Abstract
Ultraviolet, visible, and infrared spectroscopic measurements have been employed to investigate undoped binary bismuth borate glass (60 mol% Bi2O3, 40 mol% B2O3) and doped samples with 0.2% 3d transition metal oxides in order to obtain information about the role of all the constituents oxides including the dopants on the measured properties. The undoped sample shows strong extended UV-near visible absorption bands which are attributed to both trace iron impurities from raw materials used for glass preparation and Bi3+ ions. The TM-samples show the same strong UV-near visible absorption as the undoped sample beside characteristic visible bands because of TM ions. The prepared samples show obvious shielding behavior toward the effect of successive gamma irradiation especially in the visible region. The infrared absorption spectra of the prepared samples show characteristic bands related to the sharing of triangular and tetrahedral borate groups together with Bi–O groups. The IR spectra are slightly affected by gamma irradiation indicating the stability of network forming units while the modifier, OH and water bands show obvious changes in their intensities.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Recently, glasses containing heavy metal oxides have received much interest because of their manifold applications. Glasses containing high-Bi2O3 content have found uses as an efficient γ-ray absorber [1, 2], and also in scintillation detectors [3, 4] for high energy physics. The large polarisability of bismuth makes it suitable for possible nonlinear optical uses and recent environmental guidelines [5]. Moreover, bismuthate glasses are excellent precursors for the fabrication of ceramic superconductors [6, 7]. Glasses containing Bi2O3 exhibit a high refractive index and this feature is very important for advanced optical telecommunication and processing devices [8].
Although many studies have been done on physical properties of Bi2O3-containing glasses [e.g., 9–16], there are still remain many controversies about the atomic level structure and its correlation with macroscopic properties. Mogus-Milankov et al. [17–19] have assumed that Bi2O3-containing glasses consist of very deformed BiO6 groups as suggested earlier by Bishay and Maghrabi [2] while other authors believe that BiO3 are former groups and BiO6 groups are modifiers, Recent authors [11–17] postulate that both BiO6 and BiO3 units are present in Bi2O3-containing glasses and the ratio of each unit of these groups depends on the percent of other glass component oxides.
Glasses containing 3d transition metal ions exhibit interesting optical, magnetic, and electrical properties due to the possible presence of such TM ions in two or more valence or coordination states [20–23]. Recent contributions on optical studies of 3d TM ions (Ti → Cu) in various glasses have reached the conclusion that transition metal ions predominantly exhibit the high valence or tetrahedral coordination states in alkali borate, alkali silicate, cabal, bioglass [24–26], while in alkali phosphate and lead phosphate glasses [27, 28] they exist mostly in the low valence or octahedral forms.
Transition metal redox state can be changed by various methods, including laser irradiation and thermal treatment under controlled oxidized or reduced conditions by the so-called internal diffusion approach [29].
The interactions of nuclear radiation such as γ-rays and neutron beams with TM-doped glasses are of much importance. This interaction is expected to cause changes in the physical and chemical properties of the glasses [29–31]. It is generally accepted that these changes depend not only on the radiation dose but also on the type and composition of glass including the TM dopants.
This study was designed to give more information on the states of 3d transition metal ions (Ti → Cu) doped in a host of binary bismuth borate of the composition Bi2O3 60 mol%, B2O3 40 mol%. The study includes UV–visible and FTIR infrared absorption spectroscopic investigations of the prepared samples before and after successive gamma irradiation. The study aims to identify the induced defects generated in such glass system containing 3d TM ions by gamma irradiation and the mutual effects of such irradiation on optical and IR spectra.
Experimental details
Preparation of the glasses
The studied undoped bismuth borate and 3d TMs-doped samples were prepared from chemically pure materials. Bi2O3 was introduced as such and B2O3 was added in the form of orthoboric acid. Transition metals were added (0.2 wt%) in the form of their respective oxides (TiO2 V2O5, Cr2O3, MnO2, Fe2O3, CoO, NiO, or CuO) to the base undoped bismuth borate glass of the composition (60 mol % Bi2O3, 40 mol % B2O3). The weighed batches were melted in porcelain crucibles in an electric furnace at 1050 °C for 1 h. The melts were rotated several times and the homogenized melts were poured in heated stainless steel molds of the required dimensions. The prepared samples were immediately transferred to an annealing muffle furnace regulated at 350 °C. The muffle was switched off after 1 h and left to cool at a rate of 20 °C/h.
Gamma irradiation facility
A 60Co gamma cell (2000 Ci) was used as a gamma-ray source with a dose 1.5 Gy/s (150 rad/s) at a temperature of ~30 °C. The investigated samples were subjected to the same gamma dose every time using a Fricke dosimeter. The absorbed dose in water was utilized in terms of dose in glass. No cavity theory corrections were made. Each sample was successively subjected to a dose of three and finally to total 6 M rad (6 × 104 Gy).
UV–visible absorption spectra measurements
The optical absorption spectra of carefully polished samples of the undoped and TM-doped glasses of equal thickness (2 mm ± 0.1 mm) were recorded at room temperature before and after successive gamma irradiation by a recording spectrophotometer (type T 80 PG Instruments, England) in the range 200–1000 nm.
Infrared absorption measurements
The infrared absorption spectra of the undoped and TM-doped glasses were measured at room temperature in the range 400–4000 cm−1 by a Fourier transform infrared spectrometer (type Jacso FT/IR-430, Japan) using the KBr disk technique. Fine glass powder was mixed with KBr in the ratio 1:100 and the mixture was subjected to a load of 5 tons/cm2 in an evocable die for 2 min to produce clear homogeneous disks. The IR absorption spectra were immediately measured after preparing the disks. Also, the IR spectra were remeasured after subjecting the glassy powders to a specified total gamma-ray dose of 6 M rad (6 × 104 Gy).
Results
UV–visible absorption spectrum of undoped bismuth borate glass before and after gamma irradiation and induced spectrum
The optical spectrum (Fig. 1a) before irradiation reveals strong extended UV- near visible from 200 to about 500 nm and exhibiting two connected broad UV and near visible bands. The UV band shows three peaks, the first is high intense at about 235 nm and followed by two peaks in the descending lobe at about 295 and 310 nm. The second connected near-visible spectrum is a high-intense band with a peak at about 430 nm and revealing two kinks at the ascending lobe at about 380 and 395 nm. With subjecting the base glass to a dose of 3 M rad, the intensities of all the UV-near visible absorption bands increase with a slight shift of their peaks to shorter wavelengths. With further increasing the gamma dose to 6 M rad, all the bands remain identified but their intensities decrease but still higher than before irradiation.
The induced spectrum of this undoped (Fig. 1b) glass calculated by subtracting the optical densities of obtained from the glass after 3 or 6 M rad irradiation from that before irradiation shows strong UV-near visible absorption consisting of four consecutive peaks at about 205, 300, 380, 400 nm and followed by an induced distinct broad visible centered at about 530 nm with a small kink on the descending lobe at 680 nm. On subjecting this glass to a final total gamma dose of 6 M rad (6 × 104 Gy), the UV absorption is markedly decreased in its intensity but the broad visible band shows no obvious change.
UV–visible absorption spectra of TM’s-doped bismuth borate glasses before and after gamma irradiation and radiation-induced spectra
Ti-doped sample
Figure 2a illustrates the optical spectrum of Ti-sample showing strong UV and near visible absorption as the undoped sample and after being subjected to a dose of 3 M rad, the intensities of all the UV-near visible peaks markedly increase but when reaching 6 M rad, the intensities slightly decrease but still higher than before irradiation.
The induced spectrum of Ti-doped sample (Fig. 2a) calculated as described in the undoped sample shows at first with 3 M rad, the resolution of two connected strong and broad absorption bands from 200 to 500 nm. The first band consists of an induced peak at about 235 nm and succeeded by connected two small kinks at about 275 and 300 nm and then followed by an intense-induced visible band at about 430 nm with two kinks at the ascending lobe at about 380 and 400 nm. On subjecting the sample to 6 M rad, the same induced spectra are retained but with slightly lower intensities.
V-doped sample
Figure 2b illustrates the optical spectrum of V-sample showing the same strong UV and near visible absorption as the undoped sample beside the resolution of an extra small broad visible band centered at about 630 nm. On subjecting to a dose of 3 M rad, the intensities of all the observed bands increase but the small band at 630 nm decreases in intensity. When increasing the dose to 6 M rad, the intensities decrease but still higher than before irradiation.
The induced spectrum of V-doped sample (Fig. 2b) shows at first after irradiation with 3 M rad strong extended UV-induced absorption with five small kinks at 230, 275, 310, 350, 380 nm and followed by a strong-induced visible band at about 440 nm and a small kink at the descending lobe at about 680 nm. On further irradiation to 6 M rad, the intensities of the UV-induced bands markedly decrease but the visible band is slightly affected.
Cr-doped sample
Figure 2c shows the optical spectrum of Cr-sample which exhibits the same strong UV-near visible absorption bands as the undoped sample beside further resolution of an extra broad visible band centered at about 630 nm with a further kink on the descending lobe at about 730 nm. On subjecting to a dose of 3 M rad, all the spectral bands show marked decrease in their intensities but on increasing the dose to 6 M rad, the UV absorption highly increases but the visible absorption shows almost stabilization.
The induced spectrum of Cr-doped sample (Fig. 2c) shows extended UV–visible-induced bands on irradiation with 3 M rad dose consisting of low intensity peaks at 270, 290 nm beside a prominent induced band at 340 nm followed by two-induced visible bands at about 540 and 680 nm. On increasing the dose to 6 M rad, the UV absorption intensity increases with the resolution of some closer bands and the induced visible band on 540 nm is separately identified.
Mn-doped sample
This Mn-sample shows (Fig. 2d) completely the same optical spectral characteristics as the undoped sample but on gamma irradiation the spectrum shows two new additional radiation induced bands at about 520 and 580 nm.
The induced spectrum of Mn-doped sample (Fig. 2d) shows with 3 M rad, the appearance of an induced UV absorption with constant intensity revealing five peaks at about 230, 275, 310, 350, 380 nm followed by an intense broad absorption with three peaks at 510, 540, 560 nm and also with two small kinks at the ascending lobe at 440 and 460 nm.
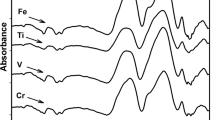
Fe-doped sample
This Fe-sample shows (Fig. 2e) also the same optical spectral characteristics as the undoped sample but the intensity of the connected UV-near visible peaks slightly increase in intensity and a further new band is observed at 350 nm. On irradiation, the UV-near visible connected peaks is observed to increase in intensities but the band at about 430 nm is slightly affected.
The induced spectrum of Fe-doped sample (Fig. 2e) shows after irradiation with 3 M rad, high-intense UV absorption consisting of five peaks at about 220, 260, 310, 350, 380 nm and followed by a medium visible band centered at about 480 and also revealing a curvature at about 600 nm.
Co-doped sample
This Co-sample gives (Fig. 2f) the same strong UV-near visible spectral characteristics as the undoped sample but the near visible band maximum moves to 460 nm beside the further appearance of a broad shoulder on the descending lobe of the visible band centered at about 650 nm. On subjecting this glass to a dose of 3 M rad, the intensities of all the bands are observed to increase and their peaks are shifted to shorter wavelengths but the band at 650 nm is observed to decrease. On further increasing the irradiation to 6 M rad, the connected UV-near visible bands are seen to decrease in intensities.
The induced spectrum of Co-doped sample (Fig. 2f) shows after irradiation with 3 M rad, low intense UV-near visible absorption beside two broad connected bands centered at about 400 and 560 nm. With a further higher dose of 6 M rad, the UV absorption highly increases in intensity revealing five peaks at about 210, 270, 310, 350, 380 nm and the visible spectrum almost remains unaffected.
Ni-doped sample
This Ni-sample reveals (Fig. 2g) the same strong UV-near visible spectral characteristics as the undoped sample but the visible band increases in its intensity and its peak moves to longer wavelength reaching about 465 nm. On subjecting this glass to a dose of 3 M rad, the intensities of the bands are observed to increase but the peaks of the visible bands are markedly shifted to lower wavelengths. With progressive irradiation to 6 M rad, the intensities of all the bands are further increased.
The induced spectrum of Ni-doped glass (Fig. 2g) shows similar behavior to that for the Co-sample exhibiting the resolution of more defined-induced spectra with progressive irradiation.
Cu-doped sample
This Cu-sample shows (Fig. 2h) the same strong UV-near visible absorption spectrum as the undoped sample but the peak of the visible band moves to about 460 nm and the rest of the visible spectrum exhibits slight upward curvature centered at about 780 nm. On subjecting this Cu-doped sample to a gamma dose of 3 M rad, the intensities of all the UV-near visible bands are observed to increase but the curvature at 780 nm is observed to disappear. With further gamma irradiation to 6 M rad, the intensities of all the peaks are observed to increase.
The induced spectrum of Cu-doped glass (Fig. 2h) shows similar behavior toward the resolution of the same induced spectra with continuous irradiation to that obtained from the two Co- and Ni-doped samples.
Infrared absorption spectra of undoped and TM-doped bismuth borate glasses and effect of gamma irradiation
Figure 3 illustrates the IR absorption spectra of undoped and transition metals doped glasses which appear complex revealing absorption bands extending from the beginning of measurements at 400 up to 4000 cm−1. The main characteristic bands are observed in the undoped bismuth borate glass at about 474, 692, 902, 1033, 1226, 1456, 1650, 2350, 3436 cm−1. The first two bands are sharp and of moderate intensity while the two subsequent bands are strong and broad and the rest bands are broad and of medium intensity. Also, there are some other small peaks identified at about 580, 625, 730, 1488 cm−1. The introduction of the 3d transition metal oxides in the doping level (0.2%) causes some minor changes which are mainly concentrated in the relative intensities of the IR bands and specifically in the first band at 480–520 cm−1 while the main characteristic bands in the region 880–1100 cm−1 remain prominent. Inspection of the IR absorption spectra reveals that most of the vibrational bands are associated with the characteristic structural units of both triangular and tetrahedral borate groups beside bismuth-oxygen groups.
Figure 4 shows the IR spectra of undoped and TM-doped glasses after being subjected to a gamma dose of 6 M rad (6 × 104 Gy). The overall IR spectra of the samples exhibit limited variations especially at the near infrared broad band at about 3445 cm−1. This band shows high response with irradiation and becomes more intensified.
Discussion
Origin of ultraviolet-near visible absorption in undoped bismuth borate glass
Sigel and Ginther [32] and Cook and Mader [33] have separately identified strong ultraviolet absorption in various undoped commercial glasses and assumed that such UV absorption bands originated from trace iron impurities within the raw materials used for the preparation of such glasses or when molten glasses came in contact with refractories during the industrial melting process for glass manufacture.
Duffy and Ingram [34–36] have recognized differently originated ultraviolet absorption in glasses. Some transition metal ions (e.g., Fe3+, Cr6+….) in glasses exhibit characteristic charge transfer ultraviolet absorption spectra even if present in the ppm level. Such metal ions in glass owe their ultraviolet spectra to an electron transfer mechanism. But certain other metal ions including Ce3+, Tb3+, U4+ as well as d10S2(ions such as Pb2+ and Bi3+) absorb radiation through electronic transitions involving orbitals essentially of the metal ion only, and the name “Rydberg” has been suggested for such spectra to distinguish them from the common charge electron transfer spectra.
Ehrt and co-worker [37–39] have confirmed in a series of successive papers that small amounts of TM ions and specifically iron impurities (even in the ppm range) cause deterioration of the UV transmission in optical glasses. They have stressed on the need for ultra pure chemicals for the preparation of special optical glasses.
Recently ElBatal et al. [16, 24–28, 40–43] have identified experimentally that the charge transfer UV absorption bands are observed in undoped phosphate, borate, silicate, cabal, bioglass, and other high lead or bismuth glasses and have confirmed that such charge transfer absorption bands are originating from unavoidable trace iron impurities even in the ppm within the raw materials used for the preparation of such different glasses. It is therefore, concluded that part of the UV absorption observed in the spectrum of the undoped studied bismuth borate glass can be related to the presence of trace iron impurities within the raw materials for the preparation of such bismuth borate glass, specifically the UV bands observed in the range 210–275 nm.
The other linked UV-near visible band extending up to 430 nm can be related to the contribution of Bi3+ ions. Paul [44] and Parke and Webb [45] early observed a UV peak when traces of Bi3+ ions were added to borate and phosphate glasses and the transition of the peak was related to 1S0 → 3P1. Duffy and Ingram [34, 35] agreed to such assignment. Reisfeld and Boehm [46] compared the absorption spectrum of Bi3+ ions in phosphate, borax, and germanate glasses and showed that the UV absorption peak lies in the three glasses at 43010, 41322, and 36764 cm−1, respectively.
Recent studies by Sanz et al. [47] and ElBatal [17], and ElBatal et al. [48, 49] have confirmed that the UV-near visible absorption bands in the range 275–440 nm observed in high bismuth borate or high-bismuth silicate glasses are correlated with Bi3+ ions. It is to be mentioned that gamma irradiation of the undoped bismuth borate glass is seen to increase the intensities of the observed UV-near visible absorption bands which are previously correlated with both trace iron impurities and Bi3+ ions. No obvious new-induced bands are observed to generate by continuous irradiation within the rest of the visible spectrum (500–1000 nm). This result can be attributed to the known shielding behavior of heavy metal Bi3+ ions [2, 10, 11, 17, 48, 49].
The previous postulation about the existence of monovalent bismuth in the Bi-doped silica glass [49] can not be expected to extend for all Bi2O3-containing glasses and specifically to Bi2O3·B2O3 glass system for the following reasons [1, 2, 11–19, 22]:
-
(i)
According to early Zachariasen’s hypothesis the glass forming oxides generally belongs to the formula: RO2 (such as SiO2, GeO2), R2O3 (such as B2O3), and R2O5 (such as P2O5). These glass forming oxides normally form tetrahedral or triangular groups [2, 12, 22]. Monovalent Bi can not easily share as structural or substitutional group except by additional oxygens.
-
(ii)
It is shown and verified by IR measurements that Bi2O3 in high-Bi2O3–B2O3 glasses denotes oxygen to B2O3 to form [BO4] tetrahedron and bismuth ions also share in forming BiO3 and/or BiO6 groups as evidenced by the same IR measurements in comparison with corresponding definite crystalline compounds [2, 6, 7, 11–19].
-
(iii)
The wide range forming ability of Bi2O3 and B2O3 to form stable glasses confirms the assumption that Bi2O3 is considered as conditional glass forming oxide [1, 2, 11–19].
-
(iv)
The ratio of the suggested units of BiO3 and BiO6 in the Bi2O3·B2O3 glass is certainly not yet conclusive and needs further studies by varying recent techniques.
The proposed states of bismuth in glasses are even controversy through several studies on optical fibers from bismuth-doped germanate, phosphate, and borate glasses [50–52]. According to these authors, the spectroscopic properties of the investigated Bi-doped glasses are different from those of the previously reported luminescence obtained from Bi3+ ions [53]. As for Bi, the most frequent oxidation number is 3 and Bi can also takes the pentavalent state. In the infrared luminescence, the presence of Bi ions in monovalent state is also proposed [54]. It is important to understand the optical properties of bismuth-doped glasses for technological applications such as optical fiber amplifier and filter laser. Recent contribution on bismuth-doped silica fiber amplifier [55] has not reached a definite conclusion to justify the specific role of Bi in various glasses and further studies are appreciated.
The observed increase of the intensities of absorption bands in the-near visible range with gamma irradiation, which are occurring within the same positions previously assumed to be related to trace iron impurities and Bi3+ ions can be correlated to several possible reasons:
-
(a)
Gamma irradiation is assumed to generate energetic electrons by Compton process [29, 30] and hence the resulting pairs of electrons and positive holes can be trapped by multivalent impurities (e.g., Fe2+, Fe3+) and cause high absorption in the vicinity of absorption of the oxidized or reduced species, It is assumed that some of the present Fe2+ ions capture positive holes and are converted to Fe3+ ions through photochemical reaction and this explains the observed increase of the intensity of the absorption bands due to Fe3+ in the UV region.
-
(b)
The presence of Bi3+ ions in high proportion in the studied glasses imparts some exceptional properties. Bi3+ cation has a high polarizability and small cation oxygen field strength. Glasses containing Bi2O3 thus have high-oxide polarizability and therefore increases optical basicity of the glass. Also, the heavy weight metal oxide (Bi2O3) present in high content in the studied glasses causes the observed shielding behavior toward successive gamma irradiation especially in the visible region.
-
(c)
Although, much work has been done on physical and chemical properties of glasses containing Bi2–O3 [1–19], yet there still remain many controversies about the atomic level of bismuth and its proper coordination in glasses. Earlier in analogy with crystalline Bi2O3 compounds, glasses containing bismuth were considered to consist of deformed BiO6 units as confirmed by X-ray radial distribution function and neutron diffraction studies [2–10].
-
(d)
However, recent studies [11–17] have reached the postulation that both BiO6 and BiO3 units are present and the ratio of each of them depends on the glass type and composition. The interpretation of the increase of intensity of the absorption bands related to Bi3+ upon gamma irradiation cannot be related to a change in the valency of bismuth cations because other valencies are unstable [56]. It is assumed that this increase is correlated with the formation of boron–oxygen-hole centers as previously suggested by several authors [48, 49, 57]. Such induced centers are generated by progressive gamma irradiation on the studied host bismuth borate glasses and specifically on the borate partner [58].
Interpretation of the UV–visible absorption spectra of TMs-doped bismuth borate glasses
It must be born in mind that the states of transition metal ions in glasses depend on the type and composition of the host glass and on melting conditions. It is generally accepted that alkali borate, alkali silicate, lead borate, and lead silicate glasses favor the presence of the TM ions in their high valence or tetrahedral coordination states while on the other hand alkali phosphate and lead phosphate glasses promote the low oxidation or octahedral coordination states [20, 58, 59]. Visual examinations and spectral data due to the prepared 3d TMs-doped bismuth borate glasses reveal that the high valence or tetrahedral coordination states are not conclusively present in noticeable abundant percent, and the same holds for the other low valence states for all the studied 3d elements. The detailed optical absorption spectra of the 3d TMs-doped samples before and after irradiation can be realized and interpreted as follows:
Titanium-doped sample
Titanium ions can exhibit trivalent and tetravalent states in glasses [51–53]. Ti3+ ion belong to the 3d1 configuration and thus exhibits a single absorption band at 480–540 nm [51–53]. In acid phosphate glasses, Ti3+ ions are assumed to be readily obtained showing an absorption band at about 540 nm and a shoulder at 650–750 nm because of Jahn–Teller distortion [58–61]. The tetravalent titanium Ti4+ions [59–62] belong to do configuration and as such will give no visible bands but exhibit ultraviolet absorption. The observed UV–visible spectrum of the Ti-doped sample supports the previous postulations about the possible existence of both trivalent and tetravalent states of titanium ions. The expected bands due to both titanium valences are assumed to be mixed and interfered with the strong bands already present by the base host bismuth borate glass but the appearance of a kink at about 680 nm confirms the presence of Ti3+ ions in this host glass [58–61].
Vanadium-doped glass
The optical spectrum of this V-sample before irradiation (Fig. 2b) retains the same spectral characteristics of the undoped sample (Fig. 1) except showing only a small curvature at about 620 nm. This behavior denotes the presence of the doping vanadium ions in the pentavalent state (V5+) but showing some indication of the trivalent V3+ state. V5+ ions are accepted to give a charge transfer band at about 370 nm [20, 58, 59] which is very close and interferes with the bands due to Bi3+ ions. It seems that the host bismuth borate glass favors the presence of vanadium as V5+ ions in noticeable percent. The observed induced spectra for V-sample (Fig. 4b) seems to be correlated with the shielding behavior of the host bismuth borate glass as evidenced by the marked decrease of the UV absorption and stability of the visible absorption with progressive gamma irradiation. A similar behavior is observed in a previous publication [49].
Chromium-doped glass
The optical spectrum of this Cr-sample reveals before irradiation (Fig. 2c) the resolution of extra visible bands beside the extended UV-near visible absorption observed from the undoped sample. Such observed spectrum indicates the presence of both hexavalent and trivalent states of chromium in noticeable percent. This is represented by the appearance of the induced bands at 540 and 680 nm indicating the trivalent chromium ions and the hexavalent chromium ions reveal a UV band close to bands due to Bi3+ ions. Gamma irradiation causes a remarkable decrease of intensities to all the UV-near visible spectrum at first. With progressive irradiation, the intensities again increase but the other separate visible spectrum reaches some stability. It is obvious that the host bismuth-borate glass favors the presence of both hexa- and tri-valent chromium states. The induced bands observed (Fig. 4b) generated by gamma irradiation can be correlated to trace iron impurities and positive hole centers originating from the host borate glass and the effect of chromium in the doping level (0.2%) causes no measurable changes in all the induced spectra.
Manganese-doped glass
The unirradiated Mn-sample shows at first the same spectral characteristics of the undoped sample. On gamma irradiation, the sample exhibits extra two-induced bands at about 540 and 580 nm. The glass shows no indication of the existence of trivalent manganese before gamma irradiation and this may be due to the shielding behavior of bismuth ions. On gamma irradiation, the generation of induced spectra can be correlated with three possible reasons: (1) The sharing of trace iron impurities. (2) The possible transformation of some divalent manganese (Mn2+) ions to trivalent (Mn3+) ions by capturing positive holes. This is supported by the appearance of the bands at 480–540 nm with irradiation which is known to be due to trivalent manganese (Mn3+) ions [58, 59]. (3) The sharing of the host borate partner through generating positive hole centers in the visible region.
Iron-doped glass
The unirradiated Fe-doped sample shows at first the same spectral characteristics of the undoped sample. With gamma irradiation, the UV-near visible spectrum sharply increases but the absorption at 460 nm slightly increases. The induced spectra reveal extended UV bands followed by a medium induced visible band at about 480 nm. This band is correlated with Fe3+ ions [20, 58, 59].
Such induced data indicate the sharing of both trace iron impurities in the UV region and the host borate glass in the visible region for generating the observed induced bands. This is the same behavior as that observed with the undoped glass containing trace iron impurities and borate partner and both promote induced defects.
Cobalt-doped glass
The unirradiated Co-doped sample shows at first the same spectral characteristics of the undoped sample. With progressive gamma irradiation, the intensities of all the absorption bands increase with 3 M rad but with 6 M rad the UV spectrum continues to increase while the visible spectrum remains unaffected. The induced spectral data obtained from Co-doped sample indicate that the curves show an increase with progressive gamma irradiation. The induced UV spectrum reveals continuous but limited increase in intensity which is correlated with the transformation of some Fe2+ ions to Fe3+ ions through photochemical reactions. The induced visible bands can be related to be due to positive hole defects generated from the host borate partner.
Nickel-doped glass
The absorption and induced spectra from Ni-doped sample are closely similar to that obtained from that obtained from Co-doped sample and thus have the same explanations to be introduced to the spectral result from Ni-sample.
Copper-doped glass
The unirradiated Cu-sample shows at first the same spectral characteristics to that obtained from the undoped glass. On gamma irradiation, this glass shows shielding effect especially within the visible spectrum while the UV absorption continues to increase with continuous irradiation. The optical and induced spectra indicate that CuO – doped sample shows the same data obtained from the undoped sample. This is certainly due to the resistance effect of Bi3+ cations and the only observed response is the sharing of trace iron impurities within the UV spectrum while the visible spectrum indicates that the base borate partner is effective in producing positive hole-induced bands.
Concluding remarks
It is evident from the comparison of all the absorption spectra of TMs-doped glasses that the combined UV-near visible absorption obtained from the undoped bismuth borate glass are repetitive to a remarkable extent in the spectra of all 3d doped TM samples. This specific combined UV-near visible absorption bands are correlated with the sharing of both trace iron impurities and bismuth Bi3+ ions. It is also evident that the various 3d transition metal ions did not sharply or distinctly impart their known characteristics bands as they normally exhibit in alkali borate, alkali silicate, lead borate and lead silicate glasses [20, 59, 60]. It seems that the presence of high percent of Bi2O3 (60 mol %) suppresses the effects of the 3d transition metal ions in their normal environments and hence the resolution of their expected specific absorption spectra.
Therefore, it is assumed that bismuth borate glass within the studied composition acts in two routes, the first route is the shielding effect toward successive gamma irradiation and hence controlling the growth of the induced bands. The second route includes the resistance of the marked resolution of the specific absorption spectra of the 3d transition metals and specifically in the doping level (0.2%). The only observed features are the appearance of some small bands with some TM ions which are mostly disappeared by progressive gamma irradiation. It seems that further work is needed to justify the role of Bi2O3 on the optical spectra of TM ions introduced in progressive increasing amounts.
Significance of radiation shielding
It is accepted that the main direction of radiation shielding is to protect personal working in a radiation environment from the harmful effects of nuclear radiation. Some materials are more effective than others in shielding a particular type of radiation. Gamma rays are known to possess relatively higher penetrating power than beta particles (electrons) or alpha particles.
Because of its low cost, barites concrete is often used in the construction of large-volume shields and most commonly used as the outer constituent of a shield, with its own activity shielded by an inner layer of other shielding material of lower activity. Apart from better compression strength, some other surface finish and high abrasion resistance, offers adequate shielding to gamma radiation in comparison with lead metal or lead and bismuth glasses [63].
Recently, various scientists [11, 48, 49, 64–66] have carried numerous studies on bismuth glasses with the aim to find out their suitability as radiation shielding materials. It is postulated that bismuth oxide and/or lead oxide glasses are recommended materials for shielding of gamma rays because of their high densities and large atomic numbers.
Contributions of the IR absorption spectra
The interpretation of IR bands observed in the studied undoped and 3d-doped bismuth borate glasses has been carried out in accordance with the concept introduced by Tarte [67] and Condrate [1, 68, 69] about the independent vibrations due to different structural groups in glass. It is assumed that the IR vibrations of characteristic network groups or atoms are independent from vibrations of other constitutional groups. This approach has been successfully applied by Dimitriev et al. [6, 70] and El Batal et al. [11, 40, 41]. Such an empirical treatment can provide significant information on the arrangement of the constitutional groups in glasses.
Bishay and Maghrabi [2] have compared, using IR studies, bismuth glasses with those containing arsenic and antimony because these three elements have similar outer electronic configuration. They have suggested that Bi2O3 in Bi2O3·B2O3 glasses can share in the structure in three different ways; it gives part of its oxygen to the boron to create a four coordinated state; it participates in the structure by forming BiO3 groups belonging to the pyramidal point group C3v; and it introduces some nonbridging oxygens. A precondition for such assumption is the presence of the IR band at 840 cm−1. This band is related to the symmetrical stretching vibrations of the Bi–O bonds in the BiO3 groups. A similar IR band, along with two others at 600 and 470 cm−1 were observed by Zheng and Mackenzie [70] in the IR spectra of Bi–Ca–Sr–O glasses. The conclusion has been drawn that both BiO3 and BiO6 groups are assumed to coexist in bismuth glasses.
Contribution of the effect of irradiation on the IR spectra
It is evident that the observed effects of gamma irradiation on the IR spectra are quite different than that on the effect of irradiation on optical absorption spectra. The main IR spectra are mostly unaffected by gamma irradiation indicating and reflecting that the structural building units remain stable and consistent and the only minor observations are the slight changes in the intensities of some bands especially that belonging to modifier units. In the case of optical absorption, induced color centers are generated which are mostly accompanied by the deepening of the colors or their change to dark brown on progressive irradiation and the net result is the induced defects or induced color centers. Table 1 summarizes infrared absorption bands and their assignments according to various authors.
In order to interpret the effect of gamma irradiation on the IR spectra, several authors have claimed the following reasonings and postulations:
-
(a)
Primak [71] had early assumed that a compaction of the Si–O–Si bond was observed to occur upon gamma irradiation. The suggested idea includes the changes in the bond angles.
-
(b)
Hobbs et al. [72] and Piao et al. [73] have proposed some mechanisms for radiation-induced defects generation including the changes in bond angles and/or bond length within the structural building units.
-
(c)
El Batal and Ezz ElDin [74] have observed that continuous gamma irradiation of borosilicate glass decreases the intensity of all the absorption spectra. They have assumed that this behavior can be related to more disorder in the already non-periodic network arrangement of such glasses.
-
(d)
Baccoro et al. [75] have assumed that gamma irradiation of lead borosilicate glasses causes structural changes by breaking of some bonds between trigonal BO3, tetragonal BO4 and tetrahedral SiO4 units.
-
(e)
ElBatal et al. [40, 41] have assumed that gamma irradiation causes minor variations of the intensities of the IR spectra of lithium diborate, sodium phosphate and lead phosphate glasses and have correlated such minor data to changes in the bond angles and/or bond lengths of the structural units.
Based on previous postulations, the effect of gamma irradiation on the present IR spectra obtained from undoped and 3d-doped bismuth borate glasses can be realized as follows:
-
(1)
It is evident that the main characteristic absorption bands for the structural building units are stable and show only slight variations in their intensities. This behavior is related to the presence of compact structural units consisting of interlinked BO4 and BO3 groups beside the presence of heavy Bi3+ cations as BiO3 and/or BiO6 units exhibiting shielding effects toward successive gamma irradiation.
-
(2)
The broad near-infrared band shows high response toward gamma irradiation and this result is related to the fact that this IR band is correlated with water vibrations and it is expected that irradiation is more effective in such glasses containing high content of heavy weight Bi3+ cations on the surface water and on the loose modifier ions which are not compactly attached to the main network structure of the glass.
-
(3)
A similar result was previously reached by one of the authors [12]
From previous considerations, the observed IR spectra vibrations of undoped bismuth borate glass can be interpreted as follows [12, 17, 77, 78]
-
(1)
The presence of high percent of Bi2O3 with B2O3 reveals the appearance of BO4 vibrational bands at about 950 and 1100 cm−1 due to the sharing of oxygens from Bi2O3 to form tetrahedral BO4 groups.
-
(2)
The persistence of the absorption band at about 700 cm−1 is indicative of the presence of BO3 or boroxol groups.
-
(3)
The low frequency band at 460 cm−1 may be related to the very deformed BiO6 groups (and possibly BiO3 according some authors) and this band is not interfered by another structural group vibrations.
-
(4)
The absorption band at 800 cm−1 coincides the BO4 groups vibration with that from Bi–O vibrations which lie in the same position.
Conclusion
UV–visible absorption spectra of undoped and 3d transition metal oxides (0.2%)-doped bismuth borate glasses were measured before and after gamma irradiation with 3 and finally 6 M rad (3 and 6 × 104 Gy). The undoped bismuth borate glass of the basic composition (60 mol % Bi2O3-40 mol % B2O3) shows before irradiation strong ultraviolet near-visible absorption bands which are attributed to the collective the presence of both trace iron impurities in the raw materials used for the preparation of the mentioned base glass and also to the presence of Bi3+ ions. 3d TM-doped samples reveal before irradiation the same strong UV bands as the undoped sample beside extra small visible bands due to the respective TM ions. On gamma irradiation, the UV-near visible spectra of all the samples show increase of the intensities of the observed bands but the visible absorption spectra reveal minor variations or retardation. It is evident from careful inspection of the optical spectra that heavy bismuth cations cause a shielding behavior toward successive gamma irradiation. This behavior is accepted to be related to the high density and large atomic number of the bismuth ions and their ability to retard the effects of gamma irradiation.
Infrared spectra of the undoped and TM-doped samples show characteristic repetitive absorption bands due to the presence of both triangular and tetrahedral borate groups beside Bi–O groups. Gamma irradiation is observed to affect the near infrared band at about 3450 cm−1 due to water while the main characteristic bands due to main structural building units remain almost unaffected. This result is correlated with the shielding behavior exhibited in the studied glasses due to the presence of heavy Bi3+ ions and to the possible changes in the bond angles or bond lengths of some structural units causing minor variations in their intensities.
References
Janakirama–Rao BHV (1962) J Amer Ceram Soc 45(11):555
Bishay A, Maghrabi C (1969) Phys Chem Glasses 10(1):1
Vankirk SE, Martin SW (1992) J Amer Ceram Soc 75(4):1028
Ford N, Holland D (1987) Glass Technol 28(2):106
Gaudagenino E, Dallgna R (1996) Glass Technol 37(3):76
Dimitriev Y, Michailova V (1990) J Mater Sci Lett 9:1251
Dimitriev Y, Michailova V (1992) Proc XVI Intern Cong on Glass Madrid 3:293
Sugimito N (2002) J Amer Ceram Soc 85:1083
Donald IW, McMillan PW (1978) J Mater Sci 13:2301. doi:https://doi.org/10.1007/BF00808042
Dumbaugh WH (1986) Phys Chem Glasses 27:119
Dumbaugh WH, Lapp JC (1992) J Amer Ceram Soc 75:2315
ElBatal FH, Azooz MA, Ezz ElDin FM (2002) Phys Chem Glasses 43:260
El-Shaarawy MG, ElBatal FH (2002) Phys Chem Glasses 43:247–253
Baia L, Stefan R, Kiefer W, Popp J, Simon S (2002) J Non-Cryst Solids 303:379
Baia L, Stefan R, Popp J, Simon S, Kiefer W (2003) J Non-Cryst Solids 324:109
Culea E, Popp L, Simon V, Neumann M, Brat L (2004) J Non-Cryst Solids 337:62
ElBatal FH (2007) Nucl Instr and Meth Phys Res (B) 254:243
Mogus-Milankov A, Santic A, Licna V, Day DE (2005) J Non-Cryst Solids 351:3235
Witkowska A, Rybicki J, Dicicco A (2002) In: Proceedings of 19th International Glass Congress, Edinburgh 43c, p 124
Stehle C, Vira C, Hogan D, Feller S, Affatigato MH (1998) Phys Chem Glasses 39:83
Bamford CR (1977) Color generation and control in glass, glass science and technology. Elsevier Publishing Company, Amsterdam, p 55
Elliot SR (1984) Physics of amorphous materials. Longman, New York
Shelby JE (1997) Introduction to glass science and technology. Royal Society of Chemistry, Cambridge
ElBatal FH, Azooz MA, Marzouk SY (2007) Opt Mater 29:1456
ElBatal FH, Azooz MA, Marzouk SY, Selim MS (2007) Physica B 398:126
ElBatal FH, Azooz MA, Marzouk SY (2006) Phys Chem Glasses European J Glass Sci Technol (B) 47:588
Marzouk SY, ElBatal FH (2006) Nucl Instr Meth Phys Res (B) 248:90
ElBatal FH, Hamdy YM, Marzouk SY (2009) J Non-Cryst Solids 355:2439
Smedskjaer MM, Yue Y (2009) Appl Suf Sci 256:202
Bishay A (1970) J Non-Cryst Solids 3:54
Friebele EJ (1991). In: Uhlmann DR, Kreidl NJ (eds) Optical properties of glass. American Ceramic Society, Westerville, p 205
Sigel GH, Ginther RJ (1968) Glass Technol 9:66
Cook L, Mader KH (1982) J Amer Ceram Soc 65:109
Duffy JA, Ingram MD (1970) Phys Chem Glasses 52:3752
Duffy JA, Ingram MD (1974) Phys Chem Glasses 15:34
Duffy JA (1997) Phys Chem Glasses 38:289
Seeber W, Ehrt D (1999) Glastech Ber Glass Sci Technol 72:295
Natura U, Ehrt D, Neumann K (2001) Glastech Ber Glass Sci Technol 74:23
Moncke D, Ehrt D (2004) Opt Mater 25:425
ElBatal FH, ElKheshen AA, Azooz MA, AboNaf SM (2008) Opt Mater 30:88
ElBatal FH, Azooz MA, ElKheshen AA (2009) Tran Ind Ceram Soc 68:81
ElBatal FH, Marzouk SY (2009) J Mater Sci 44:3061. doi:https://doi.org/10.1007/s10853-009-3406-y
ElBatal FH, Ouis MA, Morsi RM, Marzouk SY (2010) J Non-Cryst Solid 356:46
Paul A (1972) Phys Chem Glasses 13(5):144
Parke S, Webb RS (1973) J Phys Chem Solid 38:85
Reisfeld R, Boehm L (1974) J Non-Cryst Solid 16:83
Sanz O, Aro-Poinatwski EH, Gonzzlo J, Fernandez Navarro JM (2006) J Non-Cryst Solid 352:761
ElBatal FH, Marzouk SY, Nada N, Desouky SM (2007) Physica B 391:88
ElBatal FH, Marzouk SY, Nada N, Desouky SM (2010) Philosph Mag 90(6):675
Roul BK (1999) Superconductivity J 12(2):409
Meng X, Qui J, Peng M, Chen D, Zhao Q, Jiang X, Zhu C (2005) Opt Exp 13(5):1628
Peng M, Qiu J, Chen D, Meng X, Zhu C (2005) Opt Exp 13(18):6892
Suzuki T, Ohishi Y (2006) Appl Phys Lett 88:1912
Peng M, Qui J, Chen D, Meng X, Yang I, Jiang X, Zhu C (2004) Optics Lett 29:1998
Seo Y, Fujimoto Y (2010) In: Pal BB (ed) Frontiers in guided wave optics and optoelectronics. INTECH, Croatia, pp 105–118
Cotton FA, Wilkinson G, Murille CA, Bochmann M (1999) Advanced inorganic chemistry, 6th edn. John Wiley & Sons Inc., NewYork
Shkrob IA, Tadjikov BM, Trifunac AD (2000) J Non-Cryst Solids 262:6
Bates T (1962) In: Mackenzie JD (ed) Modern aspects of the vitreous state, vol 2. Butterworths, London, pp 195–254
Bamford CR (1962) Phys Chem Glasses 3:189
Ravikumar RVSSN, Chandreskhar AV, Ramoorthy L, Reddy BJ, Yamauchi J, Rao PS (2004) J Alloys Comp 364:176
Khanna A, Bhatti SS, Singh KJ, Thind KS (1996) Nucl Instr Meth Phys Res (B) 114:217
Singh K, Singh H, Khanna A, Kummar R, Nathuram R, Bhatti SS, Sahota HS (2002) Nucl Instr Meth Phys Res (B) 194:1
Singh N, Singh KJ, Singh K, Singh H (2004) Nucl Instr Meth Phys Res (B) 225:305
El Batal FH, Ezz ElDin FM (2007) Trans Ind Ceram Soc 66:4
Plionis AA, Garcia SR, Gonzales ER, Porterfield DR, Peterson DS (2009) J Radional Nucl Chem 282:239
Tarte P (1962) Spectrochim Acta 18:467
Tarte P (1964) Physics of non-crystalline solids. Elsevier, Amsterdam, p 549
Condrate R (1972) Introduction to glass science. Plenum, New York, p 101
Gattef EM, Dimitrov VV, Dimitriev YB, Wright AC (1997) In: Wright AC, Feller SA, Hannon AC (eds) Proceedings of second international conference on borate glasses, crystals and melts. Society of Glass Technology, Sheffield, p 246
Zheng H, Mackenzie J (1989) J Mater Res 4:911
Primak W (1972) J Appl Phys Phys 43:2745
Hobbs LW, Sreeam AN, Jesurum CE, Berger BA (1991) Nucl Instr Meth Phys Res (B) 116:18
Piao P, Oldham WG, Haller EE (2000) J Non-Cryst Solids 269:61
ElBatal HA, Ezz ElDin FM (2001) Arab J Nucl Sci Appl 340:77
Baccaro S, Monika SG, Third KS, Singler DP, Cecilli A (2007) Nucl Instr Meth Phys Res (B) 260:316
Kamitsos EI (2003) Phys Chem Glasses 44:79
Ardelean I, Cora S (2008) J Mater Sci Mater Electron 19:584
Pascuta P, Borodi G, Culea E (2009) J Mater Sci Mater Electron 20:360
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
ElBatal, F.H., Marzouk, M.A. & Abdel ghany, A.M. Gamma rays interaction with bismuth borate glasses doped by transition metal ions. J Mater Sci 46, 5140–5152 (2011). https://doi.org/10.1007/s10853-011-5445-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-011-5445-4