Abstract
Cationic amphiphilic polyester dendrimers possessing varied numbers of choline and alkyl chains on the periphery were synthesized and characterized. A combination of divergent and convergent synthetic routes was used to efficiently prepare the dendrimers. All of the amphiphiles bound DNA as determined by an ethidium bromide displacement assay, and the dendrimer that contained two choline and four alkyl chains exhibited the smallest charge ratio. Only this amphiphilic dendrimer formed a well-defined structure alone or with DNA in solution, while the other dendrimer compositions gave aggregates. Specifically, small vesicular structures of several 100 nanometers in diameter were observed with DNA, and this dendrimerplex exhibited the greatest transfection efficiency of the group. The results of this study highlight the important role that charge, hydrophobicity, size, and compaction ability play in binding and formation of DNA-dendrimer complexes and the resulting transfection efficiency.
Graphical Abstract
Cationic amphiphilic polyester dendrimers possessing different numbers of choline heads groups and myristic acid alkyl chains were synthesized and evaluated for gene transfection.

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The synthesis of new amphiphiles and the use of such amphiphiles to assemble supramolecular structures of various dimensions and functions are of interest for many fields, including chemistry, materials science, and biomedical engineering. In particular, the application of cationic amphiphiles to improve nucleic acid delivery efficiency to cells holds significant promise for scientific and medical breakthroughs from gene replacement to gene editing [1,2,3,4,5,6,7,8,9,10,11]. The development of highly effective synthetic delivery vectors depends upon the identification of molecules or macromolecules that bind nucleic acids (DNA, mRNA, siRNA, etc.), form supramolecular assemblies of nanometer size, and transport these assemblies into the cell for subsequent biological activity. Identified molecules and macromolecules include cationic lipids,[9, 12,13,14,15] cationic linear polymers,[16,17,18,19] and cationic dendrimers [20,21,22,23,24]. Amphiphilic synthetic vectors have been found to at least partially full-fill several requirements, including DNA complexation, cellular uptake, biocompatibility, endosomal escape, nuclear tropism, and vector unpacking via design and formulation. These efforts complement on-going work on non-ionic and intercalation strategies for complexing and delivering DNA [25,26,27,28]. Incorporating some of the most promising aspects from previous vector systems, we have prepared a family of cationic surface-block polyester dendrimers as shown in Fig. 1 and evaluated their efficiency as gene transfection agents. Specifically, we describe the: (1) synthesis of four new cationic amphiphilic polyester dendrimers with varying numbers of choline head groups and alkyl chains; (2) the physiochemical characterization of the resulting self-assembled structures in the presence and absence of DNA; and (3) transfections experiments using the reporter gene, β-galactosidase, as a model system.
The motivation for performing these studies is multi-fold. First, the synthesis of a series of new amphiphilic polyester dendrimer will increase our knowledge on these types of structures and if these dendrimers self-assemble into supramolecular structures by themselves or in the presence of nucleic acid. Second, the efficiency of DNA/RNA delivery to cells has only incrementally improved over the last 10 years using synthetic carrier or vectors. Thus, there are significant research opportunities to identify new carriers and to determine the chemical compositions and structures that are most active. Finally, successful development of a highly effective synthetic delivery vector will be of interest for a wide range of research and clinical applications, for example, to deliver the CRISPR/CAS9 for gene editing or an anti-micro RNA to treat cancer.
Dendritic macromolecules are increasingly being explored for biomedical applications,[22, 29,30,31,32,33,34,35,36] and we selected this architecture for three distinct reasons. First, the synthetic methods (divergent and convergent) [37,38,39,40,41] to prepare such macromolecules allow systematic variation of generation number as well as the number and position of functional groups with a high degree of precision. Second, the step-growth polymerization process used ensures a monodisperse system, aiding in understanding of the subsequent reactivity and properties compared to linear polymers. Third, dendrimers display a high number of end groups capable of facilitating a concerted, multifaceted interaction with DNA. Previous dendrimers investigated for gene delivery primarily focused on symmetric, cationic dendrimers [20, 21] such as poly(amidoamine),[42,43,44] poly(glycoamidoamine) [45], poly(phosphorus),[46] or poly(lysine) [47] with a few elegant examples of asymmetric amphiphilic dendrimers from the laboratories of Diederich [48] and Kono [49, 50]. For a comprehensive review of dendrimers used for gene delivery, several excellent reviews have been published [20, 22, 31, 51].
2 Experimental
2.1 Instrumentation
All solvents were dried and freshly distilled prior to use (DCM and pyridine with CaH, and THF with Na). All chemicals were purchased from Aldrich or Acros as highest purity grade and used without further purification. All reactions were performed under nitrogen atmosphere at room temperature, unless specified otherwise. NMR spectra were recorded on a Varian INOVA spectrometer (for 1H and 13C NMR, 400 and 100.6 MHz respectively). MALDI-TOF mass spectra were obtained using a PerSpective Biosystems Voyager-DE Biospectrometry Workstation operating in the positive ion mode using 2-(4-hydroxyphenylazo)-benzoic acid (HABA). Elemental analysis was obtained from Atlantic Microlab, Inc. Fluorescence spectra were recorded on a PTI Fluorimeter. Thermal transition temperatures were measured using a TA Instruments DSCQ100 modulated differential scanning calorimeter.
Abbreviations: EDCI = 1-(3-Dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride, EtOAc = ethyl acetate, DCC = dicyclohexylcarbodiimide, DCM = dichloromethane, DCU = 1,3-dicyclohexylurea, DMAP = 4-(dimethylamino)pyridine, DPTS = 4-(dimethylamino)-pyridinium p-toluenesulfonate, Pd/C = 10% palladium on activated carbon, Pd(OH)2/C = 20% palladium hydroxide on activated carbon, THF = tetrahydrofuran. DMF = Dimethylformamide.
2.2 Synthesis of the Cationic Amphiphilic Polyester Dendrimers
The synthetic procedures for the dendrimers are described below with the NMR spectra shown in the supporting information.
2.2.1 Synthesis of Dendrimer 1
2.2.1.1 Compound 6; (Scheme 1)
Compound 5 (1,3-di-O-tetradecanoylglycerol) was prepared according to the literature [52]. Compound 5 (2 g, 3.9 mmol) was dissolved in pyridine (20 mL) followed by the addition of succinic anhydride (0.46 g, 4.7 mmol). The mixture was stirred for 18 h before the pyridine was removed under vacuum at 40 °C. The residue was recrystallized in ethanol to afford compound 6 in 92% yield. 1H NMR (CDCl3): δ ppm 4.15 (m, 4H), 3.35 (t, 4H), 1.62 (t, 4H), 1.25 (m, 40H), 0.88 (t, 6H). 13C NMR (CDCl3): δ 177.18, 173.53, 171.38, 170.85, 69.89, 62.11, 34.22, 32.22, 31.49, 29.86, 29.69, 29.56, 29.47, 29.32, 29.06, 28.91, 28.59, 25.06, 22.89, 14.31. MS (FAB+): 635.45 (MNa+) (theory: 635.44). HRMS calcd. for [C35H64O8Na]+ 635.4499, found 635.4534.
2.2.1.2 Compound 8; (Scheme 1)
cis-1,3-O-Benzylidene glycerol, 7, was prepared according to the literature [53]. Compound 6 (1 g, 1.6 mmol) was dissolved in 30 mL of DCM containing compound 7 (0.34 g, 1.9 mmol), DMAP cat., and DCC (0.41 g, 2.0 mmol). The reaction was stirred for 24 h. The solution was filtered, concentrated under reduced pressure and purified by column chromatography (20% EtOAc in hexanes) to afford compound 8 in 72% yield. 1H NMR (CDCl3) δ ppm: 7.51 (m, 2H), 7.37 (m, 3H), 5.56 (s, 1H), 4.73 (s, 1H), 4.27 (m, 9H), 2.77 (m, 4H), 2.31 (m, 4H), 1.60 (t, 4H), 1.25 (m, 40H), 0.87 (t, 6H). 13C NMR (CDCl3) δ ppm: 173.56, 172.35, 171.56, 137.92, 129.20, 128.126, 126.15, 101.21, 69.54, 69.01, 66.33, 61.94, 33.99, 31.92, 29.66, 29.48, 29.33, 29.27, 29.11, 28.97, 24.81, 22.69, 14.13. MS (FAB+): 797.52 (MNa+) (theory: 797.51). HRMS calcd. for [C45H74O10Na]+ 797.5180, found 797.5174.
2.2.1.3 Compound 9; (Scheme 1)
Compound 8 (0.50 g, 0.64 mmol) was dissolved in THF (20 mL) and 10% Pd/C (0.1 g) was added. The solution was then placed in a Parr tube on a hydrogenator and shaken under 60 psi of H2 for 12 h. The solution was then filtered over wet Celite, concentrated, and placed on a high vacuum line to afford compound 9 in 97% yield. 1H NMR (CDCl3) δ ppm: 5.25 (m, 1H), 4.91 (m, 1H), 4.20 (m, 5H), 3.77 (m, 4H), 2.61 (m, 4H), 2.25 (m, 4H), 1.53 (t, 4H), 1.21 (m, 40H), 0.80 (t, 6H). 13C NMR (CDCl3) δ ppm: 173.77, 70.28, 70.01, 65.94, 63.46, 62.56, 62.08, 34.29, 32.19, 29.92, 29.89, 29.75, 29.63, 29.54, 29.38, 26.45, 25.11, 22.97, 14.38. MS (FAB+): 709.48 (MNa+) (theory: 709.48). HRMS calcd. for [C38H70O10Na]+ 709.48667, found 709.4839.
2.2.1.4 Compound 10
The N-dimethylethanolamine (10 g, 0.11 mol) was dissolved in pyridine (100 mL) followed by the addition of succinic anhydride (16 g, 0.16 mol). The mixture was stirred at room temperature for 18 h before the pyridine was removed under vacuum at 40 °C. The remaining solid was recrystallized from MeOH/Ether to afford compound 10 in 90% yield. 1H NMR (CDCl3) δ ppm: 4.44 (m, 2H), 3.36 (m, 2H), 2.86 (s, 6H), 2.55 (m, 4H); 13C NMR (CDCl3) δ ppm: 178.18, 173.13, 58.03, 56.20, 42.61, 31.31, 30.09. MS (FAB +): 190.10 (MH+) (theory: 190.10). HRMS calcd. for [C8H15NO4]+ 190.1079, found 190.1084.
2.2.1.5 Compound 1; (Scheme 1)
Compound 10 (0.2 g, 1.1 mmol), compound 9 (0.35 g, 0.51 mmol), and DMAP (catalytic amount) were dissolved in DMF. EDCI (0.23 g, 1.2 mmol) was added to the mixture and the reaction was stirred for 24 h. Upon completion of the reaction the solvent was evaporated. The crude mixture was purified by silica gel chromatography, eluting with 20% EtOAc in hexanes to afford the product in 80% yield. 1H NMR (CDCl3) δ ppm: 5.25 (m, 2H), 4.20 (m, 12H), 3.69 (m, 4H), 2.65 (m, 16H), 2.37–2.31 (m, 12H), 1.86 (m, 4H), 1.60 (m, 4H), 1.25 (m, 40H), 0.88 (t, 6H). 13C NMR (CDCl3) δ ppm: 173.51, 172.41, 172.04, 172.01, 171.81, 171.69, 171.50, 69.64, 69.40, 62.53, 62.44, 62.36, 62.33, 61.92, 45.70, 34.01, 31.94, 29.69, 29.66, 29.63, 29.49, 29.37, 29.28, 29.13, 29.05, 29.01, 28.93, 28.85, 28.75, 28.70, 24.84, 22.69, 14.11. MS (MALDI): 1027.97 m/z (M-H+) [theory: 1028.68 m/z (M+)]. HRMS calcd. for [C54H97N2O16]+ 1029.6838, found 1029.6893.
The resultig product (0.1 g, 0.09 mmol) was dissolved in CH2Cl2 and methyl iodide was added (0.12 g, 0.9 mmol). The reaction mixture was stirred for 8 h. The solvent was evaporated to afford compound 1 in quantitative yield. 1H NMR (CDCl3) δ ppm: 5.25 (m, 2H), 4.63 (m, 2H), 4.31–4.14 (m, 12H), 3.69 (m, 6H), 3.51 (m, 18H), 2.65 (m, 12H), 2.32 (m, 4H), 1.60 (m, 4H), 1.25 (m, 40H), 0.88 (t, 6H). 13C NMR (CDCl3) δ ppm: 172.73, 171.98, 171.23, 171.03, 170.72, 69.09, 68.73, 61.76, 61.35, 51.34, 33.50, 32.07, 31.41, 30.26, 29.14, 28.98, 28.85, 28.84, 28.76, 28.62, 28.39, 28.26, 24.36, 22.18, 13.61. Repeated attempts to obtain the mass spectrum of the quaternized dendrimer were unsuccessful.
2.2.2 Synthesis of Dendrimer 2
2.2.2.1 Compound 12; (Scheme 2)
Synthesis of dendrimer 2. Reagents and conditions: a myristic acid, DCC, DCM, DTPS, RT, 16 h, 89% yield; b TBAF, THF, 1 h, RT, 87% yield; c DCC, DPTS, DCM, RT, 24 h, 92% yield; d 10% Pd/C, 60 psi. H2, THF, RT, 16 h, 98% yield; e DMAP, EDCI, DMF, RT, 24 h, 81% yield; f DCM, RT, 8 h, quantitative yield
Compound 11 was prepared following a published protocol [54]. Compound 11 (0.45 g, 0.58 mmol) was then dissolved in 75 mL of CH2Cl2 with 0.63 g (2.77 mmol) of myristic acid, 0.34 g (1.16 mmol) of DPTS, and 0.72 g (3.47 mmol) of DCC. The reaction was stirred for 16 h. Next, the DCU precipitate was filtered and the solution was evaporated. The residue was re-suspended in 50 mL of ethanol, cooled to 0 °C for 6 h and filtered. The precipitate was re-suspended in 75 mL of CH2Cl2, washed with 75 mL of H2O, dried over Na2SO4, and the solvent evaporated to yield 0.84 g of product (89% yield). 1H NMR (CDCl3): δ ppm: 0.80–0.89 (t, 12H, –CH 3), 1.08 (s, 9H, t-butyl), 1.14–1.34 (m, 80H, myristic –CH 2–), 1.50–1.64 (m, 8H, C(=O)–CH2–CH 2–CH2–), 2.22–2.33 (t, 8H, C(=O)–CH 2–CH2–), 2.53–2.83 (m, 12H, succinic –CH 2–CH 2), 4.08–4.34 (m, 12H, –CH 2–CH–CH 2–), 5.18–5.30 (m, 3H, –CH2–CH–CH2–), 7.32–7.44, 7.61–7.67 (m, 10H, phenyl CH) ppm. 13C NMR (CDCl3): δ ppm 14.25, 22.67, 24.81, 26.85, 28.81, 28.79, 29.12, 29.24, 29.36, 29.53, 29.64, 31.97, 34.05, 61.88, 62.34, 69.17, 127.66, 130.13, 135.28, 138.77, 171.34, 171.69, 173.32 ppm. FAB-MS: 1620.1 m/z (MH+) [theory: 1620.29 m/z (M+)]. Elemental analysis: C, 68.84%; H, 9.69% (theory: C, 68.94%; H, 9.58%).
The product (0.81 g, 0.50 mmol) was then dissolved in 100 mL of THF. Next, 0.55 g (1.75 mmol) of tetrabutylammonium fluoride trihydrate was added to the solution. The mixture was stirred for 1 h. After 1 hour the reaction was complete as indicated by TLC. The solution was diluted with 25 mL of H2O and acidified with 1N HCl to a pH of 3. The product was extracted into EtOAc, dried over Na2SO4, rotoevaporated and dried on the vacuum line. The product, 12, was purified by column chromatography (0–3% MeOH in CH2Cl2) to afford 0.60 g of product (87% yield). Rf = 0.23 (3% MeOH in CH2Cl2). 1H NMR (CDCl3): δ ppm 0.82–0.88 (t, 12H, –CH 3), 1.20–1.31 (m, 80H, myristic –CH 2–), 1.53–1.64 (m, 8H, –C(=O)–CH2–CH 2–CH2–), 2.26–2.33 (t, 8H, –C(=O)–CH 2–CH2–), 2.60–2.68 (m, 12H, –CH 2–CH 2–), 4.11–4.34 (m, 12H, –CH 2–CH–CH 2–), 5.19–5.35 (m, 3H, –CH2–CH–CH2–) ppm. 13C NMR (CDCl3): δ ppm 14.16, 22.78, 24.98, 28.56, 28.87, 29.07, 29.24, 29.47, 29.63, 29.87, 32.01, 34.04, 62.02, 62.64, 69.16, 69.93, 171.47, 171.68, 173.51 ppm. FAB-MS: 1382.9 m/z (M-H+) [theory: 1381.9 m/z (M+)]. Elemental analysis: C, 66.72%; H, 9.91% (theory: C, 66.92%; H, 9.92%).
2.2.2.2 Compound 13 (Scheme 2)
Compound 12 (0.71 g, 0.52 mmol) was dissolved in 50 mL of CH2Cl2 with 0.11 g (0.62 mmol) of cis-1,3-O-benzylidene glycerol, 7, 0.08 g (0.26 mmol) of DPTS, and 0.16 g (0.78 mmol) of DCC. The reaction was stirred for 48 h. The DCU precipitate was filtered and the solution was evaporated. The residue was re-suspended in a minimum of EtOH and then cooled to 0 °C for 24 h. The resulting precipitate was collected via suction filtration and placed under vacuum to afford 0.73 g of compound 13 (92% yield). 1H NMR (CDCl3): δ ppm 0.81–0.92 (t, 12H, –CH 3), 1.21–1.38 (m, 80H, myristic –CH 2–), 1.51–1.69 (m, 8H, C(=O)–CH2–CH 2–CH2–), 2.24–2.35 (t, 8H, C(=O)–CH 2–CH2–), 2.58–2.80 (m, 12H, succinic –CH 2–CH 2), 4.06–4.35 (m, 16H, –CH 2–CH–CH 2–), 4.72 (s, 1H, –CH2–CH–CH2–), 5.18–5.32 (m, 3H, –CH2–CH–CH2–), 5.54 (s, 1H, CH), 7.30–7.41 (m, 3H, arom. CH), 7.45–7.51 (m, 2H, arom. CH) ppm. 13C NMR (CDCl3): δ ppm 14.00, 22.57, 24.73, 24.85, 28.56, 28.72, 28.88, 29.03, 29.18, 29.26, 29.38, 29.53, 29.56, 29.59, 31.83, 33.91, 61.79, 62.36, 66.31, 68.95, 69.53, 72.27, 101.163, 125.91, 128.31, 128.36, 129.14, 137.84, 171.40, 171.51, 171.71, 172.17, 173.40 ppm. MALDI-MS: 1566.13 m/z (M + Na+) [theory: 1544.08 m/z (M+)]. Elemental analysis: C, 67.55%; H, 9.73% (theory: C, 67.67%; H, 9.53%).
2.2.2.3 Compound 14 (Scheme 2)
Compound 13 (0.71 g, 0.46 mmol) was dissolved in THF (30 mL) and 10% Pd/C (0.7 g) was added. The solution was then placed in a Parr tube on a hydrogenator and shaken under 60 psi. H2 for 16 h. The solution was then filtered over wet Celite, concentrated, and placed on a high vacuum line to yield 0.65 g of compound 14 (98% yield). 1H NMR (CDCl3): δ ppm 0.81–0.89 (t, 12H, –CH 3), 1.17–1.35 (m, 80H, alkyl –CH 2–), 1.52–1.63 (m, 8H, C(=O)–CH2–CH 2–CH2–), 2.26–2.33 (t, 8H, C(=O)–CH 2–CH2–), 2.58–2.71 (m, 12H, succinic –CH 2–CH 2), 3.70–3.88 (m, 4H, –CH 2–CH–CH 2–), 4.08–4.38 (m, 12H, –CH 2–CH–CH 2–), 4.90–4.97 (q, 1H, –CH2–CH–CH2–), 5.17–5.30 (m, 3H, –CH2–CH–CH2–) ppm. 13C NMR (CDCl3): δ ppm 14.00, 22.59, 24.74, 28.59, 28.74, 29.03, 29.18, 29.26, 29.39, 29.57, 30.23, 31.83, 33.93, 61.79, 62.13, 62.34, 69.59, 75.72, 171.65, 171.81, 173.52 ppm. MALDI-MS: 1479.69 m/z (M + Na+) [theory: 1455.97 m/z (M+)]. Elemental analysis: C, 66.18%; H, 9.86% (theory: C, 65.99%; H, 9.83%).
2.2.2.4 Compound 2; (Scheme 2)
Compound 10 (0.13 g, 0.46 mmol), compound 14 (0.3 g, 0.21 mmol), and DMAP (catalytic amount) were dissolved in DMF. EDCI (0.09 g, 0.46 mmol) was added to the mixture and the reaction was stirred for 16 h. Upon completion of the reaction, the solvent was evaporated. The crude mixture was purified through LH20, eluting with CH2Cl2/MeOH 1:1 to afford the product in 81% yield. 1H NMR (CDCl3) δ ppm: 5.22 (m, 4H), 4.33–4.14 (m, 20H), 3.71 (m, 10H), 2.66 (m, 24H), 2.37–2.20 (m, 8H), 1.61 (m, 8H), 1.26 (m, 80H), 0.88 (t, 12H). 13C NMR (CDCl3) δ ppm: 173.34, 171.89, 171.68, 171.38, 69.74, 69.48, 62.47, 61.98, 57.77, 45.67, 34.14, 32.06, 29.79, 29.63, 29.50, 29.41, 29.28, 29.00, 28.91, 28.84, 25.00, 22.83, 14.25. MS (MALDI): 1798.3 (M-H+) (theory: 1797.18 (M+)). HRMS calcd. for [C96H168N2O28Na]+ 1820.1681, found 1820.1469.
The resulting product (0.1 g, 0.05 mmol) was dissolved in CH2Cl2 and methyl iodide was added (0.07 g, 0.5 mmol). The reaction mixture was stirred for 12 h. The solvent was evaporated to afford compound 2 in quantitative yield. 1H NMR (CDCl3) δ ppm: 5.26 (m, 4H), 4.62 (m, 4H), 4.33–4.25 (m, 20H), 4.17–4.07 (m, 4H), 3.48 (m, 10H), 2.68 (m, 18H), 2.31 (t, 8H), 1.62 (m, 8H), 1.25 (m, 80H), 0.88 (t, 12H). 13C NMR (CDCl3) δ ppm: 173.33, 171.70, 171.39, 69.68, 69.40, 62.40, 61.89, 54.93, 34.11, 32.00, 29.75, 29.57, 29.44, 29.37, 29.22, 28.96, 28.82, 24.94, 22.77, 14.20. Repeated attempts to obtain the mass spectrum of the quaternized dendrimer were unsuccessful.
2.2.3 Synthesis of Dendrimer 3
2.2.3.1 Compound 16; (Scheme 3)
cis-1,3-O-Benzylidene glycerol succinic acid monoester, 15, was prepared according to the literature [55]. Compound 14 (0.5 g, 0.34 mmol) was dissolved in 50 mL of CH2Cl2 with 15 (0.14 g, 0.75 mmol), DPTS (0.09 g, 0.31 mmol), and DCC (0.19 g, 0.94 mmol). The reaction was stirred for 16 h. The DCU precipitate was filtered and the solution was evaporated. The residue was re-suspended in a minimum of EtOH and then cooled to 0 °C for 24 h. The resulting precipitate was collected via suction filtration and placed under vacuum to afford 0.437 g of product in 65% yield. 1H NMR (CDCl3) δ ppm: 7.42 (m, 5H), 7.29 (m, 5H), 5.49 (m, 2H), 5.18 (m, 4H), 4.16 (m, 28H), 2.61 (m, 24H), 2.24 (t, 8H), 1.55 (m, 8H), 1.18 (m, 80H), 0.80 (t, 12H). 13C NMR (CDCl3) δ ppm: 172.86, 171.79, 171.38, 171.07, 137.86, 128.76, 128.00, 125.89, 100.84, 69.46, 68.71, 66.22, 62.18, 61.65, 33.79, 31.77, 29.51, 29.32, 29.20, 28.72, 24.69, 22.54, 13.97. Elemental analysis: C, 65.28%; H, 8.49%; (theory: C, 65.50%; H, 8.65%).
2.2.3.2 Compound 17; (Scheme 3)
Compound 17 (0.35 g, 0.17 mmol) was dissolved in THF (30 mL) and 10% Pd/C (0.1 g) was added. The solution was then placed in a Parr tube on a hydrogenator and shaken under 60 psi of H2 for 16 h. The solution was then filtered over wet Celite, concentrated, and placed on a high vacuum line to yield 0.3 g of product in quantitative yield. 1H NMR (CDCl3) δ ppm: 5.35 (m, 2H), 5.05 (m, 4H), 4.33 (m, 28H), 2.76 (m, 24H), 2.41 (t, 8H), 1.70 (m, 8H), 1.52 (m, 8H), 1.35 (m, 80H), 0.97 (t, 12H). 13C NMR (CDCl3) δ ppm: 173.39, 172.38, 171.75, 171.56, 171.41, 125.56, 76.98, 76.80, 75.78, 69.72, 69.48, 62.44, 61.93, 34.11, 32.02, 30.43, 29.75, 29.57, 29.44, 29.36, 29.22, 28.95, 28.79, 24.94, 22.77, 14.20.
2.2.3.3 Compound 3; (Scheme 3)
Compound 17 (0.2 g, 0.11 mmol), compound 10 (0.07 g, 0.24 mmol), and DMAP (catalytic amount) were dissolved in DMF. EDCI (0.022 g, 0.11 mmol) was added to the mixture and the reaction was stirred for 24 h. Upon completion of the reaction, the solvent was evaporated. The crude mixture was purified through LH20, eluting with CH2Cl2/MeOH 1:1 to afford the product in 60% yield. 1H NMR (CDCl3) δ ppm: 5.27 (m, 6H), 4.32–4.13 (m, 32H), 2.67–2.62 (m, 40H), 2.34 (t, 8H), 1.60 (m, 8H), 1.25 (m, 80H), 0.88 (t, 12H). 13C NMR (CDCl3) δ ppm: 173.39, 172.38, 171.75, 171.56, 171.41, 125.56, 76.98, 76.80, 75.78, 69.72, 69.48, 62.44, 61.93, 61.73, 43.56, 34.11, 32.02, 30.43, 29.75, 29.57, 29.44, 29.36, 29.22, 28.95, 28.79, 24.94, 22.77, 14.20.
The resulting product (0.1 g, 0.04 mmol) was dissolved in CH2Cl2 and methyl iodide was added (0.056 g, 0.4 mmol). The reaction mixture was stirred for 12 h. The solvent was evaporated to afford compound 3 in quantitative yield. 1H NMR (CDCl3) δ ppm: 5.24 (m, 1H), 4.64 (m, 6H), 4.29–4.08 (m, 30H), 3.70 (m, 6H), 3.41 (m, 14H), 3.32 (m, 4H), 2.67 (m, 16H), 2.34 (t, 8H), 1.60 (m, 10H), 1.25 (m, 80H), 0.88 (t, 12H). 13C NMR (CDCl3) δ ppm: 173.69, 172.04, 171.70, 69.70, 69.44, 65.55, 62.22, 61.95, 54.97, 34.12, 32.04, 29.78, 29.60, 29.48, 29.40, 29.24, 28.92, 24.94, 22.81, 14.24. Repeated attempts to obtain the mass spectrum of the quaternized dendrimer were unsuccessful.
2.2.4 Synthesis of Dendrimer 4
2.2.4.1 Compound 19; (Scheme 4)
Synthesis of dendron 25. Reagents and conditions: a DMAP, EDCI, THF, RT, 24 h, 76% yield; b 10% Pd/C, 60 psi. H2, THF, RT, 16 h, 97% yield; c pyridine, 40 °C, 18 h, 46% yield; d DMAP, EDCI, THF, RT, 24 h, 80% yield; e 10% Pd/C, 60 psi. H2, THF, RT, 12 h, 97% yield; f pyridine, 40 °C, 18 h, 90% yield
Compound 10 (3.2 g, 16.9 mmol), compound 18 (1.4 g, 7.6 mmol), and DMAP (catalytic amount) were dissolved in THF. EDCI (3.3 g, 16.9 mmol) was added to the mixture and the reaction was stirred for 24 h. Upon completion of the reaction, the solvent was evaporated. The crude mixture was purified by silica gel chromatography, eluting with EtOAc to afford compound 20 in 76% yield. 1H NMR (CDCl3) δ ppm: 7.31 (m, 5H), 4.64 (s, 2H), 4.24 (m, 8 H), 3.83 (m, 1H), 2.60 (s, 6H), 2.58 (m, 12H). 13C NMR (CDCl3) δ ppm: 172.60, 172.24, 171.99, 137.78, 128.44, 127.88, 74.26, 72.10, 63.30, 62.31, 57.58, 51.87, 45.54, 28.96, 28.78. MS (FAB+): 525.29 (MH+) (theory: 525.28). HRMS calcd. for [C26H41N2O9]+ 525.2812, found 525.2891.
2.2.4.2 Compound 20; (Scheme 4, reaction ‘b’)
Compound 19 (2 g, 3.8 mmol) was dissolved in THF (20 mL) and 10% Pd/C (0.1 g) was added. The solution was then placed in a Parr tube on a hydrogenator and shaken under 60 psi of H2 for 12 h. The solution was then filtered over wet Celite, concentrated, and placed on a high vacuum line to afford compound 21 in 97% yield. 1H NMR (CD3OD) δ ppm: 4.52 (m, 1H), 4.14 (m, 8H), 2.85 (s, 12H), 2.69 (m, 12H). 13C NMR (CD3OD) δ ppm: 173.05, 172.59, 172.43, 172.38, 72.57, 67.56, 65.30, 62.67, 60.93, 52.01, 43.56, 43.34, 29.15, 29.02, 28.89 MS (FAB+): 435.24 (MH+) (theory: 435.23). HRMS calcd. for [C19H35N2O9]+ 435.2343, found 435.2392.
2.2.4.3 Compound 21; (Scheme 4)
Compound 20 (1.5 g, 3.4 mmol) was dissolved in pyridine (20 mL) followed by the addition of succinic anhydride (0.4 g, 4.1 mmol). The mixture was stirred for 18 h before the pyridine was removed under vacuum at 40 °C. The residue was purified by silica gel chromatography, eluting with EtOAc to afford compound 22 in 46% yield. 1H NMR (CD3OD) δ ppm: 5.31 (m, 1H), 4.25 (m, 8H), 2.27–2.71 (m, 28H). 13C NMR (CD3OD) δ ppm: 175.55, 173.42, 172.81, 172.66, 172.48, 172.28, 69.29, 66.89, 64.99, 62.17, 60.70, 51.97, 51.50, 42.82, 28.83, 28.63. MS (FAB+): 535.25 (MH+) (theory: 535.25). HRMS calcd. for [C19H35N2O9]+ 535.2503, found 535.2520.
2.2.4.4 Compound 22; (Scheme 4)
Compound 21 (0.7 g, 1.3 mmol), compound 18 (0.1 g, 0.6 mmol), and DMAP (catalytic amount) were dissolved in DMF. EDCI (0.26 g, 1.3 mmol) was added to the mixture and the reaction was stirred for 24 h. Upon completion of the reaction, the solvent was evaporated. The crude mixture was purified by silica gel chromatography, eluting with EtOAc to afford compound 22 in 80% yield. 1H NMR (CDCl3) δ ppm: 7.31 (m, 5H), 5.28 (m, 2H), 4.64 (s, 2H), 4.24 (m, 20H), 3.83 (m, 1H), 2.60 (m, 56H). 13C NMR (CDCl3) δ ppm: 172.79, 172.15, 171.98, 171.65, 135.89, 128.33, 125.62, 69.42, 67.65, 65.56, 62.40, 52.07, 45.65, 34.0, 30.41, 29.08, 28.96, 28.83, 25.71. MS (FAB+): 1015.54 (MH+) (theory: 1015.53). HRMS calcd. for [C48H79N4O19]+ 1015.5339, found 1015.5364.
2.2.4.5 Compound 23; (Scheme 4)
Compound 22 (0.45 g, 0.37 mmol) was dissolved in THF (20 mL) and 10% Pd/C (0.1 g) was added. The solution was then placed in a Parr tube on a hydrogenator and shaken under 60 psi of H2 for 12 h. The solution was then filtered over wet Celite, concentrated, and placed on a high vacuum line to afford compound 23 in 97% yield. 1H NMR (CD3OD) δ ppm: 4.52 (m, 1H), 4.14 (m, 8H), 2.85 (s, 12H), 2.69 (m, 12H). 13C NMR (CD3OD) δ ppm: 172.75, 171.97, 69.23, 68.01, 67.63, 65.56, 62.42, 57.65, 52.06, 45.67, 34.31, 29.00, 28.97, 28.74, 25.75. MS (FAB+): 925.49. HRMS calcd. for [C41H73N4O19]+ 925.4809, found 925.4906.
2.2.4.6 Compound 24; (Scheme 4)
Compound 23 (0.3 g, 0.27 mmol) was dissolved in pyridine (20 mL) followed by the addition of succinic anhydride (0.032 g, 0.32 mmol). The mixture was stirred for 18 h before the pyridine was removed under vacuum at 40 °C. The residue was purified by silica gel chromatography, eluting with EtOAc to afford compound 24 in 90% yield. 1H NMR (CDCl3) δ ppm: 5.31 (m, 3H), 4.30 (m, 16H), 2.58–2.67 (m, 46H). 13C NMR (CDCl3) δ ppm: 176.91, 173.02, 172.16, 69.33, 67.02, 65.25, 62.30, 59.49, 55.83, 51.88, 51.77, 43.01, 29.57, 29.02, 28.94, 28.78, 28.67. MS (FAB+): 1225.52 (MH+) (theory: 1225.50). HRMS calcd. for [C45H77N4O19]+ 1225.5030, found 1225.5272.
2.2.4.7 Compound 26; (Scheme 5)
Compound 25 was prepared according to the literature [56]. Compound 13 (0.80 g, 0.58 mmol) was dissolved in 75 mL of CH2Cl2 with compound 27 (0.10 g, 0.24 mmol), DPTS (0.07 g, 0.24 mmol), and DCC (0.15 g, 0.72 mmol). The reaction was stirred for 24 h. The DCU precipitate was filtered and the solution was evaporated. The residue was purified by column chromatography, eluting with 35% EtOAc in hexanes to afford compound 26 in 58% yield. 1H NMR (CDCl3) δ ppm: 7.64 (m, 3Η), 7.36 ((m, 6H), 5.23 (m, 7H), 4.20 (m, 28H), 2.60 (m, 28H), 2.29 (t, 16H), 1.58 (m, 16H), 1.25 (m, 160H), 1.07 (s, 9H), 0.85 (t, 24H) ppm. 13C NMR (CDCl3) δ ppm: 173.41, 171.74, 171.43, 140.08, 135.34, 130.16, 127.79, 69.54, 69.20, 62.30, 61.79, 53.38, 33.92, 31.84, 29.60, 29.57, 29.55, 29.40, 29.27, 29.19, 29.04, 28.71, 28.54, 26.75, 24.74, 22.59, 14.02 ppm. MS (MALDI): 3181.32 (M + Na+) (theory: 3158.32 (M+)). Elemental analysis: C, 67.39%; H, 9.50% (theory: C, 67.31%; H, 9.51%).
2.2.4.8 Compound 27; (Scheme 5)
Compound 26 (0.37 g, 0.12 mmol) was dissolved in 50 mL of THF. Next, 0.11 g (0.41 mmol) of tetrabutylammonium fluoride trihydrate was added to the solution. The mixture was stirred for 1 h, until the reaction was complete as indicated by TLC. The solution was diluted with 10 mL of H2O and acidified with 1N HCl to a pH of 3. The product was extracted into DCM, dried over Na2SO4, and rotoevaporated to dryness. The resulting residue was dissolved in ethanol and placed in a freezer at -20 °C. The precipitate was isolated by suction filtration to afford compound 27 in 53% yield. 1H NMR (CDCl3) δ ppm: 5.24 (m, 7H), 4.20 (m, 28H), 2.62 (bs, 28H), 2.29 (t, 16H), 1.57 (m, 16H), 1.24 (m, 160H), 0.85 (t, 24H) ppm. 13C NMR (CDCl3) δ ppm: 173.48, 171.86, 69.57, 62.34, 61.80, 33.93, 31.84, 29.57, 29.40, 29.27, 29.19, 29.04, 28.73, 28.56, 24.74, 22.59, 14.02 ppm. MS (MALDI): 2942.90 (M + Na+) (theory: 2919.92 m/z (M+)). Elemental analysis: C, 65.98%; H, 9.61% (theory: C, 66.23%; H, 9.67%).
2.2.4.9 Compound 28; (Scheme 5)
Compound 27 (0.14 g, 0.05 mmol) was dissolved in 20 mL of CH2Cl2 with compound 7 (0.01 g, 0.06 mmol), DPTS (0.01 g, 0.04 mmol), and DCC (0.02 g, 0.07 mmol). The reaction was stirred for 4 days. The DCU precipitate was filtered and the solution was evaporated. The residue was re-suspended in DCM, filtered, and precipitated in cold ethanol to afford compound 28 in 83% yield. 1H NMR (CDCl3) δ ppm: 7.48 (m, 2H), 7.34 (m, 3H), 5.54 (s, 1H), 5.23 (m, 7H), 4.72 (s, 1H), 4.21 (m, 32H), 2.69 (m, 28H), 2.30 (t, 16H), 1.59 (m, 16H), 1.25 (m, 160H), 0.86 (t, 24H) ppm. 13C NMR (CDCl3) δ ppm: 173.30, 171.63, 171.31, 129.04, 128.26, 125.97, 101.11, 69.51, 69.22, 68.97, 66.55, 62.33, 61.79, 33.97, 31.90, 29.67, 29.64, 29.61, 29.47, 29.35, 29.26, 29.10, 28.76, 28.59, 24.81, 22.68, 14.12 ppm. MS (MALDI): 3105.10 m/z (M + Na+) [theory: 3082.10 m/z (M+)].
2.2.4.10 Compound 29; (Scheme 5)
Compound 28 (0.12 g, 0.04 mmol) was dissolved in THF (20 mL) and 10% Pd/C (0.1 g) was added. The solution was then placed in a Parr tube on a hydrogenator and shaken under 60 psi of H2 for 16 h. The solution was then filtered over wet Celite, concentrated, and placed on a high vacuum line to yield 0.11 g of product (95% yield). 1H NMR (CDCl3) δ ppm: 5.23 (m, 7H), 4.21 (m, 32H), 3.78 (m, 1H), 2.62 (m, 28H), 2.28 (t, 16H), 1.57 (m, 16H), 1.24 (m, 160H), 0.85 (t, 24H) ppm. 13C NMR (CDCl3) δ ppm: 173.34, 171.68, 171.36, 69.53, 69.25, 62.36, 61.79, 33.97, 31.90, 29.67, 29.64, 29.47, 29.35, 29.26, 29.10, 28.76, 28.61, 24.81, 22.68, 14.12 ppm. MS (MALDI): 3018.77 (M + Na+) [theory: 2994.00 (M+)].
2.2.4.11 Compound 30; (Scheme 6)
Compound 24 (0.023 g, 18.4 µmol), compound 29 (0.025 g, 8.4 µmol), and DMAP in a catalytic amount were dissolved in DMF. EDCI (1 mg, 18.4 µmol) was added to the mixture and the reaction was stirred for 24 h. Upon completion of the reaction, the solvent was evaporated. The crude mixture was purified by LH-20 chromatography, eluting with DCM/MeOH 1:1 to afford compound 30 in 41% yield. 1H NMR (CDCl3) δ ppm: 5.30 (m, 15H), 4.31–4.14 (m, 64H), 3.70 (m, 10H), 3.32 (m, 22H), 3.21 (m, 32H), 3.05 (m, 22H), 2.74 (m, 116H), 2.33 (m, 20H), 1.94 (m, 14H), 1.60 (m, 20H), 1.25 (m, 176H), 0.88 (m, 24H). 13C NMR (CDCl3) δ ppm: 173.45, 172.74, 171.99, 171.86, 171.54, 69.78, 69.52, 62.53, 62.46, 62.01, 57.90, 52.07, 45.84, 34.19, 32.11, 29.87, 29.84, 29.67, 29.54, 29.47, 29.32, 29.08, 29.03, 28.95, 28.87, 25.03, 22.88, 14.30.
2.2.4.12 Compound 4; (Scheme 6)
Compound 30 (0.02 g, 3 µmol) was dissolved in CH2Cl2 and methyl iodide was added (0.04 g, 0.3 mmol). The reaction mixture was stirred for 3 h. The solvent was evaporated to afford compound 4 in quantitative yield. 1H NMR (CDCl3) δ ppm: 5.25 (m, 24H), 4.28–4.17 (m, 82 H), 3.71 (m, 27H), 3.35 (m, 30H), 3.26 (s, 70H), 3.17 (m, 30H), 2.87 (m, 16H), 2.68 (m, 60H), 2.31 (m, 20H), 2.02 (m, 20H), 1.62 (m, 20H), 1.25 (m, 200H), 0.88 (t, 24H). 13C NMR (CDCl3) δ ppm: 173.59, 172.15, 69.80, 69.52, 62.57, 62.04, 55.06, 52.24, 34.27, 32.19, 29.93, 29.76, 29.64, 29.55, 29.39, 29.02, 25.10, 22.97, 14.41. Repeated attempts to obtain the mass spectrum of the quaternized dendrimer were unsuccessful.
2.3 Liposome Preparation
Chloroform solutions of the amphiphiles were mixed and dried to a thin film by rotary evaporation. The residual solvent was removed under vacuum for 4 h. Dried lipids were dispersed with 100 mM Tris buffer, 100 mM NaCl, pH 7.4, and sonicated for 10 min. The concentration of each lipid was 3 mM in 1 mL.
2.4 Modulated Differential Scanning Calorimetry
0.5 mg of amphiphile in 5 µL of water was hermetically sealed in an aluminum pan. The modulation was set to ±1.00 °C every 40 s, and the pan was equilibrated at −15 °C. The temperature was increased at 0.5 °C/min to 70 °C where it has held for 2 min. The temperature was then reduced to −10 °C and held at this temperature for 2 min. This heating–cooling cycle was repeated two more times before the sample was held isothermal at −10 °C for 20 min. The data collected on the third cycle were analyzed.
2.5 DNA Binding Affinities
DNA binding studies were carried out by competitive displacement fluorometric assay using ethidium bromide. This assay involved the addition of aliquots of the compound to a 3 mL solution of EthBr (1.3 µM) and calfus thymus DNA (3 µM) in buffer (100 mM NaCl, 100 mM Tris, pH 7.4) with the decrease of fluorescence (λexc = 546 nm, λem = 600 nm; 1 cm path length glass cuvette, slit width 3 nm) recorded after 5 min of equilibrium time following each addition.
2.6 X-ray Diffraction
The liposomes were centrifugated to give a hydrated pellet of multilamellar bilayers. The pellet was transferred then to a sealed quartz-glass X-ray capillary which was mounted in a temperature controllable chamber on a point-focus collimator. A stationary anode Jerrel-Ash generator (Jerrel-Ash Div., Fisher Scientific Co., Waltham, MA) was used to produce Cu Kα X-radiation.4 Diffraction patterns were obtained using a flat plate film cassette loaded with Kodak DEF X-ray film. The specimen to film distance was 10 cm with exposure times of 2–6 h. The low angle reflections were determined in accordance with Bragg’s law 2d sinθ = hλ, where λ is the wavelength (1.54 Å), d is the repeat period, h is the number of the diffraction order, and θ is the Bragg angle.
2.7 Cell Culture and Gene Transfection Experiment
Chinese hamster ovarian cells (CHO, ATCC, Manassas, VA) were cultured in complete F12K media (ATCC) containing 10% fetal calf serum (Sigma) and 1% penicillin and streptomycin (500 IU/mL and 5000 µg/mL, respectively, Mediatech, Herndon, VA) at 37 °C in 5% CO2 with humidity. When the CHO cells reached about 90% confluence, they were split into 48-well plates with a 1:4 ratio using a standard trypsin-based technique. Transfections were performed 24 h later. Briefly, plasmid DNA coding for a reporter gene, β-galactosidase (β-gal, pSV-galactosidase control vector, Promega) was first mixed with lipids in potassium phosphate buffer (PBS) at room temperature. Depending on the experimental design, the ratio of DNA and amphiphile, the pH of the buffer used, and incubation time was varied. The mixture was incubated for 15 min at room temperature before adding to the cells. The amount of DNA used was the same as used in naked DNA control and positive control (commercially available transfection reagents). After incubation at 37 °C and 5% CO2 for 2 h, medium containing the mixtures was gently removed and fresh growth medium was added. Transfection efficiencies were assessed 24–48 h post transfection depending on the experimental design. Negative controls were constructed with 1.0 mL of serum-free F12 K medium and naked DNA controls were using 1.0 mL of serum-free F12 K medium with 10.0 µL (1 µg) of reporter gene. P ositive controls were performed according to the manufacturer’s protocol. Briefly stated, 2.0 µL of escort® transfection reagent (1 mg/mL) (Sigma) was mixed with 10.0 µL (1 µg) of reporter gene in 1.0 mL of serum free F12 K medium for 15 min at room temperature before transfecting cells.
2.7.1 Reporter Gene Transfection Efficiency Assay
Reporter gene (β-gal) assay was performed with a β-galactosidase enzyme assay system (Promega, Madison, WI) following the manufacturer protocol. Briefly cells were first lysed using M-PER buffer (Pierce, Rockford, Illinois) and enzyme activities were determined.A standard curve was constructed for each experiment using dilutions of purified β-gal protein.The β-gal activities from experimental samples were determined by comparison to the standard curve (enzyme activity vs. enzyme concentration). Efficiency of each transfection was calculated as β-gal activity normalized to total protein.
2.8 Cytotoxicity
Cytotoxicity was assessed using a formazan-based proliferation assay (CellTiter 96 AQueous One Solution Cell Proliferation Assay kit, Promega) and a total protein-based assay (Pierce). Briefly cells were seeded onto a 96-well microtiter plate with an appropriate density of 1 × 104 cells per well. A predetermined amount of test chemical was added onto the cells 24 h later. After 24, 48, or 72 h, MTS (substrate) was added to each well and the plate was incubated for 4 h at 37 °C in a humidified, 5% CO2 incubator. The amount of soluble formazan produced by cellular reduction of the substrates MTS was recorded at 490 nm using a multi-well plate reader. For the total protein-based proliferation assay, cells were lysed at the same time when transfection efficiency was assayed. A 5 µL of lysates were transferred to a separate multi-well plate. The total protein content was assessed using the Coomassie Blue protein kit (Pierce, Rockford, IL) following the manufacturer protocol. Negative and positive controls were non-treated cells and commercial lipid treated cells, respectively. The proliferation results were expressed as percentages of non-treated cells.
3 Results and Discussion
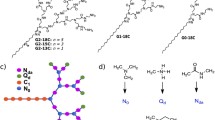
For this intended application, we established a set of five chemical design requirements to guide our studies. First, the building blocks used for the four amphiphilic surface-block dendritic macromolecules, shown in Fig. 1, are to be either natural metabolites or recognized as safe to minimize cytotoxicity. Thus, we selected glycerol, succinic acid, myristic acid, and choline. The Grinstaff group has published extensively on dendritic macromolecules composed of either natural metabolites or other monomers known to be non-toxic [54, 57,58,59,60,61]. These dendritic structures are biocompatible, with many of the compositions used in vivo for applications in wound repair,[57, 62,63,64,65] drug delivery,[66, 67] and cartilage tissue regeneration [68]. Second, to bind DNA a cationic charge was installed on the periphery of the dendrimer. The quaternized amine of choline group was chosen because it provides a cationic charge, which is unaffected by solution pH, for electrostatically complexing the negatively charged phosphate groups present in DNA. Third, to facilitate compaction with DNA an amphiphilic surface-block structure was used where myristic acid was linked to one-half of the periphery end groups. The 14 carbon chains of myristic acid provide hydrophobicity, an important feature known for compaction of the amphiphile/DNA structure and cellular uptake across the biological membrane. Fourth, the dendrimer structure should possess degradable linkages so that it can be a temporary scaffold for DNA complexation. Thus, the building blocks of the surface-block dendrimers were linked through ester bonds that are susceptible to degradation via hydrolysis or enzymatic cleavage. Finally the synthetic approach to prepare the four amphiphilic surface-block dendrimers relies on both multi-step divergent and convergent approaches to maximize efficiency, and subsequent use of various intermediate chemical structures.
These poly(glycerol–succinic acid) (PGLSA) dendrimers derivatives display different numbers of quaternized amines and alkyl chains on the periphery of the dendrimer (Fig. 1). Compound 1 has two alkyl chains of 14 carbons and two quaternary amines. It was synthesized in six reactions following a divergent route (Scheme 1). Compound 2 has two cationic charges and four alkyl chains, and was similarly prepared via a divergent approach in six reactions (Scheme 2). Dendrimer 3 has four quaternary amines and four alkyl chains and is synthesized in 8 reactions (Schemes 2, 3; compilation Scheme 7). Compound 4 has eight alkyl chains and eight quaternary amines. Its synthesis relied on using a combination of a divergent and convergent approach (Schemes 4, 5, 6). The two halves of dendrimer 4 are prepared separately in Schemes 4 and 5, respectively, and then coupled together to afford dendrimer 4.
Synthesis of dendritic amphiphile 3. Reactants and reagents: a myristic acid, DCC, DPTS, 89% yield; b TBAF, THF, 87% yield; c DCC, DPTS, DCM, 92% yield; d 10% Pd/C, H2, THF, quant. yield; e DCC, DPTS, THF, 60% yield; f 10% Pd/C, H2, THF, quant. yield; g DCC, DPTS, THF, 50% yield; h MeOH, quant. yield
The synthesis of dendrimer 3, as shown in Scheme 7, is discussed in detail as a representative example of the approach and methods used to prepare dendrimers 1 through 4. Myristic acid was coupled to 11 [56] in the presence of N,N-dicyclohexylcarbodiimide (DCC) and 4-(dimethylamino)pyridinium p-toluenesulfoxide (DPTS) in 89% yield followed by removal of the silyl protecting group with TBAF in THF in 87% yield. Compound 12 was then coupled to 7 using DCC and DTPS in 92% yield to give 13. Subsequent hydrogenolysis to cleave the benzylidene acetals of 13 afforded 14 in quantitative yield. Compound 14 was coupled with two equivalents of benzylidene glycerol succinic acid monoester, 15,[55] in the presence of DCC and DPTS to give compound 16 in 60% yield. Compound 16 was then subjected to hydrogenolysis conditions to cleave the benzylidene acetals and afford compound 17 in quantitative yield. Separately, dimethylethanolamine succinic acid monoester, 10, was prepared by reacting choline with succinic anhydride in the presence of pyridine in 90% yield. Next, compound 17 was coupled to 10 in the presence of DCC and DPTS in 50% yield. Finally, quaternization of the amines of the dendrimer with methyl iodide afforded compound 3 in quantitative yield. Key to the synthesis of all the dendrimers was the use of step-wise esterification and hydrogenolysis reactions.
Several experiments were performed to characterize the physical properties of this family of surface-block dendrimers, and the results are displayed in Table 1. Given the amphiphilic structures of these dendrimers, it seemed likely that these amphiphiles would form supramolecular structures in aqueous solution. The critical aggregation concentration (cac) for compounds 1–4 were measured using a pyrene probe [69] and determined to be 17, 13, 5, and 18 mM, respectively. Interestingly, these values are higher than those observed for carboxylic acid terminated surface-block dendrimers [56]. The milky aqueous suspensions of 1, 2, 3 or 4 were then extruded through a 100 nm polycarbonate membrane using a mini-extruder. All of the samples gave aggregates except for dendrimer 2. Dendrimer 2 formed supramolecular structures of 260 nm in diameter. When the dendrimers were extruded in the presence of DNA, large aggregates were observed with 1, 3, and 4, whereas 2 formed dendrimer/DNA assemblies of 280 nm in diameter. Differential scanning calorimeter (DSC) traces of the hydrated amphiphiles in aqueous solution showed a phase transition temperature of 32, 14, 23, and 14 °C for compounds 1–4, respectively. The structures of the assemblies formed by the dendrimers were next investigated by X-ray diffraction at 25 °C. The diffraction patterns of oriented multilayers of 1 in the presence or absence of DNA showed lamellar structures with d spacings of 6.3 nm. For dendrimer 3, we were unable to obtain a satisfactory pellet for analysis except in the presence of DNA where the d spacing was 5.5 nm. We were unable to obtain patterns from pellets of 2 and 4 with or without DNA.
To determine the interactions between the cationic amphiliphilic dendrimers and DNA, a standard ethidium bromide displacement fluorescence assay was performed. Ethidium bromide (EtBr) fluoresces intensely when intercalated within a DNA duplex. As shown in Fig. 2, the fluorescence intensity decreased rapidly upon addition of the cationic dendrimers corresponding to the formation of a dendrimer/DNA complex and displacement of EtBr. The binding curves for 1–4 indicated that a charge complex of approximately 6:1, 4:1, 10:1, 11:1 was formed between the dendrimers and the DNA, respectively. For compounds 1–4 the data suggested that the number of alkyl chains and charge of the dendrimers have an important influence on binding affinity. Compound 2 showed the lowest dendrimer/DNA charge ratio of 2:1. This may be a result of the larger alkyl chain to amine ratio (2:1) for compound 2 compared to the other dendrimers (1, 3, and 4 had an alkyl chain to amine ratio of 1:1).
Transfections experiments using the reporter gene, β-galactosidase (pSV-β galactosidase control gene, Promega) were performed next with Chinese hamster ovarian (CHO) cells. Gene transfection results were determined after 48 h as a function of both dendritic amphiphile and cation:anion ratio. As shown in Fig. 3, dendrimer 2 was the most effective synthetic vector. The transfection efficiency of dendrimer 2 was significantly greater than that of uncomplexed DNA, but less than that of the commercially available amphiphilic synthetic vectors Dotap or Escort. The transfection rates of dendrimers 1, 3, and 4 were significantly less than that of 2. Preparing formulations of the dendrimer 2 and DOPE with the DNA did not increase the transfection efficiency. Cytotoxity experiments performed with CHO cells and all of the cationic amphiphilic dendrimers showed no significant cytotoxicity as the treated samples were similar to non-treated samples.
Following the seminal paper on the use of dendrimers as gene transfection vehicles by Szoka, there has been intense activity in this area. These activities have centered around three major research efforts—dendrimer composition and structure, attachment of functionalities such as peptides and carbohydrates, and directed biological applications (e.g., plasmids, RNA, cell lines, in vivo studies). We will limit our discussion to the first, where synthesis and evaluation of gene transfection activity were the primary outcomes and have selected several representative publications for discussion. The forthcoming comparisons have several caveats as the cell lines and reporter genes (β-galactosidase vs. luciferase) used vary between the published reports, and thus an approximation is necessary to facilitate the discussion. Symmetrical PAMAM dendrimers of generation 0–10 have been explored by the groups of Szoka [42, 70], Tomalia and Baker [43, 44], Shirkoohi [71], Sadeghpour [72], and such dendrimers show high transfection activity. Transfection efficiency was dependent on generation number with larger sized dendrimers (e.g., G6+) performing better, DNA/cation charge ratio with an excess of PAMAM amines to the anionic phosphates of DNA affording greater activity, and structurally incomplete or fragmented dendrimers performing best. Significant transfection activity was observed with poly(phosphorus) based dendrimers reported by the Majoral group [46] and poly(lysine) dendrimers by the Okuda and Park groups [47]. Spermine-based dendritic structures, possessing a high density of amines, which tightly complex DNA have also been described [73]. Building upon these results, synthetic efforts were directed at preparing asymmetric dendrimers that possessed both cationic groups and hydrophophobic chains. These macromolecular amphiphiles mimic the structural characteristics of conventional gene transfection agents such as DOTAP and Lipofectamine. Such asymmetric amphiphilic dendrimers were first reported by Diederich [48] and Kono [49, 50]. In the study by Diederich, the amphiphilic surface-block dendrimers possessing a rigid diphenylethyne core and various primary amines and C12 chains showed activity of about twice that of Dotap. In comparison, amphiphile 2 has about only 50% of the activity of Dotap. Our results, in combination with others studying amphiphilic dendrimers, for gene delivery indicate that: (1) structures possessing both cations and alkyl chains complex DNA; (2) decreased aqueous solubility is observed when the number of alkyl chains surpasses the number of cationic charges; (3) the supramolecular structures with DNA are dependent on dendrimer composition with those structures possessing a smaller number of alkyl chains and cationic head groups forming discrete 100 s of nm structures as opposed to larger aggregates; (4) incorporation of a flexible core linker or increasing the length of the core linker decreases transfection efficacy, and amphiphiles possessing a relatively short and rigid core linker perform better; and (5) increased transfection activity is observed when the number of cationic head groups is two or three on the amphiphilic structure.
4 Conclusion
In summary, a series of poly(glycerol-succinate) amphiphilic dendrimers were synthesized and characterized that possess an outer layer of quaternary amines and alkyl chains. The approach described for synthesizing these dendrimers is applicable to the preparation of a variety of monodisperse macromolecules with a range of molecular weights, polarities, and surface groups. By varying the number of cationic charges and alkyl chains, it was possible to modify important structural features with respect to DNA binding and transfection. All four dendrimers bind DNA, and dendrimer 2 bound DNA with the smallest charge ratio. As the dendrimer generation increased, the charge ratio also increased. In general, these dendrimers did not form well-defined structures alone or with DNA in solution, and gave aggregates. Only dendrimer 2 gave small vesicular structures of several 100 nanometers with and without DNA. Dendrimer 2 was the best transfection agent consistent with its ability to bind DNA and form compact polyplexes, both of which facilitate the entry into the cell for subsequent transfection. From our studies, it is clear that charge, hydrophobicity, size, and compaction ability play important roles in binding and formation of DNA-dendrimer complexes and transfection efficiency. Continued research in this area will provide additional insights into the roles amphiphile composition and structure play in the optimization of effective synthetic vectors for the delivery of DNA, siRNA or oligonucleotides.
References
C.R. Safinya, K.K. Ewert, R.N. Majzoub, C. Leal, Cationic liposome-nucleic acid complexes for gene delivery and gene silencing. New J. Chem. 38, 5164–5172 (2014)
M. Collins, A. Thrasher, Gene therapy: progress and predictions. Proc. R. Soc. B 282, 20143003 (2015)
L. Naldini, Gene therapy returns to centre stage. Nature 526, 351 (2015)
D. Luo, W.M. Saltzman, Synthetic DNA delivery systems. Nat. Biotechnol. 18, 33–37 (2000)
A.V. Kabanov, P.L. Felgner, L.W. Seymour, Self-assembling complexes for gene delivery: From laboratory to clinical trial. (Willey, New York, 1998)
D. Putnam, Polymers for gene delivery across length scales. Nat. Mater. 5, 439–451 (2006)
J.P. Behr, Gene transfer with synthetic cationic amphiphiles: prospects for gene therapy. Bioconj. Chem. 5, 382–389 (1994)
W. Yan, L. Huang, Recent advances in liposome-based nanoparticles for antigen delivery. Polym. Rev. 47, 329–344 (2007)
P.P. Karmali, A. Chaudhuri, Cationic Liposomes as non-viral carriers of gene medicines: resolved issues, open questions, and future promises. Med. Res. Rev. 27, 696–722 (2007)
T.M. Reineke, M.W. Grinstaff, Designer materials for nucleic acid delivery. Mat. Res. Soc. Bull. 30, 635–639 (2005)
M.A. Mintzer, E.E. Simanek, Nonviral vectors for gene delivery. Chem. Rev. 109, 259–302 (2009)
B. Martin, M. Sainlos, A. Aissaoui, N. Oudrhiri, M. Hauchecorne, J.P. Vigneron, J.M. Lehn, P. Lehn, The design of cationic lipids for gene delivery. Curr. Pharm. Des. 11, 375–394 (2005)
A.D. Miller, Cationic liposomes for gene therapy. Angew. Chem. Int. 37, 1768–1785 (1998)
C.M. LaManna, H. Lusic, M. Camplo, T.J. McIntosh, P. Barthélémy, M.W. Grinstaff, Charge-reversal lipids, peptide-based lipids, and nucleoside-based lipids for gene delivery. Acc. Chem. Res. 45, 1026 (2012)
D. Luvino, S. Khiati, K. Oumzil, P. Rocchi, M. Camplo, P. Barthélémy, Efficient delivery of therapeutic small nucleic acids to prostate cancer cells using ketal nucleoside lipid nanoparticles. J. Control. Release 172, 954–961 (2013)
S.Y. Wong, J.M. Pelet, D. Putnam, Polymer systems for gene delivery-past, present, and future. Prog. Poly. Sci. 32, 799–837 (2007)
T.G. Park, J.H. Jeong, S.W. Kim, Current status of polymeric gene delivery systems. Adv. Drug Del. Rev. 58, 467–486 (2006)
K.W. Leong, Polymeric controlled nucleic acid delivery. MRS Bull. 30, 640–646 (2005)
Y. Kakizawa, K. Kataoka, Block copolymer micelles for delivery of gene and related compounds. Adv. Drug Del. Rev. 54, 203–222 (2002)
M. Guillot-Nieckowski, S. Eisler, F. Diederich, Dendritic vectors for gene transfection. New J. Chem. 31, 1111–1127 (2007)
Z. Sideratou, L.A. Tziveleka, C. Kontoyianni, D. Tsiourvas, C.M. Paleos, Design of functional dendritic polymers for application as drug and gene delivery systems. Gene Ther. Mol. Biol. 10, 71–94 (2006)
S. Svenson, D.A. Tomalia, Dendrimers in biomedical application–reflections on the field. Adv. Drug Deliv. Rev. 57, 2106–2129 (2005)
T. Wei, C. Chen, J. Liu, C. Liu, P. Posocco, X. Liu, Q. Cheng, S. Huo, Z. Liang, M. Fermeglia, S. Pricl, X.J. Liang, P. Rocchi, L. Peng, Anticancer drug nanomicelles formed by self-assembling amphiphilic dendrimer to combat cancer drug resistance. Proc. Natl. Acad. Sci. USA 112, 2978–2983 (2015)
X. Liu, L. Peng, Dendrimer nanovectors for SiRNA delivery. Methods Mol. Biol. 1364, 127–142 (2016)
T.R. Wilks, P.B. Anaïs, K. Nigel, E. Stulz, R.K. O’Reilly, Construction of DNA–polymer hybrids using intercalation interactions. Chem Commun (Camb) 50, 1338–1340 (2014)
L. Gallego-Yerga, L. Blanco-Fernández, K. Urbiola, T. Carmona, G. Marcelo, J.M. Benito, F. Mendicuti, C. Tros de Ilarduya,, J.M. Ortiz Mellet, G. Fernández, Host-guest-mediated DNA templation of polycationic supramolecules for hierarchical nanocondensation and the delivery of gene material. Chemistry 17, 12093–12104 (2015)
C. Ortiz Mellet, J.M. García Fernández, J.M. Benito, Cyclodextrin-based gene delivery systems. Chem. Soc. Rev. 40, 1586–1608 (2011)
J. Arigon, C.A.H. Prata, M.W. Grinstaff, P. Barthélémy, Nucleic acid complexing glycosyl nucleoside-based amphiphile. Bioconj. Chem. 16, 864–872 (2005)
C.C. Lee, J.A. MacKay, J.M.J. Fréchet, F.C. Szoka, Designing dendrimers for biological applications. Nat. Biotech. 23, 1517–1526 (2005)
E.R. Gillies, J.M.J. Fréchet, Dendrimers and dendritic polymers in drug delivery. Drug Discov. Today 10, 35–43 (2005)
H.L. Crampton, E.E. Simanek, Dendrimers as drug delivery vehicles: non-covalent interactions of bioactive compounds with dendrimers. Polym. Int. 56, 489–496 (2007)
R. Esfand, D.A. Tomalia, Poly(amidoamine) (PAMAM) dendrimers: from biomimicry to drug delivery and biomedical applications. Drug Discov. Today 6, 427–436 (2001)
G.R. Newkome, C.D. Shreiner, Poly(amidoamine), polypropylenimine, and related dendrimers and dendrons possessing different 1/2 branching motifs: an overview of the divergent procedures. Polymer 49, 1–173 (2008)
D.A. Tomalia, J.M.J. Fréchet, Dendrimers and Other Dendritic Polymers. (Wiley, Chichester, 2001)
A.W. Bosman, H.M. Janssen, E.W. Meijer, About dendrimers: structure, physical properties, and applications. Chem. Rev. 99, 1665–1688 (1999)
R. Rajasekhar Reddy, K.R. Raghupathin, D.A. Torres, S. Thayumanavan, Stimuli sensitive amphiphilic dendrimers. New J. Chem. 36, 340–349 (2012)
D.A. Tomalia, H. Baker, J. Dewald, M. Hall, G. Kallos, S. Martin, J. Roeck, J. Ryder, P. Smith, A new class of polymers: starburst-dendritic macromolecules. Polym. J. 17 117–132 (1985)
G.R. Newkome, C.N. Moorefield, F. Vögtle, Concepts, Syntheses, Perspectives, Dendritic Molecules. (VCH, New York, 1996)
D.A. Tomalia, J.M.J. Fréchet, Discovery of dendrimers and dendritic polymers: a brief historical perspective. J. Poly. Sci. Pol. Chem. 40, 2719–2728 (2002)
S.M. Grayson, J.M.J. Fréchet, Convergent dendrons and dendrimers: from synthesis to applications. Chem. Rev. 101, 3819–3868 (2001)
C.J. Hawker, J.M. Frechet, Preparation of polymers with controlled molecular architecture. A new convergent approach to dendritic macromolecules. J. Am. Chem. Soc. 112, 7638–7647 (1990)
J. Haensler, F.A. Szoka, Polyamidoamine cascade polymers mediate efficient transfection of cells in culture. Bioconj. Chem. 4, 372 (1993)
J.F. Kukowska-latallo, A.U. Bielinska, J. Johnson, R. Spinder, D.A. Tomalia, J.R. Baker, Efficient transfer of genetic material into mammalian cells using starburst polyamidoamine dendrimers. Proc. Natl. Acad. Sci. USA 93, 4897–4902 (1996)
A.U. Bielinska, C. Chen, J. Johnson, J.R. Baker Jr., DNA complexing with polyamidoamine dendrimers: implications for transfection. Bioconj. Chem. 10, 843–850 (1999)
Y.M. Liu, T.M. Reineke, Poly(glycoamidoamine)s for gene delivery. Structural effects on cellular internalization, buffering capacity, and gene expression. Bioconj. Chem 18, 19–30 (2007)
C. Loup, M.-A. Zanta, A.-M. Caminade, J.-P. Majoral, B. Meunier, Preparation of water-soluble cationic phosphorus-containing dendrimers as DNA transfecting agents. Chemistry 5, 3644–3650 (1999)
J.S. Choi, D.K. Joo, C.H. Kim, K. Kim, J.S. Park, Synthesis of barbell-like tri-block copolymers, poly(l-lysine)dendrimer-block-poly(ethylene glycol)-block-poly(l-lysine) dendrimers and its self-assembly with plasmid DNA. J. Am. Chem. Soc. 122, 474–480 (2000)
D. Joester, L.M.R. Pugin, H. Heinzelmann, E. Walter, H.P. Merkle, F. Diederich, Amphiphilic dendrimers: novel self-assembling vectors for efficient gene delivery. Ang. Chem. Int. Ed. 42, 1486–1490 (2003)
T. Takahashi, K. Kono, T. Itoh, N. Emi, T. Takagishi, Synthesis of novel cationic lipids having polyamidoamine dendrons and their transfection activity. Bioconj. Chem. 14, 764–773 (2003)
T. Takahashi, C. Kojima, A. Harada, K. Kono, Alkyl chain moieties of polyamidoamine dendron-bearing lipids influence their function as a nonviral gene vector. Bioconj. Chem. 18, 1349–1354 (2007)
P. Kesharwani, A.K. Iyer, Recent advances in dendrimer-based nanovectors for tumor-targeted drug and gene delivery. Drug Discov. Today 20, 536–547 (2015)
S.J. Cockman, C.A. Joll, Aust. J. Chem. 43, 2093–2097 (1990)
P.H.J. Carlsen, K. Sorbye, T. Ulven, K. Aasbo, Synthesis of benzylidene-protected dihydroxyacetone. Acta Chim. Scand. 50, 185–187 (1996)
N.R. Luman, K.A. Smeds, M.W. Grinstaff, The convergent synthesis of poly(glycerol-succinic acid) dendritic macromolecules. Chem. Eur. J. 9, 5618–5626 (2003)
M.A. Carnahan, M.W. Grinstaff, Synthesis and characterization of poly(glycerol-succinic acid) dendrimers. Macromolecules 34, 7648–7655 (2001)
N.R. Luman, M.W. Grinstaff, Synthesis and aqueous aggregation properties of amphiphilic surface-block dendrimers. Org. Lett. 7, 4863–4866 (2005)
M.A. Carnahan, C. Middleton, J. Kim, T. Kim, M.W. Grinstaff, Hybrid dendritic-linear polyester-ethers for in situ photopolymerization. J. Am. Chem. Soc. 124 5291–5293 (2002)
N.R. Luman, T. Kim, M.W. Grinstaff, Dendritic polymers composed of glycerol and succinic acid: synthetic methodologies and medical applications. Pure Appl. Chem. 76, 1375–1385 (2004)
M.A. Carnahan, M.W. Grinstaff, Synthesis and characterization of polyether-ester dendrimers from glycerol and lactic acid. J. Am. Chem. Soc. 123, 2905–2906 (2001)
M.A. Carnahan, M.W. Grinstaff, Synthesis of controlled layered polyester dendrimers composed of glycerol and succinic or adipic acid. Macromolecules 39, 609–616 (2006)
L. Degoricija, M.A. Carnahan, C.S. Johnson, T. Kim, M.W. Grinstaff, Synthesis and characterization of bola-type amphiphilic dendritic macromolecules. Macromolecules 39, 8952–8958 (2006)
L. Degoricija, C.S. Johnson, M. Wathier, T. Kim, M.W. Grinstaff, Photo cross-linkable biodendrimers as ophthalmic adhesives for central lacerations and penetrating keratoplasties. Invest. Ophthalmol. Visual Sci. 48, 2037–2042 (2007)
M. Wathier, P.J. Jung, M.A. Carnahan, T. Kim, M.W. Grinstaff, Dendritic macromers as in situ polymerizing biomaterials for securing cataract incisions. J. Am. Chem. Soc. 126, 12744–12745 (2004)
M. Wathier, S.M. Johnson, T. Kim, M.W. Grinstaff, Hydrogels formed by multiple peptide ligation reactions to fasten corneal transplants. Bioconj. Chem. 17, 873–876 (2006)
C. Ghobril, K. Charoen, E.K. Rodriguez, A. Nazarian, M.W. Grinstaff, A dendritic thioester hydrogel based on thiol-thioester exchange as a dissolvable system for wound closure. Angew. Chem. Int. Ed. 52, 14070–14074 (2013)
M.T. Morgan, Y. Nakanishi, D.J. Kroll, A.P. Griset, M.A. Carnahan, M. Wathier, N.H. Oberlies, G. Manikumar, M.C. Wani, M.W. Grinstaff, Dendrimer-encapsulated camptothecins: increased solubility, cellular uptake, and cellular retention affords enhanced anticancer activity in vitro. Cancer Res. 66, 11913–11921 (2006)
M.T. Morgan, M.A. Carnahan, C.E. Immoos, A.A. Ribeiro, S. Finkelstein, S.J. Lee, M.W. Grinstaff, Dendritic molecular capsules for hydrophobic compounds. J. Am. Chem. Soc. 125, 15485–15489 (2003)
S. Söntjens, D.L. Nettles, M.A. Carnahan, L.A. Setton, M.W. Grinstaff, Biodendrimer-based hydrogel scaffolds for cartilage tissue repair. Biomacromolecules 7, 310–316 (2006)
C.L. Zhao, M.A. Winnik, G. Riess, M.D. Croucher, Fluorescence probe techniques used to study micelle formation in water-soluble block copolymers. Langmuir 6, 514–516 (1990)
M.X. Tang, C.T. Redemann, F.C. Szoka, In vitro gene delivery by degraded polyamidoamine dendrimers. Bioconj. Chem. 7, 703–714 (1996)
M. Hemmati, B. Kazemi, F. Najafi, A. Zarebkohan, R. Shirkoohi, Synthesis and evaluation of a glutamic acid-modified hPAMAM complex as a promising versatile gene carrier. J. Drug Target 24, 408–421 (2016)
A. Dehshahri, H. Sadeghpour, Surface decorations of poly(amidoamine) dendrimer by various pendant moieties for improved delivery of nucleic acid materials. Colloids Surf. B 132, 85–102 (2015)
M.A. Kostiainen, J.G. Hardy, D.K. Smith, High-affinity multivalent DNA binding by using low-molecular-weight dendrons. Angew. Chem. Int. Ed. 44, 2556–2560 (2005)
Acknowledgements
The funding was provided by National Institutes of Health (Grant Nos. R21CA125327, R01GM27278).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Prata, C.A.H., Luman, N.R., Li, Y. et al. Synthesis of Cationic Amphiphilic Surface-Block Polyester Dendrimers. J Inorg Organomet Polym 28, 383–398 (2018). https://doi.org/10.1007/s10904-017-0651-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10904-017-0651-4