Abstract
A simple comparative study on the effect of doping barium borosilicate glasses by two different concentrations of TiO2 is studied. Changes occurred in some of their physical properties due to subjecting to gamma radiation or heat treatment processes are also discussed. The measured properties are Density, Optical UV absorbance and transmittance, infrared absorption spectroscopy and scanning electron microscope. Ti ions can participate in the glassy network as effective modifier ions in the form of TiO6 and TiO4 octahedral groups. Titanium ions can exist in two valence states; the trivalent Ti3+and tetravalent Ti4+ ions giving UV bands at 340 nm on the UV spectrum and at 400–480 nm after gamma irradiation. The main constituting groups BO3 and BO4 units give IR bands at 1250–1500 and 800–1200 cm−1, respectively. In Ti-doped glasses, vibrational bands of SiO4, TiO4 or (Si–O–Ti) vibrations are detected at 600–800 cm−1. Heat treatment process at 525 °C for 6 h gives more ordered glassy structures with good properties; higher density, lower UV absorbance and higher transmittance, on the contrary, the effect of radiation that weakens the glassy structure and creates more non-bridging oxygens NBOs.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Boron oxide B2O3 is an excellent glass network forming oxide not only because of easily preparation method of borate glasses but also because it is a good host for many modifying oxides such as transition metals (TM). Boron is able to modify its coordination number with oxygens to be three or four so it can form two main super structural units of borate glasses; the planar triangle BO3 and the tetrahedral BO4 units. These units may be present in many structural groups such as diborate, tetraborate, pentaborate or broxol ring…etc based on the percent of bridging or non-bridging oxygens in the glassy network [1]. This gives borate glasses an advantage over silicate and phosphate glasses, which can form only tetrahedral coordinated units with oxygens.
However, borate glass has very low durability even in the ordinary atmosphere; water vapor can cause it to be corroded. So borosilicate glass can solve this problem, while the introduction of an alkali oxide into borate glasses such as BaO enhanced its durability by modifying B2O3 from the triangular borons to tetrahedral coordination BO4 [1].
On the other hand doping glass with transition metal (TM) such as titanium oxide acquires many wide industrial applications, where it has special electrical and optical properties. Titanium ions can participate in the glassy network in two oxidation states Ti3+ and /or Ti4+ ions with the electronic configurations 3d1 and 3d0 respectively. The ratio of the two valence states depends mainly on the glass composition and glass preparation conditions [2].
Optical and spectroscopic studies of 3d TM doped barium borate and strontium borate glasses show some shielding effects to gamma radiation as it was reported by Marzouk et al. [3]. Barium titanate BaTiO3 glasses have [4] great interesting optical, electrical and magnetic properties that are varied according to their preparation conditions and modification processes. BaTiO3 glasses have wide applications e.g. underwater transducers, sensors, heaters, high capacity reversible electrodes [5] inhibiting bacterial growth [6] optical glasses, textile or continuous filament fiberglass [7] and recently as bioactive materials [8, 9].
As a trial for having better physical and chemical properties of glasses, heat treatment process is an effectual route in order to give more ordered glassy systems or in sometimes crystalline glasses with improved properties. For instance, the formation of transparent heat-treated glasses (HTG) or glass ceramics from BaTiO3 is with great interest due to their characteristic physical properties compared with their parent glasses. Some of these properties are high charge storage capacity, ferroelectric behavior, good insulating property, high dielectric constant, and relatively low phonon energy [10].
The main target of this work is to study some physical properties of a certain composition of barium borosilicate glass before and after doping it with titanium ions. Then study the effect of gamma radiation or heat treatment process on the studied properties.
2 Experimental Procedure
Glass samples were prepared using the melting annealing technique, with the compositions listed in Table 1. The used ingredients were chemically pure H3BO3, SiO2, BaCO3 and TiO2. The three batches were accurately weighed by using an electronic balance, mixed thoroughly and ground to a fine powder, and then they were melted in porcelain crucibles in an electric furnace at 1100–1200 °C for 2 h with rotating melts for homogeneity. After casting melts were immediately transferred to a muffle furnace regulated at 550 °C for annealing. After 1 h, the muffle was switched off to decrease its temperature to the room temperature with a rate of 25 °C/h.
Differential thermal analysis (DTA) of glass samples is investigated by a Shimadzu–50 DTA using 10 mg. powder heated from 30 to 600 °C at a rate 10 °C/min. DTA was carried out to determine the glass transition temperature (Tg) and crystallization peak temperature (TC). Al2O3 was taken as a reference material.
Heat treatment of glasses was made in a muffle furnace regulated at 525 °C for 6 h then it was switched off to decrease to the room temperature with a rate of 25 °C/h. Selection of heating temperature depends on the glass transition and crystallization temperatures detected from the DTA measurement.
X-ray Diffraction measurements (XRD) were made by using a Shimadzu XD-DI diffractometer where the X-ray was operated at 40 kV and 30 mA through the measurements. XRD was measured on glasses before and after heat treatment process.
Density of glasses was measured at room temperature, using the suspended weight method based on Archimedes principle. Xylene was used as immersion liquid. All measurements were made three times with maximum error 0.0002 g/cm3.
Optical UV absorbance and transmission spectra of the three highly polished samples G1, G2 and G3 with the dimensions 1 × 4 × 0.2 cm3 were recorded at room temperature before and after successive gamma irradiation in the dose range from 5 to 50 kGy. The optical measuring was carried out by using a recording spectrophotometer in the range 200–1100 nm type JASCO, Corp., V-570, Rel-00, Japan.
Infrared absorption spectra (FTIR) of glass samples were measured at room temperature at the wave number range 400–4000 cm−1 using spectrometer type VERTEX 70, FT/IR-430, Japan. Glass samples were measured before and after being subjected to 5 kGy of gamma radiation doses as well as after being heat treated at 525 °C for 6 h.
Scanning electron microscope SEM of type (Jeol-JSM 5400) was utilized to study the surface morphology of glass specimen. The measuring was carried out at high magnifications by means of high energetic electrons scan the specimen surface and a high resolution image of screen on T.V monitor shows the surface shape. The surface morphology of a selected glass sample G2 before and after gamma radiation and heat treatment process is clarified.
Gamma irradiation process takes place by using Co60 gamma cell (2000 Ci) as a gamma ray source with a dose rate of 1.5 Gy/s at 30 °C. The glass samples were placed in the gamma cell in the manner that each sample was subjected to the same gamma dose.
3 Results and Discussion
3.1 Differential Thermal Analysis DTA
Figure 1 investigates the DTA curves of G2 and G3 glass samples. It shows the thermal behavior of the prepared barium borosilicate glasses doped titanium oxide. The curves show the glass transition temperatures of both glasses at about (Tg = 262 °C). While the onset of crystallization appears at about Tc = 562 °C for G2 and 436 °C for G3. And Crystallization temperature range and melting temperature range (Tf) of G2 and G3 is observed at 762 and 786 °C respectively. It can be resolved from the curve that G2 with the lower concentration of titanium ions (5 wt%) has higher thermal stability than G3 with 6.5 wt% concentration of titanium ions.
3.2 X-ray Diffraction (XRD)
Figure 2 shows the amorphous structure natures of the prepared glasses where no sharp peaks are detected. Although, TiO2 is one of the most efficient nucleating agents in the preparation of glass ceramics, it shows the absence of formation of crystalline phases after this heat treatment process. Many glass researchers [11,12,13,14] have investigated that TiO2 doped glasses with a concentration range from 5 to 13.6% acts as a nucleating agent producing some variations in the constituting structural glass units rather than crystallization [3]. Also, it can be assumed that the perfect conditions required for the crystallization of this certain composition is not that selected, either the required temperature or heating duration. The glass after this process is so called as heat treated glass (HTG).
3.3 Density
Studying density of glasses is an important physical parameter for helping in the exploring of tightness and structural changes of the material [1]. Usually, Density of glass is defined as a match between masses and volumes of their internal structural groups. So, density is directly related to the chemical composition of the glass and the way in which its atomic groups are connected [15]. Therefore addition of an alkali or modifier oxide should change the density values of glass depending on the concentration of the introduced ions and the route of their interconnection with the other glass constituents as well as the composition of the host glass network.
Since our glass compositions contain large Ba2+ ions which can break the network structure causing the formation of more NBOs, more holes, and vacancies. Then the observed increase in density of glass containing TiO2 as shown in Table 2 can be referred to the participation of Ti ions in the glass matrix in the form of TiO4 and TiO6 that also can charge balance the percent of NBOs, originating more compact and denser structures [16, 17].
However, this behavior is limited because, at higher concentration of Ti ions (6.5%), a decrease in the glass density is observed rather than the glass with a lower concentration (5%), although it is still higher than the base glass as shown in Table 2. This can be deduced to the incapability of the defects already present in the base glass to accommodate such high Ti ion concentration. Also, there will be a chance given to the conversion of BO3 to [BO4]− units. Thus a glass framework with a large volume is formed showing a slight decrease in density values [18]. Consequently, G3 with higher Ti ion concentration gives slightly lower density values than G2 with the lower Ti ions concentration.
3.3.1 Effect of Irradiation on the Density of Glasses
As shown in Table 2 subjecting glass samples to gamma radiation with a dose of 5 kGy leads to a slight decrease in their density values. This can be due to the effect of ionizing radiation on glasses, where it leads to atomic movements, electronic defects and/or breaking in the network bonds. Therefore the creation of more NBOs and conversion of BO3 into [BO4] − groups may take place giving more amorphous open structures and the net result would be a lowering of density values [19, 20].
3.3.2 Effect of Heat Treatment on the Density of Glasses
It can be noticed that heat-treated glasses have higher density values than the untreated as shown in Table 2. This may be due to the rearrangement of ions in the network during the heating process, where the temperature was increased to be between the glass transition and crystallization temperatures. This rearrangement or reordering may be due to the formation of nuclei or microcrystalline phases in the glass structure. Consequently, a more blocked or compact structure can be formed and the reduction of the free volume takes place in the network structure [21]. So the net result would be a slight increase in density values after the heat treatment process.
3.4 Optical UV Spectra (Absorbance and Transmittance)
Properties of glasses are directly related to the interatomic forces and potentials in the glass lattice so changes in the lattice due to doping of an element or irradiation can be detected by optical UV spectroscopy which gives information concerning to radiation-induced defect centers [22]. For instance, doping with a transition metal can deal with intrinsic defects in the glassy matrix by trapping the radiolytic electrons or holes, so they are called as potential traps. As well as they can affect the formation rates and intrinsic color centers recovery rates [23].
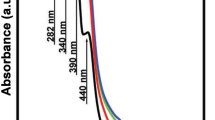
According to results shown in Fig. 3, the optical absorption spectra of barium borosilicate base glass (G1) showed a sharp peak at 240 nm and a knick at 320 nm. However, introducing of TiO2 with either 5 or 6.5% in G2 and G3, respectively leads to increasing the intensity of the peaks to be more obvious and sharp at 240 and 340 nm. Many authors have reported that the observed peak at about 240 nm is related to the presence of traces of iron impurities Fe3+, which cannot be avoided during glass preparation [3, 24]. It was recommended that the preparation of optical glasses requires the need for ultra-pure materials that are free from trace impurities specifically ferric ions even in the ppm level because it could weaken the usefulness of recent optical glasses [25, 26].
Titanium ions can exist in two valence states; the trivalent Ti3+and tetravalent Ti4+states [1, 2, 17]. Ti3+ has the electronic configuration of 3d1 and it can give visible bands at 400–480 nm in phosphate and borosilicate glasses. On the other hand, Ti4+ ions have the electronic configuration of 3d0 so it does not exist UV visible bands but show only a UV band at about 340 nm. Therefore it can be expected that most of Ti ions are present in their tetravalent states due to the observed band at 340 nm as shown in Fig. 3 [27].
3.4.1 Interpretation of Effect of Radiation on the Optical UV Spectra
It is obviously observed from Fig. 4 the increase in intensity of peaks at 240 and 340 nm as well as the appearance of bands at 400–440 nm in Ti containing glasses—especially at the high irradiation doses—that may be assigned to the charge transfer transition between Ti3+ and Ti4+ ions as it was reported by [28]. Another band observed from the transmission spectra shown in Fig. 5 at about 570 nm may be assumed the tetragonal distortion of the octahedral legands around trivalent Ti3+titanium ions [29, 30].
It is well known that the change occurred in glass due to irradiation resulting from the formation of pairs of negative electron centers and positive hole centers. The formation of these defects can cause many changes in the structure of the glass lattice such as the creation of more NBOs then the conversion of triangular BO3 to the tetrahedral [BO4]− units. This can be one of the reasons of the observed increase in absorbance intensity as shown in Fig. 4 or decrease in transmittance intensity as shown in Fig. 5 because of the formation of color centers in the host glass after irradiation [31,32,33,34]. Furthermore, the presence of transition metal ions either traces of Fe2O3 or TiO2 can inhibit the formation of hole centers by trapping the excited electrons induced due to irradiation by photo-oxidation of lower valence ions [34]. So the intensity of the bands at 240 and 340–360 nm is increased after irradiation due to the oxidation of some Fe2+ ions that present in the network to Fe3+, or Ti3+ to Ti4+ ions [35, 36]. On the other hand, Fe3+ ions can be reduced to Fe2+ and Ti4+ to Ti3+ according to Moncke and Ehrt [25, 26]. Such oxidation–reduction variations cause the observed changes in the UV spectra of the investigated glasses.
The additional peaks observed in the optical transmittance in the range 500–600 nm shown in Fig. 5, may be related to the formation of more non-bridging hole centers (NBOHs) or the formation of boron hole centers [2].
3.4.2 Effect of Heat Treatment on Optical Spectra of the Prepared Glasses
Figures 6 and 7 show the optical absorption and transmission of the investigated glass samples before and after heat treatment process. It can be noticed that the absorbance intensity of the bands is decreased for the heat-treated glasses as shown in Fig. 6; however, their transmittance is increased according to Fig. 7. Since heat treatment gives glass structure the chance for rearrangement and the ability of healing some defects found in its network to get rid of free H2O molecules or OH groups [37], so the glass transmittance can be enhanced and the absorption decreased due to the more compact structure caused by the better ordering of the network. The last result can be also provided by the density results shown in Table 2.
3.5 Infrared Absorption Measurements
The FTIR spectra of the glasses is studied in the range 4000–400 cm−1 and the active vibrational IR bands assigned to [BOn, n = 3 and/or 4], [BaOn, n = 2 and 4] [TiOn n = 2, 4 and 6] and [SiO4] structural units, are located in the mid-infrared region, i.e. in the spectral range 1600–400 cm−1. It is observed that glasses show the prominent absorption bands in the following three regions.
The FTIR absorption spectrum of the unirradiated Ba-borosilicate glass holding different TiO2 concentrations is illustrated in Fig. 8. The figure shows obvious peaks at 660, 830 and 1030 cm−1 which become broader with a remarkable decrease in their intensities after the addition of TiO2 as shown for G2 and G3 glasses.
Figure 9 shows the FTIR spectral features of the glasses after successive gamma irradiation with 5 kGy where all glasses gives higher intensities values than those before irradiation however they maintained relatively in their spectral positions. The main obvious peaks are centered at about 540, 680, 830, 1380, 1600 cm−1 and small broad bands at about 1750, 2000–2100, 2338, 2920 and 3700–3800 cm−1.
Figure 10 shows IR spectral features of the prepared glasses after heat treatment process where it shows different behaviors than those before and after irradiation. It is obvious that Ti-doped HTGs have lower IR spectral intensities than the undoped HTG. Some variations are observed in Fig. 10; small sharp peaks at 880–1060 cm−1 appear broader in Ti-doped HTGs and the appearance of strong medium peaks at 1200 and 1380 cm−1.
3.5.1 The IR Spectra of Unirradiated Glasses
The IR spectra of unirradiated glasses shown in Fig. 8 reveal two weak absorption bands; at 411 cm−1 that can be ascribed to the silicate bending rock mode and a small peak at 540 cm−1 that can be considered to be superposed with the borate deformation modes, such as the in-plane bending of boron-oxygen triangles and normal vibration of Ti–O bond in the deformed TiO6 octahedral units [38]. A sharp and distinct band at 660–680 cm−1 is due to bending vibrations of Si–O–B bridges [39]. Another band at around 830 cm−1 can be related to the symmetrical stretching vibration of the Ti–O bonds in the [TiO3] groups [40]. The bands observed around 1000 cm−1 are due to the combined stretching vibrations of Si–O–Si and B–O–B network of tetrahedral structural units. The region between 1000 and 1500 cm−1 shows prominent absorption bands at 1030, 1240 and 1380 cm−1. The band at around 1030 cm−1 may be assigned to the stretching vibrations of B–O in BO3 units from meta and orthoborate groups [41, 42]. The small absorption band at 1240 cm−1 is specific to the B–O stretching vibrations of BO3 triangular units with non-bridging oxygen (NBO) atoms. The peak at around 1380 cm−1 is assigned to anti-symmetrical stretching vibrations with three NBOs of the B–O–B groups [43].
All IR spectral features of the investigated glasses are tabulated in Table 3.
The IR spectral features of the unirradiated glasses can be interpreted in the following points;
-
(1)
To define the vibrational modes for the main building units in titania [TiO4 units] and identify the role and vibrational modes of Ti–O linkages as glass former or modifier, new researchers studied FTIR spectra of the pure TiO2 and they concluded that (1) glasses containing high TiO2 shows that titanium ions give absorption of tetravalent (Ti4+) ions with no distinct indication of Ti3+ ions. (2) The tetravalent titanium ions reveal vibrations in the wave number range 460–850 cm−1 very near and interfering with SiO4 groups vibrations. (3) The complex IR bands are almost due to both SiO4 and TiO4 or (Si–O–Ti) vibrations [27].
-
(2)
The bands observed in region 830–1200 may be related to the B–O bond stretching of the tetrahedra BO4 units that are connected by the titanium cations. Therefore the existence of [BO4]− units indicates that the addition of TiO2 into the glass causes a progressive conversion of BO3 to [BO4] units [44]. In other words, the symmetrical stretching vibrations of the Ti–O bond in the TiO3 polyhedral and the vibrations of the various arrangements are containing BO4 units [45].
-
(3)
TiO2 in Ba-borosilicate glasses can share in the structure by different manners; it creates a four-coordinated state by giving part of its oxygen to the boron, secondly, it participates in the glass structure by forming TiO3 groups and thirdly, it introduces some NBOs in the glass network [46]. The addition of TiO2 contents either 5 or 6.5 wt% causes some limited variations. Such as the increase in the intensity of the band at 660 cm−1 that may be related to the determination of extra vibrational bands due to Ti–O–Ti or Ti–O–B [2].
-
(4)
The presence of Ti ions favored the presence of B2O3 oxide in the form of tetraborate groups at 800–1200 cm−1 rather than triangular borate groups at 1250–1650 cm−1 according to Kamitos [47]. It is obviously shown from the IR spectral curves in Fig. 8 that the relative intensities of the absorption bands of the triangular borate groups at (1250–1650) cm−1 are lower and broader in Ti containing glasses (G2 and G3) than Ti free glass (G1). This behavior can be assumed to the transformation of the boron coordination from three to four terminates after the certain compositional range. This behavior can explain increasing the density of Ti-containing glasses rather than the base glass because of the conversion to the more attached tetrahedral BO4 units.
-
(5)
El-Damrawi et al. [48] show that increasing behavior of relative area (around 800 cm−1) may specify an increase of non-bridging oxygen atoms upon addition of a transition metal in silicate glass. They reported that the higher area refers to the higher concentration of modifier cations in the silicate network. This behavior may explain increasing the relative area around 800 cm−1 after increasing the percent of introduced titanium ions from 5 to 6.5% as shown in Fig. 8. So it is expected that most of the titanium ions participate in the borosilicate network as an effective modifier. Consequently, it can be also assumed why the density of the glass enhanced by the addition of 5% TiO2 rather than 6.5% TiO2 as shown in Table 2. Since the further addition of TiO2 caused a decrease in density of the glass, which may be due to the introducing of some NBOs as the amount of Ti ions becomes more than the required for healing the defects in the network, therefore, a decrease in the network connectivity and a decrease in glass density takes place.
-
(6)
Increasing the number of NBOs due to the presence of the divalent cations in the glass e.g. BaO results in a disruption of the continuity of the silicate glassy network due to the creation of local defects leading to the formation of non-bridging silicon-oxygen bonds (Si–O–NBO). Jincheng and Cormack [49] concluded that such a decrease in network connectivity causes the changes in ring size distribution. Hence the percentage of NBO in the first coordination shell of introduced alkali ions increases with increasing alkali contents, but the smaller size of the alkali ions also means higher field strength, which requires the coordination of NBOs to screen their charges. The higher field strength of alkali ions is required to increases the ability to capture oxygen ions to create free oxygen ions [50]. The mechanism of the entrance of modifier through glass matrix is suggested by Sharma et al. [51].
$${\text{2}}{{\text{e}}^{-}}{+^+}{\text{O}} - {\text{Si}} \equiv {\text{ }}{ \to ^ - }{\text{O }} - {\text{ Si }}\left( {{\text{NBO}}} \right)$$(2)$${\text{2}}{{\text{e}}^{-}}{+^+}{\text{O }} - {\text{ Si }} \equiv {\text{ }} \to {\text{ O }}-{\text{ Si}} \equiv \left( {{\text{neutral NBO}}} \right)$$(3) -
(7)
The rest of the bands extending from 2400 to 4000 cm−1 are related to the absorption bands due to B–OH, Si–OH, OH and water vibrations as it was attributed by El Batal et al. [22].
3.5.2 Effect of Gamma Radiation on IR Spectra of the Prepared Glasses
Figure 9 shows higher IR absorbance for the irradiated glasses than those before irradiation which may be due to the occurrence of some possible changes in bond angles and/or bond lengths of the building groups due to radiation. There are some mechanisms can discuss the variation of IR spectral features after gamma radiation;
-
(1)
Hobbs et al. [52], described the changes in the radiation-induced structure by assuming that generation of defects breaks the connectivity of the network because of the tendency to more randomness or amorphicity of the structure. As well as Primak [53] explained early that radiation causes compaction in the glass by the decrease of the bond angle of the building units such as Si–O–Si, B–O–B or Si–O–B. This may explain the higher intensities of IR vibrational bands after irradiation shown in Fig. 9.
-
(2)
Piao et al. [54] defined the mechanism of radiation-induced defects by supposing that during irradiation; the ionization process creates electron–hole pairs, and providing paths for bond rearrangements, decreasing the constraints and structural relaxation. The relaxation process reliefs some of the excess energy stored in the structure accompanied by a decrease in the average bridging bond angle. Because of the absence of ordered structure in the glass, the relaxation involves long-range effects.
The last assumptions can discuss the slight lowering in density values of the irradiated glasses comparing with the unirradiated ones as shown in Table 2. Because of the creation of defects that reduce the connectivity of the network forming more disordered structures.
3.5.3 Effect of Heat Treatment on IR Spectra of the Prepared Glasses
It has been shown by some authors [55, 56] that infrared spectroscopy can be used to interpret structural modifications in glass exerted by thermal or mechanical treatment. Previously, Bell et al. [57] showed in their structural models that the positions of the Si–O stretching band depend primarily on the average of a Si–O–Si bond angle in the glass structure. The Si–O band was found to shift to higher frequencies with the increase in the average of Si–O–Si [58].
Figure 10 shows the effect of heat treatment on IR spectral features where the small bands at 2000–2500 cm−1 that may be correlated to silanol group are detected more obviously. The Si–O–Si bond is broken during the heat treatment process allowing the formation of silanol group (SiOH) at about 2200 cm−1. Doremus [59] discussed the mechanism of interaction of H2O with silicate glasses, and attributed the high wavenumber bands as a result of the distribution of oxygen as molecule by S jumping within the glass network vacancies however other molecules can transport oxygen in silicate glasses forming Si–OH as a result of the following reaction:
By comparing IR spectra of glasses before and after heat treatment process as shown in Fig. 8 and inset of Fig. 10 respectively, it is observed that the intensities of peaks at 830, 980, 1380 and 1600 cm−1 are increased after heat treatment process. This increase in the intensity may be due to either water evaporation or some residual gasses release due to the heat treatment process. Figure 10 shows also some changes in the intensity of peaks at higher wavenumber range (1600–4000 cm−1) like the peaks at 1730, 2000, 2200, 2950 and 3700–3800 cm−1, these changes may be related to the formation of water vapor after the fracture of the –OH bond [37, 60].
From Fig. 10 it is obviously observed in Ti free glass (G1), that the intensities of vibrations of the triangular BO3 units at (1250–1650) cm−1 is higher than the intensities of vibrations of the tetrahedral borate BO4 units at (800–1200) cm−1 because of the change in the number of bridging and non-bridging oxygen atoms due to the heat treatment process. However, the relative intensities of tetrahedral borate groups are slightly higher or nearly equal to the triangular units in Ti-doped glasses (G2 and G3). This behavior may be attributed to the introducing of the nucleating agent TiO2 which promotes the heat treatment process and allows the more ordered structure to be formed with lower NBOs. According to our results, the heat treatment process enhanced some glass properties by giving more connected and ordered network structures with higher density values.
3.6 Scanning Electron Microscope SEM
Figure 11 depicts SEM images of the glass surface of G2 before and after both gamma radiation with 5 kGy and heat treatment at 525 °C for 6 h. The images show that after irradiation the surface becomes rough with non-uniformities while the HTG2 image shows more uniform and pure surface. The SEM images can interpret the effect of radiation and heat treatment where the first produce more NBOs because of the atomic displacement producing a slightly rough surface. On the other hand, heat treatment process gives arranged or ordered structures with more uniform surface morphology as shown in Fig. 11.
4 Conclusion
Ti ions can participate in barium borosilicate glasses in the form of TiO6 and TiO4 octahedral groups. Introducing of such transition metal leads to some improvements of glasses with limitations. When its concentration is 5 wt% an increase in density takes place because of the modification in the glassy network caused by introducing such TM. However, when it reaches 6.5%, more randomness with higher NBO are formed a giving large framework to house theses large ions thus a decrease in density values takes place. Irradiation causes a decrease in density values due to the atomic displacement and defects caused by radiation leads to more NBO and more open structure while heat treatment process give more arranged glassy systems with more blocked and denser glasses. Titanium ions can exist in two valence states; the trivalent Ti3+and tetravalent Ti4+states with the electronic configurations 3d1 and 3d0 respectively. Ti3+ can give visible bands at 400–480 nm while Ti4+ does not exit UV, visible bands. Optical UV spectra show that observed peaks at 240 nm are correlated to the unavoidable trace iron impurities Fe3+.While the extra observed bands at 340 nm observed in Ti-doped glasses are due to the absorption of tetravalent titanium ions Ti4+. Gamma irradiation shows an increase in UV absorbance intensities and a decrease in transmittance because of the formation of induced defect centers or color centers. The intensity of the formed induced bands at 240 and 340 nm after irradiation are increased due to the photo-oxidation reaction of Fe2+ to Fe3+ions or Ti3+ to Ti4+. Radiation causes also the appearance of bands at 400–440 nm in Ti containing glasses due to the charge transfer transitions between Ti3+ and Ti4+. Heat treatment gives glass structure the chance for rearrangement and the ability to heal some defects like the existing of H2O molecules or OH groups leading to an increase in the glass transmittance.
FTIR spectral features show that BO3 and [BO4]− groups are the main constituting building units in borate glasses and their vibrating sites are 1250–1500 and 800–1200 cm−1, respectively. Introducing of titanium ions in borosilicate system gives complex IR bands that are due to both SiO4 and TiO4 or Si–O–Ti vibrations where TiO2 participates in borosilicate network as an effective modifier. The higher intensities of IR bands after irradiation are correlated to the creation of local defects leading to the formation of non-bridging silicon-oxygen bonds Si–O–NBO. Heat treatment process causes the formation of silanol group (SiOH) at about 2200 cm−1. As well as increasing the intensities of peaks 800–1600 cm−1 due to the water evaporation or releasing of some residual gasses.
In brief, by comparing some of the physical properties in undoped and Ti-doped barium borosilicate glasses before and after gamma irradiation and heat treatment process, we concluded that radiation causes defects and decreases slightly the desired properties of the studied glasses however the heat treatment process enhances and improves them.
References
B. Ashok, V. Kumar, R. Kistaiah, J. Non-Cryst. Solids 426, 47 (2015)
A.M. Abdelghany, H.A. El Batal, J. Non-Cryst. Solids 379, 214 (2013)
M.A. Marzouk, F.H. ElBatal, H.A. ElBatal, J. Opt. Mater. 57, 14 (2016)
R. Harizanova, M. Abrashev, I. Avramova, L. Vladislavova, Ch Bocker, G. Tsutsumanova, G. Avdeev, Ch. Rüssel, Solid State Sci. 52, 49 (2016)
M. Tutsumisago, A. Hayashi, In Proceedings of the 5th international conference on borate Glasses, Crystals & Melts, Glass Technology (2006) p. 6
D. Lippley, B.A. Melhus, M.R. Leonards, S.A. Feller, M.A. Affatigato, Eur. J. Glass Sci. Technol. A 47, 127 (2006)
K.L. Loewenstein, The Manufacturing Technology of Continuous Glass Fibers, Glass Science and Technology, 3rd edn (Elsevier, Amsterdam, 1993)
A.M. Abdelghany, H.A. ElBatal, F.M. EzzElDin, Ceram. Int. 38, 1105 (2012)
M.A. Ouis, A.M. Abdelghany, H.A. ElBatal, Process. Appl. Ceram. 6, 141 (2012)
E.A. Ferreira, F.C. Cassanjes, G. Poirier, Opt. Mater. 35, 1141 (2013)
T.I. Chuvaeva, O.S. Dymshits, V.I. Petrov, M.Y. Tsenter, A.V. Shashkin, A.A. Zhilin, V.V. Golubkov, J. Non-Cryst. Solids 282, 306 (2001)
T. Kasuga, Acta Biomater. 1, 55 (2005)
A.S. Monem, H.A. El Batal, E.M.A. Khalil, M.A. Azooz, Y.M. Hamdy, J. Mater. Sci. Mater. Med. 19, 1097 (2008)
C. Rajyasree, D.K. Rao, J. Mole. Struct. 1007, 168 (2012)
N.A. El-Alaily, T.D. Abd-Elaziz, L. Soliman, J. Silicon 5, 1 (2015)
G. Paramesh, K.B.R. Varma, Int. J. Appl. Glass. Sci. 4, 248 (2013)
G. Paramesh, K.B.R. Varma, J. Non-Cryst. Solids 380, 128 (2013)
H. Scholze, Glass Nature, Structure and Properties, (Springer, New York, 1991)
N.A. El-Alaily, J. Glass Technol. 44, 30 (2003)
F.M. Ezz Eldin, N.A. El- Alaily, H.A. El Batal, J. Radioanal. Nucl. Chem. 163, 267 (1992)
N.A. EL-Alaily, Glass Technol. 44, 30 (2003)
F.H. El Batal, A.A. El Kheshen, M.A. Azooz, S.M. Abo-Naf, J. Opt. Mater. 30, 881 (2008)
K. Arshak, O. Korostynska, J. Microelectron. Int. 19, 30 (2002)
E.M.A. Khalil, F.H. El-Batal, Y.M. Hamdy, H.M. Zidan, M.S. Aziz, A.M. Abdelghany, J. Silicon 2, 49 (2010)
D. Moncke, D. Ehrt, Opt. Mater. 25, 425 (2004)
D. Moncke, D. Ehrt, J. Non-Cryst. Solids 352, 2631 (2006)
F.H. ElBatal, M.A. Marzouk, H.A. ElBatal, J. Mol. Struct. 1121, 54 (2016)
L.E. Bausá, J.G. Solé, A. Durán, J.M.F. Navarro, J. Non-Cryst. Solids 127, 267 (1991)
B.V. Raghavaiah, C. Laxmikenth, N. Verraiah, Opt. Commun. 235, 341 (2004)
S. Baccaro, G. Sharma, D.P. Singh, Nucl. Instrum. Methods Phys. Res. B 266, 594 (2008)
F.H. El Batal, A.M. Abdelghany, R.I. Elwan, J. Mol. Struct. 1000, 103 (2011)
S.Y. El-Zaiat, M. Medhat, F. Mona, A. Marwa, J. Opt. Commun. 370, 176 (2016)
A.M. Abdelghany, H.A. ElBatal, F.M. Ezz-Eldin, J. Mol. Struct. 1147, 33 (2017)
D. Moncke, D. Ehrt, In Photoionization of Polyvalent Ions, ed. by H.P. Glick, D. Moncke, D. Ehrt (Nova Science Publishers Inc. 610, New York, 2007)
A. Bishay, J. Non-Cryst. Solids 3, 54 (1970)
E.J. Friebele, in Uhlamann DR and Kreidl NJ American Ceramic Society Optical Absorption of Glass (Westerville, OH, USA, 1991), p. 205
A.H. De Aza, X. Turrillas, M.A. Rodriguez, T. Duran, P. Pena, J. Eur. Ceram. Soc. 34, 1409 (2014)
R. Kaur, S. Singh, O.P. Pandey, J. Mol. Struct. 1049, 386 (2013)
K. El-Egili, Physica B 325, 340 (2003)
H.A. Saudi, A.G. Mostafa, N. Sheta, S.U. El Kameesy, H.A. Sallam, Physica B 406, 4001 (2011)
S.G. Motka, S.P. Yawale, S.S. Yawale, Bull. Mater. Sci. 25, 75 (2002)
E.I. Kamitsos, A.P. Patsis, M.A. Karakassides, G.D. Chryssikos, J. Non- Cryst. Solids 126, 52 (1990)
Y.B. Saddeek, E.R. Shaaban, E.I.S. Moustafa, H.M. Moustafa, Physica B 403, 2399 (2008)
I. Pal, A. Agarwal, S. Sanghi, Indian J. Pure Appl. Phys. 50, 237 (2012)
G. Chryssikos, L. Liu, C. Varsamis, E. Kamitsos, J. Non-Cryst. Solids 235, 761 (1998)
F.H. ElBatal, M.A. Marzouk, A.M. Abdelghany, J. Mater. Sci. 46, 5140 (2011)
E.I. Kamitsos, G.D. Chryssikos, Solid State Ionics 105, 75 (1998)
G. El-Damrawi, A. Hassan, R. Ramadan, S. El-Jadal, New J. Glass Ceram. 6, 47 (2016)
D. Jincheng, A.N. Cormack, J. Non-Cryst. Solids 351, 2263 (2005)
J. Du, L.R. Corrales, J. Non-Cryst. Solids 352, 3255 (2006)
A. Sharma, H. Jain, A.C. Miller, Surf. Interface Anal. 31, 369 (2001)
L.W. Hobbs, A.N. Streeram, C.E. Jesurum, B.A. Berger, Nucl. Instrum. Methods Phys. Res. B 116, 18 (1996)
W. Primak, Appl. Phys. J. Phys. Chem. Glass 43, 2745 (1972)
F.W. Piao, G. Oldham, E.E. Haller, J. Non- Cryst. Solids 276, 61 (2000)
V. Davine, in Campbell 1 edition—first (Published Houston County, Georgia, 1992), p. 1831
R. Agrawal, G. Psaila, E.L. Wimmers, M. Zait, in Querying Shapes of Histories (Proc. of the 21st Int’l Conference, IBM Research Report), p. 3
J. Bell, P. Dean, Discuss. Faraday Soc. 50, 1 (1970)
F.H. El-Batal, F.M. Ezz-Eldin, Trans. Ind. Ceram. Soc. 66, 175 (2007)
R.H. Doremus, J. Non-Cryst. Solids 349, 242 (2004)
M.M. Salem, Int. Sci. Res. 3, 74 (2014)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
El-Alaily, N.A., Hussein, E.M.A. & Ezz Eldin, F.M. Gamma Irradiation and Heat Treatment Effects on Barium Borosilicate Glasses Doped Titanium Oxide. J Inorg Organomet Polym 28, 2662–2676 (2018). https://doi.org/10.1007/s10904-018-0934-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10904-018-0934-4