Abstract
Development of a highly active, stable, and facile-synthesized photoelectrocatalyst for water oxidation (OER) is very challenging and has attracted great research attention. In this article, highly efficient MOF-based photoelectrocatalysts (MOF-5 and amine-functionalized MOF (NH2-MOF-5)) have been synthesized at room temperature and have been successfully characterized. For the photoelectrochemical studies, working electrodes are prepared by coating the synthesized photoelectrocatalysts on Ni-foam. All the synthesized materials have been successfully characterized via powder X-ray diffraction (PXRD), Fourier-transform infrared (FTIR) spectroscopy, scanning electron microscopy (SEM), energy-dispersive X-ray (EDX) spectroscopy, elemental mapping, and ultraviolet-visible (UV-Vis) spectroscopy. Photoelectrochemical measurements for oxygen evolution reaction are performed via cyclic voltammetry and linear sweep voltammetry. It has been observed that among all the synthesized catalysts, Co3O4@NH2-MOF-5/NF has emerged as an efficient, stable, and highly active photoelectrocatalyst towards oxygen evolution reaction (OER) as compared to all other synthesized catalysts. It requires just 223 mV overpotential to deliver the 10 mA cm−2 current density and exhibits the lowest Tafel slope 52 mV dec−1 as compared to all other synthesized samples and some of the previously reported catalysts. Furthermore, long-term catalytic stability is studied via continuous linear sweep voltammetry and chronoamperometric measurements. This study encourages the development of a more efficient MOF-based catalyst for different photoelectrochemical studies.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the twenty-first century, society is facing a great challenge to deal with environmental pollution and energy crisis due to dependence on carbon-based fuels. So, it motivated a huge number of scientists and researchers to search for an alternate source of energy [1]. Different strategies have been proposed to solve this and one of them is hydrogen production from water splitting. Water splitting is divided into two half-reactions: cathodic half-reaction (where hydrogen evolution reaction (HER) takes place) and anodic half-reaction (where oxygen evolution reaction (OER) takes place) [2]. Both HER and OER are kinetically sluggish reactions and huge overpotential is required to occur at a suitable rate. OER is four electron-proton coupled reaction (4OH− → 2H2O + 4e− + O2) while HER is just a two-electron transfer reaction (2H+ + 2e− → H2). So, OER is kinetically more sluggish and requires higher overpotential as compared to HER to overcome the energy barrier and splits the water [3].
Therefore, extensive research has been focused on the development of highly efficient OER catalysts. Among various active OER catalysts, RuO2 and IrO2 are considered as highly active OER catalysts both in acidic and basic electrolytes because they require very less overpotential for oxygen evolution from water splitting [4, 5]. But, they cannot be used in large-scale practical applications due to their scarcity and expensive nature. So, researchers and scientists have devoted their research attention to the development of efficient, stable, and cost-effective non-noble metal OER catalysts, i.e., 3d-transition metal oxides, perovskites, transition metal sulfides, hydroxides, and non-metal compounds [6,7,8,9,10].
Metal-organic frameworks (MOFs) are porous crystalline-materials, composed of inorganic metal and an organic ligand. Due to their unique properties such as high porosity and large surface area, they have been widely used in a large number of potential applications such as gas adsorption/separation [11], magnetism [12], optoelectronics [13], and catalysis [14]. In recent years, due to their tunable pore structure and easy functionalization, MOFs are emerging as promising catalysts in a basic medium for water splitting [15]. Recently, highly efficient MOF-based OER catalysts have been reported such as NiFe-MOF-74 supported on Ni-foam delivered the benchmark of 10 mA cm−2 at 223 mV overpotential [16]. Similarly, Fe-doped Ni-MOF FeII0.1Ni0.4-MOF, Co-BPDC/Co-BDC heterojunction, UTSA-16, and Co-MOF have been reported as efficient OER catalysts and they delivered the 10 mA cm−2 current density at 294, 335, 408, and 360 mV overpotential, respectively [17,18,19,20]. Therefore, metal-organic frameworks (MOFs) have been used as potential catalysts for oxygen evolution reaction (OER), oxygen reduction reaction (ORR), and hydrogen evolution reaction (HER) such as NiO-MOF/rGo, rGO-NiO/CuO MOF, Ni-BTC-MOF/rGO, Co-BTC MOF/rGO, MnBDC@rGO, Cu-ZIF-67, NiCo/graphene, NiCo-ZIF, Cu-MOF/GO, and NiCo/NCNTs [21,22,23,24,25,26,27,28,29,30]. It has been also observed that catalytic efficiency of MOFs can be further increased by using 2-aminoterephthalic acid as an organic linker instead of terephthalic acid because amine functionalization shifts the band gap towards the visible region, increases the absorption of visible light, improves the charge separation, and reduces the e−/h+ (electron-hole pair) recombination [31].
First row 3d-transition metal oxide nanoparticles have been widely used in catalytic applications due to their low band gap, non-irritating, easy availability, and cost-effectiveness. Transition metal oxide (Mn, Fe, Co, Ni, and Cu) is also used as efficient HER and OER catalysts [32].
Therefore, in this research, we report the facile and low-cost synthesis of highly efficient OER MxOy@MOF-5 and MxOy@NH2-MOF-5 (MxOy = Co3O4, NiO, CuO, and ZnO) catalysts at room temperature via in situ incorporation of four metal oxide nanoparticles into host MOFs. It is observed that amine functionalization has increased the photoelectrocatalytic activity towards OER. MxOy@NH2-MOF-5 photoelectrocatalysts show better OER activity as compared to MxOy@MOF-5 and some of previously reported MOF-based OER catalysts.
Experimental
Chemicals
Zinc acetate dihydrate (Zn(CH3COO)2·2H2O, Merck, 99.99%), 1,4-benzenedicarboxylic acid (H2BDC, Merck, 99%), 2-aminoterephthalic acid (Merck, 99%), N,N-dimethylformamide (DMF, Merck, 99.8%), sodium hydroxide (NaOH, Merck, 97%), ethanol (CH3CH2OH, Merck, 95%), oleic acid (C18H34O2, Merck, 99%), iron sulfate heptahydrate (Fe2(SO4)3·7H2O, Merck, 21-23% Fe basis), cobalt acetate tetrahydrate (Co(C2H3O2)2·4H2O, Merck, 97%), ammonia solution (NH4OH, Merck, 28%), nickel chloride hexahydrate (NiCl2·6H2O, Merck, 97%), copper nitrate trihydrate (Cu(NO3)2·3H2O, Merck, 99%), and deionized water.
Synthesis of transition metal oxide (MxOy) nanoparticles
Four transition metal oxide (MxOy = Co3O4, NiO, CuO and ZnO) nanoparticles were synthesized [33,34,35,36]. Except for Co3O4 nanoparticles, the remaining metal oxide (NiO, CuO, and ZnO) nanoparticles were synthesized by using the precipitation method. In brief, a particular amount of metal salt was dissolved in 100 mL distilled water by continuous stirring for 30 min. After that, 0.2 M NaOH was added dropwise and stirred for a specific period of time at a specific temperature as mentioned in Table 1. Then, precipitates were obtained, separated by centrifugation, washed with distilled water, and dried at 50 °C. After that, dried precipitates were calcinated at 500 °C for 2 h, while Co3O4 nanoparticles were prepared by using the one-step hydrothermal method. Cobalt acetate tetrahydrate was dissolved in distilled water by continuous stirring for 30 min. After that, 10 mL of ammonia solution was added dropwise under continuous stirring and transferred the whole mixture into Teflon-lined autoclave and heated at 180 ° C for 12 h. Finally, the precipitates of Co3O4 nanoparticles were obtained.
Synthesis of amine-functionalized MOF-5
MOF-5 was synthesized at room temperature. In a round-bottom flask, 2.7 g of Zn(CH3COO)2·2H2O was dissolved into 100 mL DMF by continuous stirring. Then, 50 mL DMF solution containing 0.8 g H2BDC was added dropwise to the mixture under constant magnetic stirring. The whole mixture was stirred at room temperature for 24 h. Precipitates were obtained, collected by centrifugation, and washed with distilled water and DMF several times. The resulting product was dried at 50 °C in a vacuum oven for 3 h.
Amine-functionalized MOF-5, NH2-MOF-5, was synthesized under the same conditions as mentioned above for MOF-5 by using 2-aminoterephtahlic acid instead of 1,4-benzenedicarboxylic acid as an organic linker.
Synthesis of MxOy@MOF-5 and MxOy@NH2-MOF-5 composites
MxOy@MOF-5 and MxOy@NH2-MOF-5 composites were synthesized at room temperature via in situ incorporation of pre-synthesized metal oxide nanoparticles into host MOFs. In a typical procedure, 0.8 g of 1,4-benzenedicarboxylic acid was dissolved in 20 mL DMF by continuous stirring for 30 min. After that, dropwise added 10 mL DMF suspension of 0.01 g metal oxide nanoparticles and 2.7 g/20 mL DMF solution of Zn(CH3COO)2·2H2O, respectively. Then, the resultant solution was continuously stirred for 24 h and precipitates were separated by centrifugation, washed with distilled water and DMF, and dried overnight at 50 °C in an oven under vacuum. In the same way, MxOy@NH2-MOF-5 composites were synthesized by using 0.8 g/20 mL DMF of 2-aminoterephthalic acid instead of 1,4-benzenedicarboxylic acid.
Instruments
Powder X-ray diffraction spectra (PXRD) were obtained in 2θ range 5 to 80° via Shimadzu X-ray diffractometer with Cu-Kα radiation (λ = 0.15406 nm) at 40 kV and 40 mA. A Nicolet Nexus 870 spectrometer was used to collect FTIR spectra in the wavelength range from 400 to 4000 cm−1. Morphology and elemental composition of synthesized samples were evaluated by using Philips XL30 Environmental SEM attached with an Oxford Instrument Inca 500 energy-dispersive X-ray spectrometer (EDX). Optical properties were studied by using Shimadzu UV-2600 spectrophotometer in the range of 200–900 nm.
Formation of working electrodes
The slurry of the catalyst was uniformly pasted on a piece (1 cm2) of Ni-foam (NF) to form working electrodes. Before pasting slurry, Ni-foam was cleaned with acetone and water via sonication and dried at 50 °C in an oven for 2 h. To prepare the slurry, 10 mg of synthesized sample was dispersed in 1 mL deionized water via sonication for 30 min. After that, it was uniformly pasted on Ni-foam to form a photoanode for OER.
Photoelectrochemical studies
Photoelectrochemical studies towards OER were studied in a common three-electrode setup with Ag/AgCl as a reference electrode, Pt as a counter electrode, and catalyst coated on Ni-foam as photoanode. The photoelectrochemical studies were studied both in dark and in presence of visible light. The electrodes were dipped in 1M KOH (aq) electrolyte solution. A Uniscan instrument 3100 Poteniostat/Galvanostat attached with NOVA 1.10 software for automated data taking and recording was used for OER analysis. OER activity was studied via cyclic voltammetry (CV) and linear sweep voltammetry (LSV) measurements at 30 and 1 mV s−1, respectively. All the potential data was converted into reversible hydrogen electrode (RHE) potential by using the following equation.
For OER, overpotential (ƞ) was calculated by using the following equations
Tafel slope was calculated from the Tafel plot by using the following equation
where ƞ is the overpotential, j is the current density, and b is the Tafel slope
Furthermore, the stability of working electrodes was found via continuous 1000 LSV sweeps and chronoamperometric studies at an applied potential of 1.5 V in the presence of visible light.
Results and discussion
Crystallographic analysis
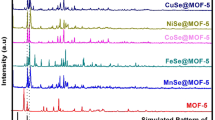
The powder X-ray diffraction patterns (PXRD) of bare MOF-5 and MxOy@MOF-5 in comparison with the simulated pattern of MOF-5 generated from original CIF file are represented in Fig. 1a. It can be seen that all the characteristic diffraction peaks of MOF-5 are consistent with the simulated pattern and it reveals the successful synthesis of Zn-based metal-organic framework, MOF-5. After the incorporation of metal oxide nanoparticles into MOF-5, MxOy@MOF-5 spectra exhibit some new diffraction peaks besides the diffraction peaks of MOF-5, which are in good agreement with the diffraction pattern of metal oxide (Co3O4, NiO, CuO, and ZnO) nanoparticles. Figure 1b represents the crystallographic patterns of NH2-MOF-5 and MxOy@NH2-MOF-5 composites. It is observed that NH2-MOF-5 has a different crystal structure and PXRD pattern as compared to MOF-5 due to the use of 2-aminoterephtalic acid instead of simple terephthalic acid. However, like MxOy@MOF-5, PXRD spectra of MxOy@NH2-MOF-5 indicate that amine-functionalized MOF, NH2-MOF-5, maintains its crystal structure and remains intact after incorporation of metal oxide nanoparticles. No extra diffraction peaks for impurity are observed, which indicates the single-phase formation of all these samples. From powder XRD analysis of MxOy@MOF-5 and MxOy@NH2-MOF-5, it can be concluded that incorporation of metal oxide nanoparticles does not significantly affect the crystalline structure of host MOFs, and there is no chemical interaction between incorporated nanoparticles and a central metallic cluster of MOFs; they are intact with each other via physical interaction.
Fourier-transform infrared spectroscopic analysis
The surface functional groups and formation of pure Zn-based MOFs MOF-5 and NH2-MOF-5 as well as their composites MxOy@MOF-5 and MxOy@NH2-MOF-5 are confirmed by FTIR spectroscopy. Figure 2a represents the FTIR spectra of MOF-5 and MxOy@MOF-5 composites. In the FTIR spectrum of MOF-5, two strong transmittance peaks related to symmetric and asymmetric stretching vibrations of the carboxylic group are observed at 1575 and 1375 cm−1. A weak transmittance peak can also be seen at 642 cm−1 due to symmetric stretching vibration of the central Zn4O6+ cluster of MOF-5 [37]. However, in the FTIR spectrum of NH2-MOF-5, a weak peak is observed at 1242 cm−1 for C–N stretching vibration of the aromatic amine group and two strong transmittance peaks are also observed at 1540 and 1378 cm−1 indexed to the vibrational motion of carboxylate group of 2-aminoterephthalic acid, shown by Fig. 2b [38], while FTIR spectra of MxOy@MOF-5 and MxOy@NH2-MOF-5 composites exhibit some additional peaks at 576, 517, 535, and 555 cm−1 for vibrations of Co–O, Ni–O, Cu–O, and Zn–O [39,40,41], respectively in addition to vibrational bands of both Zn-based MOFs MOF-5 and NH2-MOF-5, which indicates that metal oxide nanoparticles have been successfully incorporated into MOFs.
Morphological and compositional analysis
The morphology and composition of all the synthesized samples have been studied by scanning electron microscopy images, SEM-based energy-dispersive X-ray (EDX) analysis, and elemental mapping. Figure 3A (a–e) represents the SEM images of synthesized bare MOF-5 and MxOy@MOF-5 composites. It appears that synthesized MOF-5 has grown in well-defined rectangular morphology and is uniformly distributed, shown by Fig. 3A (a). Similarly, the incorporated samples, MxOy@MOF-5, have also grown in almost rectangular morphology but due to the incorporation of metal oxide nanoparticle coarse surfaces, variations in size and slight agglomerations are also observed, represented by Fig. 3A (b-e). Figure 3A (a′–e′) represents the SEM-based mix elemental maps of synthesized MOF-5 and its composites. It indicates that metal oxide nanoparticles have been successfully incorporated into cavities of MOF-5 and these particles are uniformly distributed.
The SEM images reveal that bare amine-functionalized MOF NH2-MOF-5 and its composites MxOy@NH2-MOF-5 have grown in spherical shapes with spongy smooth surfaces, as shown in Fig. 3B (a–e). However, as a result of the incorporation of metal oxide nanoparticles, some variations in size and agglomeration are observed in incorporated samples. Mix elemental maps of MxOy@MOF-5 and MxOy@NH2-MOF-5 indicate that metal oxide nanoparticles have been successfully incorporated into cavities of both Zn-based MOFs and these particles are uniformly distributed, as shown in Fig. 3B (a′–e′). Individual elemental maps of basic components of bare MOFs (MOF-5 and NH2-MOF-5) and their composites (MxOy@MOF-5 and MxOy@NH2-MOF-5) are shown in Figure S1 and S2.
The composition of all the synthesized samples has been studied through SEM-based EDX images. Figure S3 and S4 represent the EDX images of MOF-5/MxOy@MOF-5 and NH2-MOF-5/MxOy@NH2-MOF-5, respectively. It is evaluated from EDX images of bare MOF-5 and NH2-MOF-5 that they only contain diffraction peaks of basic elements Zn, C, and O of MOF-5 and Zn, C, O, and N of NH2-MOF-5, respectively. Similarly, incorporated samples contain all basic diffraction peaks of their respective MOF and they also contain additional diffraction peaks of Co, Cu, Ni, and Zn. It indicates the successful incorporation of metal oxide nanoparticles into both Zn-based MOFs. No additional peak of impurity is observed, which indicates a high degree of purity of these synthesized samples.
Optical analysis
The UV-Vis absorption spectra of as-synthesized bare Zn-based MOF MOF-5 and amine-functionalized MOF NH2-MOF-5 as well as their composites are represented by Fig. 4a, b. Figure 4a shows that MOF-5 exhibits maximum absorption at 207 nm with an absorption edge at 320 nm due to the organic linker terephthalate and inorganic central Zn4O13 cluster. It indicates that in MOF-5 ligand-to-metal charge transfer (LMCT) takes place. Upon loading with metal oxide nanoparticles into MOF-5, a redshift is observed in the UV region with maximum absorption at 235, 213, 245, and 256 nm in Co3O4@MOF-5, NiO@MOF-5, CuO@MOF-5, and ZnO@MOF-5, respectively. It has been also observed that in MxOy@MOF-5, absorption edges have extended up to 400 nm and new absorption peaks are also observed in the visible region with λmax at 695 and 754 nm in Co3O4@MOF-5 and CuO@MOF-5, respectively. The band gap of all these synthesized samples has been calculated from the Tauc plot, Fig. 4c, and is 4.03,3.01, 3.94, 3.48, and 3.70 eV for MOF-5, Co3O4@MOF-5, NiO@MOF-5, CuO@MOF-5, and ZnO@MOF-5, respectively. All these MOF-5-based samples exhibit low efficiency towards water splitting due to a large effective band gap. Therefore, their efficiency has been further improved by the amine-functionalization of MOF-5. The water-splitting efficiency of NH2-MOF-5 is significantly improved due to amine functionality. NH2-MOF-5 exhibits absorption edge at around 438 nm and upon loading with metal oxide nanoparticles, MxOy@NH2-MOF-5 composites, absorption edges extend up to 600 nm, falling in the visible region, as shown in Fig. 4b. So, a significant redshift is observed. Their calculated band gaps are 3.45, 2.78, 3.39, 3.23, and 2.89 eV for NH2-MOF-5, Co3O4@NH2-MOF-5, NiO@NH2-MOF-5, CuO@NH2-MOF-5, and ZnO@NH2-MOF-5, respectively. It indicates that amine functionalization reduces the band gap, increases the charge separation, and improves the catalytic activity.
a UV-Vis absorption spectra of MOF-5, Co3O@MOF-5, NiO@MOF-5, CuO@MOF-5, and ZnO@MOF-5; b UV-Vis absorption spectra of NH2-MOF-5, Co3O4@NH2-MOF-5, NiO@NH2-MOF-5, CuO@NH2-MOF-5, and ZnO@NH2-MOF-5; c Tauc plot of MOF-5, Co3O@MOF-5, NiO@MOF-5, CuO@MOF-5, and ZnO@MOF-5; d Tauc plot of NH2-MOF-5, Co3O4@NH2-MOF-5, NiO@NH2-MOF-5, CuO@NH2-MOF-5, and ZnO@NH2-MOF-5
Photoelectrochemical studies towards oxygen evolution reaction
Photoelectrochemical studies towards oxygen evolution reaction are studied by cyclic voltammetry (CV) and linear sweep voltammetry (LSV) at 30 and 1 mV s−1, respectively. Photoelectrochemical studies towards OER are studied both in dark and in the presence of visible light. Figure 5a represents the CV curves of MOF-5/NF and MxOy@MOF-5/NF electrodes. It is observed that almost zero current density is produced in dark due to negligible OER activity. However, all the synthesized samples exhibit anodic current density under illumination which further increases gradually with the incorporation of pre-synthesized nanoparticles. The bare MOF-5/NF generates only 0.012 mA cm−2 current density and after incorporation of metal oxide nanoparticles, a tremendous increase in current density has been observed in MxOy@MOF-5/NF electrodes and among all these catalysts, Co3O4@MOF-5/NF shows the lowest onset potential and generates maximum current density for OER. The catalytic OER activity of these synthesized samples has been further increased by amine functionalization of terephthalic acid. Figure 5b represents the CV curves of synthesized NH2-MOF-5/NF and MxOy@NH2-MOF-5/NF. It can be observed from CV curves that after amine functionalization, lower onset potential and higher current density are achieved towards OER as compared to bare MOF-5/NF and MxOy@ MOF-5/NF, respectively. Like MxOy@MOF-5/NF, among MxOy@NH2-MOF-5/NF electrodes, Co3O4@NH2-MOF-5/NF shows the lowest onset potential and generates the highest 34.48 mA cm−2 current density as compared to bare NH2-MOF-5/NF (6.33 mA cm−2), NiO@NH2-MOF-5/NF (8.06 mA cm−2), CuO@NH2-MOF-5/NF (16.77 mA cm−2), and ZnO@NH2-MOF-5/NF (20.67 mA cm−2), respectively.
Figure 5c and d represent the LSV curves of bare MOF-5 and amine-functionalized MOF, NH2-MOF-5/NF-based electrodes, and it can be seen that amine-functionalized-based electrodes exhibit better OER activity as compared to non-amine-functionalized working electrodes. It is also observed that the Co3O4@NH2-MOF-5/NF electrode exhibits the largest current density (32.93 mA cm−2 at 1.49 V vs. RHE) for OER as compared to all other electrodes. For comparison, overpotential (η) requires to achieve 5 mA cm−2 current density is considered, represented by Fig. 5e, f. It is found that Co3O4@NH2-MOF-5/NF needs only 203 mV overpotential to deliver 5 mA cm−2 as compared to all other amine-functionalized-based electrodes NH2-MOF-5/NF (η5 = 353 mV), NiO@NH2-MOF-5/NF (η5 = 243 mV), CuO@NH2-MOF-5/NF (η5 = 223 mV), and ZnO@NH2-MOF-5/NF (η5 = 333 mV) as well as non-amine-functionalized-based electrodes Co3O4@MOF-5/NF (η5 = 323 mV), NiO@MOF-5/NF (η5 = 353 mV), CuO@MOF-5/NF (η5 = 343 mV), and ZnO@MOF-5/NF (η5 = 333 mV). Furthermore, the overpotential required to reach the benchmark of 10 mA cm−2 current density is compared and it has been found that Co3O4@NH2-MOF-5/NF requires the lowest 223 mV overpotential to deliver 10 mA cm−2 as compared to both amine-functionalized and non-amine-functionalized-based electrodes CuO@NH2-MOF-5/NF (η10 = 253 mV), ZnO@NH2-MOF-5/NF (η10 = 353 mV), Co3O4@MOF-5/NF (η10 = 343 mV), NiO@MOF-5/NF (η10 = 363 mV), and CuO@MOF-5/NF (η10 = 353 mV), represented by Fig. 6a. This indicates that the Co3O4@NH2-MOF-5/NF electrode is a highly efficient photoelectrocatalyst for photoelectrochemical OER as compared to all other synthesized photoelectrocatalysts as well as previously reported Co-based and MOF-based OER catalysts, as represented in Table 2.
Tafel slope is an important parameter to understand the reaction kinetics. So, the Tafel plot is derived from LSV curves of MOF-5/NF and NH2-MOF-5/NF-based electrodes, as shown by Fig. 6b, c. It is revealed from Tafel plots that amine-functionalized electrodes show a lower Tafel slope as compared to non-amine-functionalized-based electrodes, which indicates that amine-functionalized electrodes have more favorable electron transfer and better OER activity. It is observed that Co3O4@NH2-MOF-5/NF exhibits the lowest Tafel slope 52 mV dec−1 as compared to all other synthesized catalysts as well as previously reported MOF-based OER catalysts as shown in Table 2. This result agrees with the highest current density of Co3O4@NH2-MOF-5/NF towards OER activity. So, it can be concluded that Co3O4@NH2-MOF-5/NF is a highly efficient catalyst for OER as compared to all synthesized photoelectrocatalysts. It is expected that in basic medium, OER is occurred by the following equations
So, this overall catalytic OER study reveals that amine functionalization of MOF-5 by using 2-aminoterephthalic acid has shifted the band gap towards the visible region, increased the charge separation, enhanced the absorption of visible light, and improved the photoelectrocatalytic OER activity. Thus, amine functionalization of MOF-5 has significantly enhanced the OER activity via water splitting.
The stability of the working electrode is another important parameter for photoelectrochemical studies. Therefore, the stability of Co3O4@MOF-5/NF and Co3O4@NH2-MOF-5/NF is studied by 1000 continuous LSV sweeps. Figure 7a and b represent the 1st and 1000th LSV cycle of Co3O4@MOF-5/NF and Co3O4@NH2-MOF-5/NF, respectively. It reveals that there is no degradation in current density before and after the 1000th LSV cycle. In addition to 1000 LSV cycles, long-term photoelectrocatalytic stability is also important for commercial applications. The long-term stability of all the synthesized samples is carried out for 6000 s by using the chronoamperometry technique. Figure 7c and d show the current vs. time graph of all the synthesized working electrodes at the static potential of 1.5 V vs. RHE for MOF-5-based and NH2-MOF-5-based working electrodes, respectively in the presence of visible light. The stability study reveals the satisfactory and superior stability of Co3O4@NH2-MOF-5/NF photoelectrocatalyst towards OER in alkaline solution.
Another important parameter to study reaction kinetics is impedance spectroscopy (EIS). It gives an idea about solution induced resistance as well as resistance at electrode/electrolyte interface (charge transfer resistance). The small diameter of semicircle indicates low induced resistance, low charge transfer resistance, and higher catalytic activity. Figure S5 represents the Nyquist plot of Co3O4@NH2-MOF-5/NF and bare MOF NH2-MOF-5. It shows that Co3O4@NH2-MOF-5/NF has a small diameter of the semicircle as compared to bare MOF NH2-MOF-5/NF, which reveals that Co3O4@NH2-MOF-5/NF has low charge transfer resistance, maximum conductance, and better OER activity as compared to bare MOF NH2-MOF-5/NF.
Proposed mechanism of photoelectrochemical oxygen evolution reaction
In MOFs, organic linkers act as an antenna, capture the visible light, and generate the photogenerated electrons. Therefore, it is hypothesized that photogenerated electrons are transferred from the valence band of NH2-MOF-5 to its conduction band and subsequently transferred to the energy level of incorporated Co3O4 nanoparticles. Furthermore, these electrons are transferred to the counter electrode via an external circuit and increased the charge separation, reduced the electron-hole pair recombination, and enhanced the catalytic activity. The resulting holes at the surface of the photoanode cause the photooxidation of water into O2 and H+. The H+ is transported towards the counter electrode via electrolyte where they undergo photoreduction by photogenerated electrons and generate hydrogen gas, as shown in Fig. 8.
Conclusion
In summary, the photoelectrocatalytic activity towards water oxidation (OER) of MOF-5-based photoelectrocatalysts has been successfully increased by amine functionalization. So, NH2-MOF-5/NF and MxOy@NH2-MOF-5/NF photoelectrocatalysts exhibit better and enhanced catalytic activity towards OER as compared to MOF-5/NF and MxOy@MOF-5/NF catalysts. Among all the synthesized photoelectrocatalysts, Co3O4@NH2-MOF-5/NF exhibits excellent catalysis towards OER in alkaline medium and it requires the lowest overpotential 203 mV and 223 mV to delivers 5 and 10 mA cm−2, respectively. In addition, it exhibits the lowest Tafel slope of 52 mV dec−1 and excellent long-term stability as compared to all other synthesized catalysts. A large number of active sites, fast electron transfers, and reduce electron-hole pair recombination lead to enhance the OER activity of Co3O4@NH2-MOF-5/NF as compared to all other synthesized catalysts and even some of the previously reported catalysts. This study encourages the development of more nanoparticles incorporated amine-functionalized MOF-based photoelectrocatalysts and used them in electrocatalytic applications.
References
Panwar NL, Kaushik SC, Kothari S (2011) Role of renewable energy sources in environmental proection: a review. Renew Sust Energ Rev 15:1513–1524
Zhang L, Xiao J, Wang H, Shao M (2017) Carbon-based electrocatalysts for hydrogen and oxygen evolution reactions. ACS Catal 7:7855–7865
Zhao X, Pattengale B, Fan D, Zou Z, Du J, Huang J, Xu C (2018) Mixed-node metal-organic framework as efficient electrocatalysts for oxygen evolution reaction. ACS Energy Lett 3:2520–2526
Zhao LL, Cao Q, Wang AL, Duan JZ, Zhou WJ, Sang YH, Liu H (2018) Iron oxide embedded titania nanowires-an active and stable electrocatalyst for oxygen evolution in acidic media. Nano Energy 45:118–126
Wang L, Wu YZ, Cao R, Ren LT, Chen MX, Feng X, Zhou JW, Wang B (2016) Fe/Ni metal-organic frameworks and their binder-free thin films for efficient oxygen evolution with low overpotential. ACS Appl Mater Interfaces 8:16736–16743
Lu Z, Wang H, Kong D, Yan K, Hsu PC, Zheng G, Yao H, Liang Z, Sun X, Cui Y (2014) Electrochemical tuning of layered lithium transition metal oxides for improvement of oxygen evolution reaction. Nat Commun 5:4345
Sun Y, Gao S, Lei F, Liu J, Liang L, Xie Y (2014) Atomically-thin non-layered cobalt oxide porous sheets for highly efficient oxygen-evolving electrocatalysts. Chem Sci 5:3976–3982
Gao M, Sheng W, Zhuang Z, Fang Q, Gu S, Jiang J, Yan Y (2014) Efficient water oxidation using nanostructured α-nickel-hydroxide as an electrocatalyst. J Am Chem Soc 136:7077–7084
Jung JI, Jeong HY, Lee JS, Kim MG, Cho J (2014) A bifunctional perovskite catalyst for oxygen reduction and evolution. Angew Chem Int Ed 53:4582–4586
Delgado D, Minakshi M, Senanayake G, Kim D (2015) Modified electrolytic manganese dioxide (MEMD) for oxygen generation in alkaline medium. J Solid State Electrochem 19:1133–1142
Rosi NL, Eckert J, Eddaoudi M (2003) Hydrogen storage in microporous metal-organic frameworks. Science 300:1127–1129
Zhang WX, Yang YY, Zai SB (2008) Syntheses, structures and magnetic properties of dinuclear copper (II)-lanthanide (III) complexes bridged by 2-hydroxymethyl-1-methylimidazole. Eur J Inorg Chem 2008:679–685
Moore EG, Samuel APS, Raymond KN (2009) From antenna to assay: lessons learned in lanthanide luminescence. Acc Chem Res 42:542–552
Jahan M, Liu Z, Loh KP (2013) A Graphene oxide and copper-centered metal organicframework composite as a tri-functional catalyst for HER, OER, and ORR. Adv Funct Mater 23:5363–5372
Xiaohua Z, Brian P, Donghua F, Zehua Z, Yongqing Z, Jing D, Jier H, Cailing X (2018) Mixed-node metal-organic frameworks as efficient electrocatalysts for oxygen evolution reaction. ACS Energy Lett 3:2520–2526
Xing J, Guo K, Zou Z, Cai M, Du J, Xu C (2018) In situ growth of well-ordered NiFe-MOF-74 on Ni foam by Fe2+ induction as an efficient and stable electrocatalyst for water oxidation. Chem Commun 54:7046–7049
Dandan Z, Renxing H, Huaming X, Ruizhen L, Xingyong L, Ming P, Ying L (2020) Effect of the valence state of initial iron source on oxygen evolution activity of Fe-doped Ni-MOF. Chemical Paper
Qingqing Z, Feifei Y, Guoxu Q, Yonghong N (2020) Cobalt-based MOF-on-MOF two-diemensional heterojunction nanostructures for enhanced oxygen evolution reaction electrocatalytic activity. Inorg Chem 59:1295–1305
Jiang J, Huang L, Liu MX, Ai HL (2017) Bioinspired cobalt-citrate metal-organic framework as an efficient electrocatalyst for water oxidation. ACS Appl Mater Interfaces 9:7193–7201
Meng GQ, Yang JJ, Ma XS, Zhai JM, Lu TJ (2017) A porous cobalt (II) metal-organic framework with highly efficient electrocatalytic activity oxygen evolution reaction. Polymers 9:676
Noor T, Zaman N, Nasir H, Iqbal N, Hussain Z (2019) Electro catalytic study of NiO-MOF/rGO composite for methanol oxidation reduction. Electrochim Acta 307:1–12
Noor T, Pervaiz S, Iqbal N, Nasir H, Zaman N, Sharif M, Pervaiz E (2020) Nanocomposites of NiO/CuO based MOF rGO: an efficient and robust electrocatalyst for methanol oxidation in DMFC. Nanomaterials 10:1601
Yaqoob L, Noor T, Iqbal N, Nasir H, Zaman N (2019) Development of nickel-BTC-MOF-derived nanocomposite with rGO towards electrocatalytic oxidation of methanol and its product analysis. Catalysis 9:856
Yaqoob L, Noor T, Iqabal N, Nasir H, Sohail M, Zaman N, Usma M (2020) Nanocomposite of cobalt benzene tricarboxylic acid MOF with rGO: an efficient and robust electrocatalyst for oxygen evolution reaction (OER). Renew Energy 156:1040–1054
Wahab A, Iqbal N, Noor T, Ashraf S, Raza MA, Ahmad A, Khan UA (2020) Thermally reduced mesoporous manganese MOF@reduced graphene oxide nanocomposite as bifunctional electrocatalyst for oxygen reduction and evolution. RSC Adv 10:27728–27742
Haider MD, Iqbal N, Rizvi SAM, Noor T, Hanif S, Anwar R (2021) ZIF-67 derived Cu-doped electrocatalyst for oxygen reduction reaction. J Electrochem Energy Conv Stor 18:21001
Sarwar E, Noor T, Iqbal N, Mehmood Y, Ahmed S, Mehek R (2018) Effect of Co-Ni ratio in graphene based electro-catalyst for methanol oxidation. Fuel Cells 18:189–194
Hanif S, Shi X, Iqbal N, Noor T, Anwar R, Kannan AM (2019) ZIFderived PtNiCo/NC cathode catalyst for proton exchange membrane fuel cell. Appl Catal B Environ 258:117947
Noor T, Ammad M, Zaman N, Iqbal N, Yaqoob L, Nasir H (2019) A highly efficient and stable copper BTC metal organic framework derived electrocatalyst for oxidation of methanol in DMFC application. Catal Lett 149:3312–3327
Hanif S, Iqbal N, Shi X, Noor T, Ali G, Kannan AM (2020) NiCo-N-doped carbon nanotubes based cathode catalyst for alkaline membrane fuel cell. Renew Energy 154:508–516
Horiuchi Y, Toyao T, Saito M, Mochizuki K, Iwata M, Higashimura H, Anpo M, Matsuoka M (2012) Visible light promoted photocatalytic hydrogen production by using an amino-functionalized Ti (IV) metal-organic framework. J Phys Chem C 116:20848–20853
Fang S, Lichen B, Aliki M, Seunghwa L, Chao H, Laurent L, Xile H (2018) Transition metal oxides as electrocatalysts for the oxygen evolution reaction in alkaline solutions: an application-inspired renaissance. J Am Chem Soc 140:7748–7759
Yao Y, Yang Z, Sun H, Wang S (2012) Hydrothermal synthesis of Co3O4-graphene for heterogeneous activation of peroxymonosulfate for decomposition of phenol. Ind Eng Chem Res 51:14958–14965
Rahdar A, Aliahmad M, Azizi Y (2015) NiO nanoparticles: synthesis and characterization. J Nanostruct 5:145–151
Phiwadang K, Suphankij S, Mekprasart W, Pecharapa W (2013) Synthesis of CuO nanoparticles by precipitation method using different precursors. Energy Procedia 34:740–745
Adam RE, Pozina G, Willander M, Nur O (2018) Synthesis of ZnO nanoparticles by co-precipitation method for solar driven photodegradation of Congo red dye at different pH. Photonics Nanostruct Fundam Appl 32:11–18
Bordiga S, Lamberti C, Ricchiardi G, Regli L, Bonino F, Damin A, Lillerud KP, Bjorgen M, Zecchina A (2004) Electronic and vibrational properties of a MOF-5 metal-organic framework: ZnO quantum dot behavior. ChemCommun 20:2300–2301
Hu YH, Zhang L (2010) Amorphization of metal organic framework MOF-5 at unusually low applied pressure. Phys Rev B 81:174103–174107
Hongyan X, Zhenyin H, Jiangtao D, Qiang Z, Libo G, Danfeng C, Junbin Z, Jun L, Chenyang X (2015) Synthesis and microwave absorption propertied of core shell structured Co3O4-PANI nanocomposite. J Nanomater 2015. https://doi.org/10.1155/2015/845983
Vinod V, Thekkae P, Miroslav C (2013) Green synthesis of copper oxide nanoparticles using gum karaya as a biotemplate and their antibacterial application. Int J Nanomedicine 8:889–898
Kalpana H, Sanjay B, Amit H, Prakash C, Kakasaheb M, Jalinder A, Nishigandh P, Vasant C (2014) Novel green route of synthesis of ZnO nanoparticles by using natural biodegradable polymer and its application as a catalyst for oxidation of aldehydes. J Macromol Sci A Pure Appl Chem 51:941–947
Acknowledgments
The authors acknowledge Prof. Duncan H. Gregory, School of Chemistry, University of Glasgow, UK, for providing the lab facilities.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOCX 2093 kb)
Rights and permissions
About this article
Cite this article
Fiaz, M., Hussain, D. & Athar, M. Synthesis of transition metal oxide incorporated MOF-5 and NH2-MOF-5 as efficient photoanode for oxygen evolution reaction. Ionics 27, 759–770 (2021). https://doi.org/10.1007/s11581-020-03866-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-020-03866-1