [21].
In this reported research work TiO2, CuO nanoparticles and their nanocomposite TiO2/CuO were synthesized and successfully incorporated into MOF-5 by in-situ incorporation of pre-synthesized precursors into MOF-5. Further they were used for photoelectrochemical studies towards oxygen evolution reaction (OER) and photodegradation of methylene blue and methyl orange. Amongst, CuO/TiO2@MOF-5 exhibits excellent catalytic activity towards both OER and photodegradation of organic dyes.
2 Experimental Work
2.1 Chemicals
Zinc (II) chloride (ZnCl2, Merck, 98%); Titanium (IV) chloride (TiCl4, Merck, 99%); Copper (II) chloride dihydrate (CuCl2·2H2O, Merck, 99.99%); Terephthalic acid (1,4-H2BDC, Merck, 98%); N,N-dimethylformamide (DMF, Merck, 99.8%); Sulphuric acid (H2SO4,Merck, 98%); Sodium hydroxide (NaOH, 97%), Potassium hydroxide (KOH, Merck, 85%); Ammonium hydroxide (NH4OH, Merck, 32%); Acetic acid (CH3COOH, Merck, 99%).
3 Preparation of CuO Nanoparticles
Nanoparticles of CuO were synthesized by using sol-gel method as reported in the literature [22]. 0.2 M aqueous solution of CuCl2·2H2O was prepared by dissolving 3.40 g of CuCl2.2H2O in 100 mL de-ionized water. 1 mL of glacial acetic acid was added to above solution and heated the whole mixture at 100 °C under constant stirring. Then 8M NaOH was added drop-wise until pH reached 7. Black precipitates were obtained, which were separated by centrifugation, washed with de-ionized water and dried at 50 °C for 24 h.
3.1 Preparation of TiO2 Nanoparticles
TiO2 nanoparticles were synthesized by hydrolysis of TiCl4 as reported previously [23]. In brief, 1 mL of TiCl4 was added to 10% sulphuric acid at 0 °C with continuous stirring for 30 min. A gray solution was formed. It was then heated upto 60 °C to form a clear solution. Then, NH4OH was added drop-wise to above solution until pH reached 7 and a white solution formed. At last, the white solution was cooled to room temperature and gelled for 12 h. After that, white powder of hydrousTiO2 was formed, which was separated by centrifugation, washed with de-ionized water and dried in air. The dried precipitates were calcinated at 400 °C to get off-white precipitates of TiO2 nanoparticles.
3.2 Preparation of CuO/ TiO2 Nanocomposite
The CuO/TiO2 nanocomposite was synthesized by precipitation method. 6 mL of concentrated sulphuric acid was added to 10 mL of distilled water and cooled to 0 °C. Then, 2.88 mL of TiCl4 and 3.10 g of CuCl2·2H2O were added in above solution and temperature was maintained at 35 °C. Afterthat, 5M NaOH was added drop-wise until black precipitates were obtained, which were separated by centrifugation, washed with distilled water and dried in air. The dried precipitates were calcinated at 400 °C for 3 h to get CuO/TiO2nanocomposite.
3.3 Preparation of MOF-5
For the synthesis of MOF-5, 1.410 g of ZnCl2 was dissolved in 20 mL of DMF by sonication for 1 h. In another beaker, 1.295 g of 1, 4-BDC was dissolved in 20 mL DMF and added dropwise to the above mixture under continuous stirring. Then, whole mixture was stirred for 12 h. White precipitates were obtained, separated by centrifugation, washed with de-ionized water/DMF and dried at 60oC for 6 h. Finally, the dried precipitates were ground and stored for further process.
3.4 Incorporation of CuO/TiO2intoMOF-5
CuO/TiO2@MOF-5 composite was synthesized by in-situ incorporation of pre-synthesized CuO/TiO2 composite into MOF-5 at room temperature. Typically, 1.410 g of ZnCl2 and 0.020 g of CuO/TiO2 composite were dissolved in 20 mL of DMF by sonication for 1 h. In another beaker, 1.295 g of 1, 4-BDC was dissolved in 20 mL DMF and added dropwise to the above mixture under continuous stirring. Then, whole mixture was stirred for 12 h. Light green precipitates were obtained, separated by centrifugation, washed with de-ionized water/DMF and dried at 60oC for 6 h. Finally, the dried precipitates were ground and stored for further process.
3.5 Measurement of Dye Degradation by Prepared Samples
1 × 10− 4 M dye solutions were prepared by dissolving 0.035 g of methylene blue and 0.033 g of methyl orange in 1.0 L distilled water separately. The λmax value of methylene blue and methyl orange were measured by UV-visible spectrophotometer. λmax of methylene blue and methyl orange were measured as 663.5 nm and 465.6 nm respectively.
0.2 g of Sample was mixed in the 100 mL dye solution with maximum absorbance and kept in dark with constant stirring for 30 min. 5 mL of sample was taken and to remove the solid particles centrifuged and its absorbance was measured by spectrophotometer. The remaining solution was kept in a closed reactor under UV light. UV–Vis absorbance of the solution was measured after every 10 min interval by taking out 5 mL sample from the stock.
3.6 Preparation of Working Electrodes
The working electrodes were prepared on a piece (1 cm × 1 cm) of nickel foam. The piece of Ni-foam was cleaned by washing with acetone and distilled water, dried at 50° for 2 h. The slurry of synthesized samples was prepared by adding 0.01 g of sample in 2 mL distilled water and sonicated for 30 min to form uniform slurry. Then, slurry was pasted uniformly on a pre-cleaned piece of Ni-foam and dried in an oven at 50° for 6 h.
3.7 Electrochemical Studies for Oxygen Evaluation Reaction (OER)
Electrochemical studies were performed by using a common three electrode set-up containing Ag/AgCl as reference electrode, Pt-wire as counter electrode and catalyst@Ni-foam as working electrode in 1 M KOH (aq) electrolyte. The cyclic voltammetry and linear sweep voltammetry were performed to evaluate the catalytic activity towards OER at 50mVs− 1 and 1mVs− 1, respectively. All the potential data is converted into reversible hydrogen electrode (RHE) by using the following equation [24].
Overpotential (η) for OER has been calculated from linear sweep voltammetry (LSV) by using following equation.
3.8 Characterizations
Powder X-ray diffraction patterns (P-XRD) of all the synthesized samples were obtained by using Shimadzu X-ray diffractometer with Cu-Kα radiation (λ = 0.15406 nm) in 2θ range 5° to 55°. The morphology and composition of synthesized samples were evaluated through scanning electron microscopy (SEM) images and energy dispersive X-ray (EDX) spectra by using Philips XL30 Environmental SEM attached with an Oxford Instrument Inca 500 Energy Dispersive X-ray Spectrometer (EDX). The optical properties of synthesized samples were measured through Shimadzu UV-2600 spectrophotometer in wavelength range 200–900 nm.
4 Results and Discussion
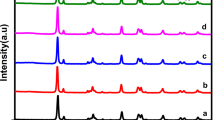
The PXRD patterns of all synthesized samples are represented in Fig. 1. In the PXRD pattern of TiO2and CuO all the diffraction peaks are in well agreement with the reported pattern of TiO2 and CuO, respectively. In the same way, diffraction pattern of MOF-5 is in good agreement with reported pattern of MOF-5. It indicates successful synthesis of crystalline Zn-based MOF MOF-5, TiO2and CuO nanoparticles.
PXRD pattern of CuO/TiO2 composite contains diffraction peaks indexed to both CuO and TiO2 nanoparticles. Diffraction peak at 2θ about 25.20° indexed to TiO2 anatase structure and matched well with JCPDS card number 21-1272 and diffraction peaks at 2θ about 32.62°, 35.58°,38.87° and 48.74° indexed to CuO. PXRD diffraction pattern of CuO/TiO2@MOF-5 contains most of diffraction peaks indexed to MOF-5 and some additional peaks are also observed related to CuO/TiO2composite. However, it is also observed that in PXRD pattern of CuO/TiO2@MOF-5, the two main diffraction peaks of CuO around 35° to 40° are not more visible because these are overlaid by MOF-5. It shows that MOF-5 maintains its crystalline structure and shows its dominance after the incorporation of CuO/TiO2 composite.
The morphology of all the synthesized samples is studied by scanning electron microscopy (SEM) images, represented by Fig. 2a––e. SEM images of CuO and TiO2 show that they have been grown in small irregular particles, which are highly agglomerated, shown by Fig. 2a, b. Figure 2c represents the SEM image of CuO/TiO2 composite, which shows that it has grown in block shape crystals. While SEM image of MOF-5, Fig. 2d, reveals that MOF-5 has grown in uniform and well distributed rectangular shape crystalline form with smooth surfaces and no agglomeration is observed. Similarly, it can be observed from SEM image of CuO/TiO2@MOF-5 that it has also grown in rectangular shaped crystalline form with smooth surfaces, represented by Fig. 2e.
The composition of all the synthesized samples has been studied by SEM based EDX analysis, represented by Fig. 3(a-e). Figure 3a represents the EDX spectrum of CuO, it shows that it contains the basic peaks of Cu and O (from CuO nanoparticles) and no other peak is observed. In the same way, EDX spectrum of TiO2 contains basic diffraction peaks of Ti and O as shown in Fig. 3b. Similarly, EDX spectrum of CuO/TiO2 reveals that it contains peaks index to both CuO and TiO2nanoparticles, represented by Fig. 3c. It can be seen from the EDX spectrum of MOF-5 that it contains diffraction peaks related to Zn, C and O and the EDX spectrum of CuO/TiO2@MOF-5 shows that it contains additional peaks for Cu and Ti in addition to peaks of MOF-5. It indicates the successful incorporation of CuO/TiO2 composite into MOF-5, shown by Fig. 3d and e respectively. In all these EDX spectra, no extra peak for impurity is observed which indicates high purity and single phase formation of these samples.
The optical properties measured by UV-Vis Spectrophotometry, Fig. 4a represent the UV-Vis spectra of all the synthesized samples. It can be observed from UV-Vis spectra of MOF-5 that it shows maximum absorption in UV-region with λ max at 256 nm and shows no absorption in visible region. However, after the incorporation of CuO/TiO2 composite into MOF-5, CuO/TiO2@MOF-5 shows a shift towards lower wavelength in UV-region with λ max at 230 nm and a new absorption peak is also observed in the visible region with λ max at 697 nm. Tauc plot is derived from UV-Vis spectra of the synthesized samples to study the band gap, represented by Fig. 4b. The band gap of all these samples has been calculated from Tauc plot by extrapolating the absorption edges by using following equation [25].
where α = absorption coefficient, h = Planck’s constant, ν = wavenumber.
The band gap of CuO and TiO2 are 1.44 and 3.23 eV, respectively while their composite CuO/TiO2 has intermediate band gap of 2.10 eV. Bare MOF-5 has band gap of 3.78 eV and after the incorporation of CuO/TiO2 nanocomposite, band gap of CuO/TiO2@MOF-5 is 3.45 eV. It indicates that incorporation of CuO/TiO2 nanocomposite into MOF-5 has shifted the band gap towards more visible region due to synergistic effect and hetero-junction formation between CuO/TiO2 and central metallic cluster [Zn4O]6+ of MOF-5, which increased the absorption of visible light, reduced the electron-hole pair recombination and enhanced the photocatalytic activity.
4.1 Photoelectrochemical studies towards OER
All the synthesized samples have been used for photoelectrochemical studies towards oxygen evolution reaction (OER) via water splitting, both in the presence of dark and visible light. OER measurements have been observed by cyclic voltammetry (CV) and linear sweep voltammetry (LSV) at 50mVs− 1 and 1mVs− 1 scan rate, respectively. The CV curves of all the synthesized samples are shown in Fig. 5a within potential range of 1.0 to 2.0 V vs. RHE. It is observed from CV curves that all the synthesized samples show zero current density in dark. It can be seen from the CV curves that MOF-5/NF does not show any OER activity and generates almost zero current density, both in dark as well as in the presence of visible light. However, after the incorporation of CuO/TiO2 nanocomposite into MOF-5 and in the presence of visible light prominent increase of current density was observed due to OER activity. The CV curves reveal that among all the synthesized samples, CuO/TiO2@MOF-5/NF shows lowest onset potential and maximum current density as compared to all other samples. It indicates that CuO/TiO2@MOF-5/NF shows better OER activity as compared to all other samples due to hetro-junction formation between CuO/TiO2 and MOF-5.
Further, photoelectrochemical activity of synthesized samples towards OER is evaluated by linear sweep voltammetry (LSV) measurements, represented by Fig. 6b. Like CV, LSV curves also evaluated that MOF-5/NF does not show any OER activity. For comparative studied, overpotential required to achieve 2 mA cm−2 current density is considered. It is observed that CuO/TiO2@MOF-5 needs only 153 mV overpotential to reach the current density of 2 mA cm−2as compared to all other synthesized samples CuO/NF (η2 = 183 mV), TiO2/NF(η2 = 273 mV) and CuO/TiO2/NF(η2 = 163 mV), represented by Fig. 6c.It can also be observed that among all synthesized samples only CuO/TiO2@MOF-5/NF delivers the benchmark of 10 mA cm−2 current density at just 263 mV overpotential, which is superior than some of the previously reported Cu-based OER catalysts as shown in Table 1. It reveals that CuO/TiO2@MOF-5/NF shows lowest onset potential, lowest overpotential to deliver 2 mA cm−2 current density and better OER activity as compared to all other synthesized samples. Further, the excellent OER activity of CuO/TiO2@MOF-5/NF is understand by Tafel plot derived from LSV curve, represented by Fig. 6d. Tafel plot is derived from LSV curve by using equation [26];
where η = overpotential, j = current density and b = Tafel slope.
The calculated Tafel slope of CuO/TiO2@MOF-5/NF is just 91 mV dec−1, which is lowest as compared to all other synthesized samples CuO/NF (269 mV dec−1), TiO2 (368 mV dec−1) and CuO/TiO2 (286 mV dec−1). It indicates thatCuO/TiO2@MOF-5/NF has faster and better electron transfer as compared to all other synthesized samples, which enhanced its activity as compared to other synthesized samples.
4.2 Photodegradation of Organic dyes
All the synthesized samples have been used for the degradation of methylene blue (MB) and methyl orange (MO) in the presence of visible light. Methylene blue and methyl orange are effectively oxidized by CuO, TiO2, CuO/TiO2, MOF-5 and CuO/TiO2@MOF-5, as shown in Fig. 7. It can be observed from Fig. 7 (a) and (b) that with the passage of time due to degradation of MB and MO, concentration and absorbance are decreased.
Transition metal oxide nanoparticles are considered as active photocatalysts due to their suitable band gap for visible light harvesting but their activities are still less due to rapid electro-hole pair recombination [27, 28]. So, individual nanoparticles of CuO and TiO2 do not effectively photodegrade MB and MO but after the formation of CuO/TiO2 composite, effective photodegradation is observed. However, after the incorporation of CuO/TiO2 composite into MOF-5, CuO/TiO2@MOF-5 shows better photocatalytic activity as compared to individual CuO, TiO2, MOF-5 and CuO/TiO2 composite due to decrease of electron-hole pair recombination and increase of charge separation. It can also be observed form Fig. 7c and d that after 60 min CuO/TiO2@MOF-5 shows minimum absorption of 0.012 and 0.087 as compared to all other synthesized samples CuO (0.184, 0.273), TiO2 (0.193, 0.207), CuO/TiO2 (0.165, 0.140) and MOF-5 (0.206, 0.214), respectively for methylene blue (MB) and methyl orange (MO). It indicates that MB and MO have been successfully degraded by the synthesized samples in the presence of visible light and maximum activity has been observed by CuO/TiO2@MOF-5.
4.3 Proposed Possible Mechanism of Photodegradation of Organic Dyes:
The possible mechanism for photocatalytic degradation of Methylene blue is as follows. The schematic representation of Possible Mechanism of Photodegradation of Organic Dyes by synthesized samples is shown by Fig. 6.
Depending upon above discussions, it is proposed that upon irradiation of visible light a number of photoexcited electrons are transferred from valence band (VB) to conduction band (CB) of MOF-5 leaving behind the positive hole (h+) in valence band (VB). The photoexcited electrons are further transferred from CB of MOF-5 to CuO/TiO2 and oxidized the oxygen molecules into O2•−radicals. These O2•− radicals directly participate in the degradation of methylene blue and methyl orange. On the other side, positive hole (h+) in the valance band also participates in the degradation of methylene blue as well as methyl orange.
5 Conclusions
In this reported article, highly efficient and stable catalyst CuO/TiO2@MOF-5/NF has been synthesized by in-situ incorporation of pre-synthesized precursors into MOF-5. It has been observed that incorporation of precursors has increased the charge separation and catalytic activity of CuO/TiO2@MOF-5 due to hetro-junction formation as compared to all other synthesized samples. The synthesized samples have been used for the study of photodegradation and electrochemical studies. Results indicate that CuO/TiO2@MOF-5/NF has better ability for photodegradation of methylene blue as well as photoelectrochemical OER activity as compared to all other synthesized samples.
References
M. Fiaz, M. Athar, JOM. 1–7 (2019)
P.W. Menezes, A. Indra, C. Das, C. Walter, C. Geobel, V. Gutkin, D. Schmeiber, M. Driess, ACS Catal. 7, 103–109 (2017)
Y. Jiao, Y. Zhang, M.T. Jaroniec, S.Z. Qiao, Chem.Soc. Rev. 44, 2060–2086 (2015)
S.H. Ye, Z.X. Shi, J.X. Feng, Y.X. Tong, G.R. Li, Angew. Chem. Int. Ed. 57, 2672–2676 (2018)
K.L. Materna, B. Rudshteyn, B.J. Brennan, M.H. Kane, A.J. Bloomfield, D.L. Huang, D.Y. Shopov, V.S. Batista, Adv. Energy Mater. 4, 1701475 (2017)
Y. Zeng, R. Hao, B. Xing, Y. Hou, Z. Xu, Chem. Commun. 46, 3920–3922 (2010)
M. Li, T. Liu, X. Bo, M. Zhou, L. Guo, S. Guo, Nano Energy 33, 221–228 (2017)
S. Peng, L. Li, X. Han, W. Sun, M. Srinivasan, S.G. Mhaisalkar, F. Cheng, Q. Yan, J. Chen, S. Ramakrishna, Angew. Chem. In. Ed. 126, 12802–12807 (2014)
C. Xia, Q. Jiang, C. Zhang, G. Cui, Coord. Chem. Rev. 257, 1946–1956 (2013)
C. Wang, H.Y. Li, G.L. Guo, P. Wang, Transit. Metal Chem. 38, 275–282 (2013)
J. Lee, O.K. Farha, J. Roberts, K.A. Scheidt, S.T. Nguyen, J.T. Hupp, Chem. Soc. Rev. 38, 1450–1459 (2009)
J.R. Li, J. Yu, W. Lu, L.B. Sun, J. Sculley, P.B. Balbuena, H.C. Zhou, Nat. Commun. 4, 1538 (2013)
R.J. Kuppler, D.J. Timmons, Q.R. Fang, J.R. Li, T.A. Makal, M.D. Young, D. Yuan, D. Zhao, W. Zhuang, H.C. Zhou, Coord. Chem. Rev. 253, 3042–3066 (2009)
K. Sumida, D.L. Rogow, J.A. Mason, T.M. McDonald, E.D. Bloch, Z.R. Herm, T.H. Bae, J.R. Long, Chem. Rev. 112, 724–781 (2011)
H.C. Zhou, J.R. Long, O.M. Yaghi, Chem. Rev. 112, 673–674 (2012)
H.B. Wu, X.W. Lou, Sci. Adv. 3, 9252 (2017)
M. Alvaro, E. Carbonell, B. Ferrer, F.X. LlabresiXamena, H. Garcia, Chem-Eur. J. 13, 5106–5112 (2007)
L.R. Hou, C.Z. Yuan, Y. Peng, J. Hazard. Mater. 139, 310–315 (2007)
A.H. Jawad, A.F. Alkarkhi, N.S.A. Mubarak, Desalin. Water Treat. 2014, 1–12 (2014)
A.N. Ejhieh, M.K. Shamsabadi, Appl. Catal. A 477, 83–92 (2014)
Y. Fang, Y.R. Wang, G. Jiang, H. Jin, Y. Wang, X. Sun, S. Wang, T. Wang, Bull. Mater. Sci. 35, 495–499 (2012)
J.M. Kshirsager, R. Shrivastava, P.S. Adwani, Thermal Sci. 2, 233–242 (2017)
W. Li, T. Zeng, PLoS ONE 6, 21082 (2011)
M. Fiaz, M. Kashif, S. Majeed, M.N. Ashiq, M. Athar, M.A. Farid, ChemistrySelect 4, 6996–7002 (2019)
J.H. Shah, M. Fiaz, M. Athar, J. Ali, M. Rubab, R. Mahmood, S.U. U. Jamil, R. Djellabi, Environmental Technology, 2019
M. Fiaz, M. Athar, ChemistrySelect, 2019, 4, 8508–8515
C.M. Magdalane, K. Kaviyarasu, N. Matinise, N. Mayedwa, N. Mongwaketsi, D. Letsholathebe, G.T. Mola, N.A. Al-Dhabi, M.V. Arasu, M. Henini, J. Kennedy, M. Maaza, B. Jeyaraj, S. Afr. J. Chem. Eng. 26, 49–60 (2018)
C.M. Magdalane, K. Kaviyarasu, G.M. Priyadharisni, A.K.H. Bashir, N. Mayedwa, N. Matinise, A.B. Isaev, N.A. Al-Dhabi, M.V. Arasu, S. Arokiyaraj, J. Kennedy, M. Maaza, J. Mater. Res. Technol. 8(3), 2898–2909 (2019)
Acknowledgements
Author acknowledges Higher Education Commission (HEC) of Pakistan and Institute of Chemical Sciences, Bahauddin Zakariya University, Pakistan for providing lab facilities to carry out this research work as well as Dr. Muhammad Athar for his nice suggestion and supervisions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jabbar, A., Fiaz, M., Rani, S. et al. Incorporation of CuO/TiO2 Nanocomposite into MOF-5 for Enhanced Oxygen Evolution Reaction (OER) and Photodegradation of Organic Dyes. J Inorg Organomet Polym 30, 4043–4052 (2020). https://doi.org/10.1007/s10904-020-01550-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10904-020-01550-5