Abstract
The applications of dendrimer-based vectors seem to be promising in non-viral gene delivery because of their potential for addressing the problems with viral vectors. In this study, generation 3 poly(propyleneimine) (G3-PPI) dendrimers with 1, 4-diaminobutane as a core initiator was synthesized using a divergent growth approach. To increase the hydrophobicity and reduce toxicity, 10% of primary amines of G3-PPI dendrimers were replaced with bromoalkylcarboxylates with different chain lengths (6-bromohexanoic and 10-bromodecanoic). Then, to retain the overall buffering capacity and enhance transfection, the alkylcarboxylate–PPIs were conjugated to 10 kDa branched polyethylenimine (PEI). The results showed that the modified PPI was able to form complexes with the diameter of less than 60 nm with net-positive surface charge around 20 mV. No significant toxicity was observed in modified PPIs; however, the hexanoate conjugated PPI–PEI (PPI-HEX-10% PEI) and the decanoate conjugated PPI–PEI (PPI-DEC-10%-PEI) showed the best transfection efficiency in murine neuroblastoma (Neuro-2a) cell line, even PPI-HEX-10%-PEI showed transfection efficiency equal to standard PEI 25 kDa with reduced toxicity. This study suggested a new series of hyperbranched (PEI)–dendrimer (PPI) architectural copolymers as non-viral gene delivery vectors with high transfection efficiency and low toxicity.

A schematic representative of hyperbranched PEI conjugated to poly(propyleneimine) dendrimers
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Recently, the human gene therapy has gained great attention (Ginn et al. 2013). However, safe and efficient transfers of genetic materials are serious problems in gene therapy (Verma and Somia 1997). The application of viral vectors has some limitations such as small capacity, immunogenicity, pathogenicity, and difficult and expensive production routs (Liu and Muruve 2003; Sun et al. 2003).
Non-viral vectors such as cationic liposomes (Allon et al. 2012; Malaekeh-Nikouei et al. 2009) and a number of cationic polymers including poly-L-lysine (Askarian et al. 2015), poly(ethylenimine) (PEI) (Mahmoudi et al. 2014), polyamidoamine (PAMAM) dendrimer (Ayatollahi et al. 2015a), hyperbranched poly(amino ester) (Lim et al. 2001), and polyallylamine (Oskuee et al. 2015; Oskuee et al. 2014) have been developed as alternatives to viral gene delivery carriers due to their safety and cost advantages. However, low transfection efficiency of non-viral vectors compared with the viral ones and toxicity problems have limited their use in clinical trials, which needs to be overcome for in vivo applications. Many researchers have attempted to solve those problems by redesigning the existing non-viral vectors (Dehshahri et al. 2012a; Dehshahri et al. 2012b). Among the non-viral delivery systems, dendrimer-based vectors due to their unique structural features seem to be promising candidates for addressing the above problems (Lee et al. 2005; Svenson and Tomalia 2005).
Dendrimers and their derivatives are cationic polymers which could condense the negatively charged DNA in physiological environment and form complexes (polyplexes) (Tack et al. 2006). Due to the difficulty of synthesizing dendrimers and advantages of dendrimers for delivery systems, great attention has been focused on the modification of the existing dendrimers, instead of the development of novel dendrimers (Kim et al. 2007). Thus, the modification of the surface groups of basic dendrimers by molecular engineering (Calderón et al. 2010; Paleos et al. 2007) can result in dendrimers with enhanced properties, which could better fit gene delivery systems.
The cytotoxicity of dendrimers such as PAMAM and PPI has been found to be generation-dependent, with higher generation dendrimers being the most toxic, which is mostly related to the primary amino group (Fischer et al. 2003; Jevprasesphant et al. 2003). Therefore, it is very promising to chemically modify the terminal groups of dendrimers to create transfection agents with low toxicity, enhanced water solubility, and improved aqueous stability (Tack et al. 2006).
It has been reported that hydrophobic–hydrophilic balance plays an important role in enhancing the gene transfection ability of polycation-based non-viral vectors (Nimesh et al. 2007; Takahashi et al. 2003). PAMAM dendrimer itself and also PAMAM dendrimers modified with PEG, amino acids, fatty acids, and ligands have been extensively used and examined as transfection agents for gene delivery, both in vitro and in vivo (Askarian et al. 2015; Choi et al. 2006; Choi et al. 2004; Hu et al. 2014). By contrast, the applications of PPI dendrimers for gene delivery systems have been limited to a small number of works. For instance, plain G3 and quaternized G2 PPI dendrimers were reported to be suitable for targeting genes to the liver (Schatzlein et al. 2005).
In the present work, we synthesized PPI dendrimer, alkyl-conjugated PPI dendrimers, and PPI-alkyl-PEI copolymers and evaluated their characterizations including transfection efficiency and cytotoxicity. The used approach was, low, partially, and complete surface derivatization of PPI dendrimers with bromoalkylcarboxylic acids (with different chain lengths). It was followed by conjugation of terminal carboxylate moieties of PPI derivatives with branched PEI (molecular weight (MW) 10 kDa) which resulted in hyperbranched (PEI)–dendrimer (PPI) architectural copolymers with the terminal amino moieties.
Materials and methods
Materials
1, 4-Diaminobutane, acrylonitrile, and Raney Nickel were purchased from Merck. Branched polyethylenimine (PEI; average MW 10 kDa) was obtained from Polyscience, Inc. (Warrington, USA). Branched polyethylenimine (PEI; average MW 25 kDa), 6-bromohexanoic acid, 10-bromodecanoic acid, 1-ethyl-3-[3-dimethylaminopropyl] carbodiimide hydrochloride (EDC) were purchased from Sigma–Aldrich (Munich, Germany). Plasmid pRL-CMV-luc under the control of cytomegalovirus (CMV) promoter and a luciferase assay kit were obtained from Promega (Madison, USA).
All solvents and chemicals were supplied by Sigma–Aldrich (Munich, Germany) and were of the highest grade available. Dialysis was carried out using Spectra/Por dialysis membranes (Spectrum Laboratories, Houston, USA).
Synthesis of poly(propyleneimine) dendrimer
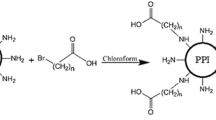
Poly(propyleneimine) dendrimers with 1, 4-diaminobutane as a core initiator was synthesized using a divergent growth approach. G3-PPI was obtained through repetitive double Michael addition of acrylonitrile to primary amine end-groups from a 1, 4-diaminobutane core, followed by heterogeneously catalyzed hydrogenation of nitrile groups to primary amino groups (Scheme 1). The divergent route followed in synthesis was according to a study reported elsewhere (de Brabander-van den Berg and Meijer 1993).
Synthesis of PPI-R-COOH
PPI-R-COOH was synthesized via the reaction between PPI and a series of bromoalkylcarboxylic acids with different chain lengths (Scheme 2). Briefly, various amounts of 6-bromohexanoic acid or 10-bromodecanoic acid dissolved in dimethylformamide (DMF). This solution was dropwised to the stirring solution of PPI (0.1 g in 5 ml DMF) over 3 h. The reaction was stirred for 24 additional hours at room temperature (RT). Then, to remove unreacted alkylating agents, the reaction mixture was dialyzed once against 0.25 M NaCl and twice against water (1000 Da cutoff Spectra/Por dialysis tubing). Finally, the resulting solution was freeze-dried.
Synthesis of PPI-R-PEI
To restore the primary amine density of PPI-R derivatives, the carboxylic acid end groups of PPI-R were conjugated to the branched PEI using amide linkage formation. Briefly, 5 ml aqueous solution of carboxyalkylated PPI was mixed with 3 M excess solution of either 1.8 or 10 kDa PEI. In order to activate the terminal carboxylic acid of PPI-R, 1 mol equivalent of EDC were dropwised to the reaction mixture. The reaction was stirred for 24 h at RT. Then, the reaction mixture was purified using 10,000 Da cutoff Spectra/Por dialysis tubing by dialyzing against three changes of water. The aqueous dialysate was lyophilized.
Characterization of synthesized products
The synthesis products were characterized by FTIR and NMR. The FTIR spectra of the dendrimers were recorded on a Shimadzu FTIR spectrometer as potassium bromide pellets. NMR spectra were recorded in CD3Cl on a Bruker ARX 300 spectrometer (Bruker Daltonik GmbH, Bremen, Germany).
Estimation of the primary amine content of PPI derivatives by a TNBS assay
The primary amine content of synthesized PPI derivatives was determined using quantification of accessible primary amines by coupling with 2,4,6-trinitrobenzene sulfonic acid (TNBS).
Standard PPI solutions and test solutions containing PPI derivatives were serially diluted in 0.1 M sodium tetraborate to a final volume of 100 μl using a 96-well plate. To each well, 2.5 μl of TNBS (75 nmol, 22 μg; diluted in water) was added. TNBS reacts with primary amino groups to form colored trinitrophenylated derivatives. After 10 min at RT, absorption was measured at 405 nm using a microplate reader.
Preparation and purification of the plasmid DNA
pRL-CMV-luc was transformed into Escherichia coli bacterial strain DH5α, propagated in selective Luria–Bertani (LB) medium, centrifuged, and extracted from the cell pellets using the Qiagen Endofree Mega Plasmid Kit (QIAGEN, Hilden, Germany) according to the manufacturer’s protocols.
The purity of the plasmid DNA was detected by measuring the UV absorbance at 260 nm with an UV spectrophotometer. Results of the A260/A280 absorbance were higher than 1.8 that indicated no protein was present.
Polycation/DNA complex (polyplex) formation
PPI and various derivatives (C) were separately diluted into 50 μl of HEPES-buffered glucose solution (20 mM HEPES in 5% aqueous glucose solution), added to 50 μl solutions of the plasmid (P) in the same solvent (2 μg/50 μl), mixed together, and allowed to be incubated at room temperature for 30 min to form nanoparticles at different charge ratios between the polymer and plasmid DNA (C/P or w/w or weight/weight).
Gel retardation assay
The complex formation ability of the vectors was analyzed using an agarose gel retardation assay. Polycation/plasmid DNA (pDNA) complexes (polyplexes) were prepared as indicated before and placed into a 1% (w/v) agarose gel in TBE buffer (Tris base 10.8 g, boric acid 5.5 g, disodium EDTA 0.75 g and water) containing Green viewer dye. Electrophoresis was performed at 80 V for 40 min and visualized under UV-illuminator.
Measurement of polyplex size and zeta potential
The hydrodynamic size and zeta potential of polyplexes at various C/P ratios were analyzed using a Zetasizer Nano-ZS analyzer (Malvern Instruments, UK) in a salt-free buffer (HBG). Different amounts of vectors were diluted in 125 μl of buffer and added to an equal volume of the same buffer containing DNA. After 20-min incubation, polyplex sizes were measured. Data analysis was performed in an automatic mode, and the results are presented as mean ± SD, n = 3. Each mean represents the average value of 30 runs.
Measurement of buffering capacity of modified PPI
A solution of 0.4 mg/ml of each PPI derivative in DDW was prepared and adjusted to the pH of 12 using 1 M NaOH. Five microliter aliquots of 1 M HCl were subsequently added until the pH was reduced to 2.5. The reciprocal slopes of graphs plotting pH versus the amount of HCl added were used to determine the buffering capacity of PPI derivatives.
Transfection experiments
Neuro-2a (N2a) cells were seeded at a density of 1 × 104 cells/well in 96-well plates 1 day prior to transfection experiments and grown in the DMEM medium with 10% fetal bovine serum. Different dendrimer/plasmid DNA weight ratios, in the range 2:1 to 6:1 (w/w), were used to prepare the polycation/plasmid complexes (i.e., polyplexes). Polyplexes were prepared by adding 50 μl of a solution of polycation at varying concentrations in HBG to 50 μl of a solution of plasmid DNA (20 μg/ml) in HBG with mixing by pipetting up and down followed by incubating for 30 min at room temperature. Transfection was performed by adding 10 μl of polyplex solution (equivalent of 200 ng pDNA) to the wells of 96-well plates containing 60–90% confluent cultures of cells in complete medium containing 10% FBS. After 4 h, the medium was replaced with a fresh complete medium and gene expression was assayed 24 h later. Cells were lysed, and the luciferase activity was measured using the Promega Renilla Luciferase Assay kit (Madison, WI) and a luminometer (Berthold Detection Systems, Pforzheim, Germany). The results were presented as relative light units (RLU) per number of seeded cells, mean ± SD, n = 3. Branched 25 kDa PEI was used as a positive control for transfection experiments.
Cytotoxicity assay
The metabolic activity of the cells treated with polyplexes was evaluated by the method using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT). Briefly, N2a cells were seeded at a density of 1 × 104 cells per well in a 96-well plate and incubated for 24 h. Thereafter, the cells were treated with the same amounts of polyplexes used for transfection experiments, and after 4 h, the medium was replaced with a fresh complete medium. After 24 h of incubation, 20 μl of 5 mg/ml MTT in the PBS buffer was added to each well, and the cells were further incubated for 4 h at 37 °C. The medium containing unreacted dye was discarded, and 100 μl of DMSO was added to dissolve the formazan crystals formed by live cells. Optical absorbance was measured at 590 nm (reference wavelength 630 nm) using a microplate reader (Statfax–2100, Awareness Technology, USA), and cell viability was expressed as a percentage of viable treated cells relative to untreated control cells. Values of metabolic activity were presented as means ± SD of triplicates.
Statistical analysis
The statistical significance was determined using Student’s t test. p values of ≤0.05 were considered significant.
Results
Synthesis and characterization of PPI dendrimers
Schematic synthesis pathway for PPI dendrimers is presented in Scheme 1. PPI dendrimers were successfully synthesized according to 1H-NMR and FTIR analysis (spectra not shown).
The FTIR spectrum of 0.5 G PPI showed band at 2245 cm−1, which was assigned to (CN) stretching vibrations. Absorption band at 3355 cm−1 (due to N–H stretching vibrations) confirmed the generation of 1.0 G PPI. The FTIR spectrum of 3.0 G PPI showed bands at 1226, 2827, 2962, 1577, and 3344 cm−1, which were assigned to C–N stretching of CH2–NH2, C–H aliphatic stretching vibrations, N–H bending vibrations of amine, and N–H stretching of primary amine, respectively.
1H-NMR spectrum of 3.0 G PPI showed protons from alkane (1.3–1.53 ppm) and alkyl amines (2.3–2.5 ppm).
Synthesis and characterization of PPI-R-PEI
Schematic synthesis pathway for PPI-R-PEI dendrimers is presented in Scheme 2. A two-step process was used for the synthesis of the modified dendrimers. In the first step, PPI G3 dendrimer was modified by the conjugation of ω-bromoalkylcarboxylic acids (6-bromohexanoic acid and 10-bromodecanoic acid) to the amine groups of PPI G3 dendrimers (PPI-R). In the next step, PEI was attached to the provided terminal functional carboxylate groups.
The synthesized products were characterized by FTIR and 1H-NMR. As an example, the FTIR spectrum of PPI-R-PEI showed bands at 1640 cm−1 and 1530 cm−1, which were assigned to the carbonyl group of amide and N-H bending vibrations of amide, respectively. The obtained data from 1H-NMR (CDCl3) spectrum of PPI-R-PEI was δ (ppm) 2.3–2.29 (–CH2NH2–, –CH2NH–, –CH2N–), 2.2–2.9 (–CH2–CO), and 3.0–3.2 (–CH2–NHCO).
Estimation of primary amine content of the products
The extent of PPI primary amine group substitution by alkylcarboxylate moieties (which is equal to the number of alkylcarboxylate groups conjugated to the PPI dendrimer) as well as the extent of PPI-alkylcarboxylate grafted with PEI was calculated by the TNBS assay.
The results for G 3.0 dendrimer possessing 16 primary amine groups showed that for the initial feed mole percent of 10 and 100, the achieved degrees of substitution were 10 and 12 mol% (Table 1).
Approximately, for 10 and 100% modification, 1.6 and 11.3 molecules of ω-bromoalkylcarboxylic acid were found to be conjugated to the dendrimer possessing 16 primary amines. The reaction yields for the coupling of PEI to carboxylate groups were also less than 100% so that the final derivatives contained the average of 78.5 ± 0.7% (76–81%) of the primary amine content of the parent PPI.
The modified dendrimers have been abbreviated as PPI-X-%-PEI, in which X, %, and PEI represent the number of carbons in the alkyl chain (HEX, hexanoic acid; DEC, decanoic acid), percentage (%) of primary amines substituted with alkyl chains, and 10 kDa PEI coupled to the modified PPI structure, respectively.
Buffering capacity
Buffering capacity is essential for the swelling of endocytic vesicles and escape from endosome into the cytoplasm (proton sponge effect). Therefore, solutions of PPI and its derivatives were titrated from pH 12 to 2 with the stepwise addition of HCl (1 N). The protonation profiles of PPI derivatives were markedly affected. Generally, the addition of PEI to the PPI structure resulted in an increase in the buffering capacity, and PPI-HEX-10%-PEI showed the best protonation profile (Fig. 1).
Agarose gel retardation
Condensation of pDNA into nanoscale structures is necessary for both protecting the DNA from serum nucleases and taking up by cells, often through non-receptor-mediated endocytosis. Therefore, the inhibition of DNA migration in agarose gel electrophoresis (as a result of dendrimer ability to condense DNA) was tested. Polyplexes were prepared at various dendrimer/DNA ratios and electrophoresed on agarose gel 1% (Fig. 2).
Unmodified PPI was unable to completely inhibit the migration of plasmid DNA, even at the C/P ratio of 4. As the primary amine content of the modified PPI was increased, lower amounts of dendrimer were required to prevent DNA migration.
Specifically, PPI-HEX-10%-PEI and PPI-DEC-10%-PEI completely inhibited the migration of pDNA, at the C/P ratio of 1 which was significantly better than unmodified PPI (Fig. 2).
Size and zeta potential
The results demonstrated that at higher weight ratios of polyplexes, size was decreased and zeta potential was increased. Also, modification of PPI produced polycations with the ability to form complexes with the diameter of less than 60 nm (Fig. 3).
In the case of PPI-HEX-10%-PEI at the weight ratios of 2, 4, and 6, the particle size of the polyplexes was ranged from 29 to 39 nm.
The zeta potential of PPI and unmodified PPI complexes was measured at polycation/pDNA ratios of 2, 4, and 6 (Fig. 4). The zeta potential of the polyplexes was gradually increased in accordance with the increase of weight ratios. The net surface charge of the modified PPIs was more than that of the unmodified PPI. For instance, zeta potential of PPI-HEX-10%-PEI compared with that of unmodified PPI showed an increase from 1.3 to 22.3 mV at C/P ratio of 4.
MTT assay
The cytotoxicity of the PPI derivatives at various weight ratios was examined by an MTT assay in N2a cells to measure cell survival. The cytotoxicity of PPI derivatives at various weight ratios was compared with that of PPI G3, and the results were shown in Fig. 5. Among all the derivatives, PPI-HEX-10%-PEI exhibited relatively the best cell viability (over 80%) at C/P ratio of 2. In each sample with the increase in weight ratio, all the PPI derivatives and PEI showed increasing cytotoxicity.
Cytotoxicity of polyplexes formed with PPI G3 and its derivatives and 200 ng of plasmid DNA in HBG over the indicated range of cationic polymer to plasmid DNA (C/P) (w/w) ratios. Polyplexes prepared with PPI and 10 kDa PEI in HBG were included as controls at the indicated C/P ratios, as well as the polyplex prepared with 25 kDa PEI in HBG at C/P 0.8. Metabolic activities are presented as the mean ± SD of triplicates
Transfection efficiency
The transfection efficiency of the newly synthesized PPI dendrimer was investigated on N2a cells by a Renilla luciferase assay system. PPI and derivative polyplexes were prepared at varying weight ratios ranging from 2:1 to 6:1 (polycation:plasmid). PEI 25 kDa, PEI 10 kDa, and PPI G3 polyplexes (at the same w:w ratios as that of the modified PPI), were also used as controls.
The transfection data showing newly synthesized PPI derivative had remarkable transfection efficiency compared with those showing the parent PPI G3.
As shown in Fig. 6, PPI-HEX-10% and PPI-DEC-10% polyplexes indicated the maximal gene expression efficiency at the weight ratios of 4and 6, respectively.
Transfection efficiency of polyplexes formed with PPI G3 and its derivatives and 200 ng of plasmid DNA in HBG over the indicated range of cationic polymer to plasmid DNA (C/P) (w/w) ratios. Polyplexes prepared with PPI and 10 kDa PEI in HBG were included as controls at the indicated C/P ratios, as well as the polyplex prepared with 25 kDa PEI in HBG at C/P 0.8. Luciferase activities are presented as the mean ± SD of triplicates. Three asterisks indicate p < 0.001 modified PPI compared to PPI G3. Letter a indicate p > 0.05 modified PPI compared to PEI25
The transfection efficiency of PPI-HEX-10%-PEI and PPI-DEC-10%-PEI increased drastically comparing to that of the unmodified PPI G3 and was three times more than that of 10 kDa PEI.
One explanation for the deducted gene transfer ability was the presence of the unreacted carboxylic acid groups on a PPI-R-PEI surface, assigned to the repulsion with the negative charge of DNA. These results corresponded to the results of gel electrophoresis.
Discussion
Polyamidoamine (PAMAM) and PPI are the most investigated dendrimers, which exhibit potentials in medical applications such as drug delivery, gene delivery, and imaging (Martinho et al. 2014). Dendrimers consist of interior shells and multivalent surfaces; their inner nanoenvironments can be utilized as nanoscale containers for drugs that are protected from outside by the surface (Boas et al. 2001). PPI dendrimers have a nonpolar pocket which is able to increase the solubility of hydrophobic drugs (Cheng et al. 2008b). Because of high density of surface groups, dendrimers can be functionalized with different molecules such as aptamers for targeting purposes (Pednekar et al. 2012) or hydrophobic molecules to enhance the transfection efficiency (Yoo and Juliano 2000).
Although some reports have suggested PPI dendrimers as non-viral gene delivery vectors (Zinselmeyer et al. 2002), their application has been limited due to weak transfection efficacy and cytotoxicity. However, the cytotoxicity of dendrimers has been found to be generation-dependent and higher generations are more toxic (El-Sayed et al. 2002). For instance, some studies have reported that high-generation PPI dendrimers display higher cytotoxicity than low-generation PPI on many cell lines (Jain et al. 2010).
PAMAM and PPI dendrimers compromise primary amines on the surface, which allows for electrostatical attachment to negatively charged molecules as well as the cell membrane and may destabilize it and induce cytotoxicity (Cheng et al. 2008a). Different modifications have been performed on PPI to improve transfection efficacy and reduce cytotoxicity, such as conjugation of peptide (Taratula et al. 2009), oligoethylenimine (Russ et al. 2008), amino acids (Aldawsari et al. 2011; Kim et al. 2007), guanidine (Tziveleka et al. 2007), and fluorination (Liu et al. 2014).
Among cationic polymers, PEIs are nominated as effective non-viral vectors. They are able to greatly condense nucleic acids and protect them from environment. Due to high buffering capacity, they can trigger a proton sponge mechanism and escape from endosome to deliver their gene cargoes into the cytoplasm. Cytotoxicity of PEIs challenges their applications in many mammalian cell lines although it is dependent on molecular weight, degree of branching, ionic strength of the solution, zeta potential, and particle size (Paul et al. 2014). Low molecular weight PEIs have shown low cell toxicity but also low transfection ability (Kunath et al. 2003). However, there are many reports, in which the advantages of PEIs have been utilized to improve transfection efficacy such as pullulan-PEI (Rekha and Sharma 2011) or chitosan-PEI (Parhiz et al. 2013; Park et al. 2013).
In this study, we took advantage of 10 kDa PEI and synthesized hyperbranched (PEI)–dendrimer (PPI) architectural copolymers including PPI-HEX-10%-10 kDa and PPI-DEC-10%-10 kDa to produce efficient and low cytotoxic gene delivery carriers based on cationic PPI dendrimer. The synthesis approach was dendritic hybridization involving (a) architectural PPI generation 3, (b) dendrimers surface derivatization with bromoalkylcarboxylic acids, (c) conjugation of terminal carboxylate moieties of PPI core with branched-PEI (MW 10 kDa) which resulted in core–shell dendritic copolymers with the terminal amino moieties.
The DNA condensation ability characterized by a gel retardation assay showed that unmodified PPI weakly condenses plasmid while modified polymers showed similar behavior like PEI which is in agreement with other studies reporting that by an increase in amine content, condensation ability increased (Ayatollahi et al. 2015b; Dehshahri et al. 2009).
Although plain PPI exhibited low positive charge at low C/P ratios which resulted in the formation of larger polyplexes, the synthesized polymers formed polyplexes below 60 nm with appropriate positive charge density (15.6–29.5 mV) which were suitable for efficient gene delivery. Previously, it was shown that carbon chain length and substitution degree have a direct role in the change of average particle size (Tziveleka et al. 2007).
The buffering capacity seemed to be increased in the modified polymers compared with PPI, and the similar pattern to the unmodified PEI indicated effective endosomal escape ability after cellular uptake.
Excellent non-viral carriers require high gene transfection efficacy with minimum cytotoxicity. However, these properties are in the opposite direction in most of the polycationic gene vectors. High transfection ability correlates with cytotoxicity and better biocompatible vectors show weak transfection efficacy (Breunig et al. 2007).
In our study, PPI-HEX-10%-10 kDa and PPI-DEC-10%-10 kDa succeeded to associate between transfection efficacy and cell viability, especially at C/P ratio of 4. Cytotoxicity might be induced by electrostatic interactions between polycations and cell membranes (Kloeckner et al. 2006). Thus, by increasing the polycation/plasmid DNA weight ratios, the amount of excessive positive charge of polyplexes increased resulting in a slightly increase in cytotoxicity (Gholami et al. 2014).
Transfection efficiency of synthesized polymers was compared with 10 kDa PEI and PPI dendrimers (over the same range of C/P ratios), and 25 kDa PEI at the C/P ratio of 0.8 was utilized as optimal delivery vector. The transfection results demonstrated imperfect transfection ability of the unmodified PPI at all C/P ratios, while PPI-HEX-10%-10 kDa and PPI-DEC-10%-10 kDa improved transfection efficacy by 2.9- and 3.5-fold, respectively, compared with the unmodified 10 kDa PEI. It seems that 10% substitution of primary amines of dendrimers with PEI was sufficient to utilize the advantages of PEI and avoid the accumulation of amines and induction of cytotoxicity. The 25 kDa PEI showed higher transfection efficiency even better than 10 kDa PEI, but exhibited greater cytotoxicity than 10 kDa PEI. Interestingly, transfection efficacy of PPI-HEX-10%-10 kDa at C/P ratio of 4 was approximately equal to that of 25 kDa PEI with less cytotoxicity.
There are some reports in which the effect of different alkyl chain length conjugations on transfection efficacy of polycations has been studied and hexanoate has been found to have optimal length, while alkyl chains longer than 10-carbone have resulted in dendrimeric structure deficiencies and reduction in transfection activity (Oskuee et al. 2009). In our study, length of alkyl chain did not show a significant effect on the transfection efficacy at C/P ratio of 2, but the optimal alkyl chain length was found to be 6 (hexanoate) at C/P ratio of 4. The obtained results were in agreement with those of the former studies, in which hexanoate modification of PEIs mainly improved transfection efficacies (Dehshahri et al. 2009). Improved transfection efficiency of PPI derivatives was due probably to more favorable hydrophobic–hydrophilic balance in the structure and greater buffering capacity, which promotes the cytosolic release and early endosomal escape of polyplexes (Nimesh et al. 2007; Tziveleka et al. 2007).
Conclusion
In this study, we produced PPI-alkyl-PEI polymers in order to synthesize efficient and low cytotoxic gene delivery vectors based on cationic PPI dendrimer. PPI-HEX-10%-10 kDa and PPI-DEC-10%-10 kDa condensed pDNA effectively and formed polyplexes below 60 nm. However, cell viability of N2A cells treated with the synthesized vectors was above 75%, and the transfection efficiency significantly increased by about 3.5-fold compared with that of the unmodified 10 kDa PEI. Further investigations could be done on the ability of these modified dendrimers for other drug delivery or targeting purposes.
References
Aldawsari H, Edrada-Ebel R, Blatchford DR, Tate RJ, Tetley L, Dufes C (2011) Enhanced gene expression in tumors after intravenous administration of arginine-, lysine- and leucine-bearing polypropylenimine polyplex. Biomaterials 32:5889–5899. doi:10.1016/j.biomaterials.2011.04.079
Allon N, Saxena A, Chambers C, Doctor BP (2012) A new liposome-based gene delivery system targeting lung epithelial cells using endothelin antagonist. J Control Release 160:217–224. doi:10.1016/j.jconrel.2011.10.033
Askarian S, Abnous K, Taghavi S, Oskuee RK, Ramezani M (2015) Cellular delivery of shRNA using aptamer-conjugated PLL-alkyl-PEI nanoparticles. Colloids Surf B: Biointerfaces 136:355–364. doi:10.1016/j.colsurfb.2015.09.023
Ayatollahi S et al (2015a) Synthesis of efficient gene delivery systems by grafting pegylated alkylcarboxylate chains to PAMAM dendrimers: evaluation of transfection efficiency and cytotoxicity in cancerous and mesenchymal stem cells. J Biomater Appl 30:632–648. doi:10.1177/0885328215599667
Ayatollahi S et al (2015b) Synthesis of efficient gene delivery systems by grafting pegylated alkylcarboxylate chains to PAMAM dendrimers: evaluation of transfection efficiency and cytotoxicity in cancerous and mesenchymal stem cells. J Biomater Appl 30:632–648. doi:10.1177/0885328215599667
Boas U, Karlsson AJ, de Waal BF, Meijer EW (2001) Synthesis and properties of new thiourea-functionalized poly(propylene imine) dendrimers and their role as hosts for urea functionalized guests. J Org Chem 66:2136–2145
Breunig M, Lungwitz U, Liebl R, Goepferich A (2007) Breaking up the correlation between efficacy and toxicity for nonviral gene delivery. Proc Natl Acad Sci U S A 104:14454–14459
Calderón M, Quadir MA, Strumia M, Haag R (2010) Functional dendritic polymer architectures as stimuli-responsive nanocarriers. Biochimie 92:1242–1251. doi:10.1016/j.biochi.2010.02.017
Cheng Y, Wu Q, Li Y, Xu T (2008a) External electrostatic interaction versus internal encapsulation between cationic dendrimers and negatively charged drugs: which contributes more to solubility enhancement of the drugs? J Phys Chem B 112:8884–8890
Cheng Y, Xu Z, Ma M, Xu T (2008b) Dendrimers as drug carriers: applications in different routes of drug administration. J Pharm Sci 97:123–143. doi:10.1002/jps.21079
Choi JS, Nam K, Park JY, Kim JB, Lee JK, Park JS (2004) Enhanced transfection efficiency of PAMAM dendrimer by surface modification with L-arginine. J Control Release 99:445–456
Choi JS, Ko KS, Park JS, Kim YH, Kim SW, Lee M (2006) Dexamethasone conjugated poly(amidoamine) dendrimer as a gene carrier for efficient nuclear translocation. Int J Pharm 320:171–178
de Brabander-van den Berg E, Meijer E (1993) Poly (propylene imine) dendrimers: large-scale synthesis by hetereogeneously catalyzed hydrogenations. Angew Chem Int Ed Engl 32:1308–1311
Dehshahri A, Oskuee RK, Shier WT, Hatefi A, Ramezani M (2009) Gene transfer efficiency of high primary amine content, hydrophobic, alkyl-oligoamine derivatives of polyethylenimine. Biomaterials 30:4187–4194
Dehshahri A, Oskuee RK, Ramezani M (2012a) Plasmid DNA delivery into hepatocytes using a multifunctional nanocarrier based on sugar-conjugated polyethylenimine. Gene Ther Mol Biol 14:62–71
Dehshahri A, Oskuee RK, Shier WT, Ramezani M (2012b) β-Galactosylated alkyl-oligoamine derivatives of polyethylenimine enhanced pDNA delivery into hepatic cells with reduced toxicity. Curr Nanosci 8:548–555
El-Sayed M, Ginski M, Rhodes C, Ghandehari H (2002) Transepithelial transport of poly(amidoamine) dendrimers across Caco-2 cell monolayers. J Control Release 81:355–365
Fischer D, Li Y, Ahlemeyer B, Krieglstein J, Kissel T (2003) In vitro cytotoxicity testing of polycations: influence of polymer structure on cell viability and hemolysis. Biomaterials 24:1121–1131
Gholami L, Sadeghnia HR, Darroudi M, Kazemi Oskuee R (2014) Evaluation of genotoxicity and cytotoxicity induced by different molecular weights of polyethylenimine/DNA nanoparticles. Turk J Biol 38:380–387. doi:10.3906/biy-1309-51
Ginn SL, Alexander IE, Edelstein ML, Abedi MR, Wixon J (2013) Gene therapy clinical trials worldwide to 2012—an update. J Gene Med 15:65–77. doi:10.1002/jgm.2698
Hu W et al (2014) Redox and pH-responsive poly (amidoamine) dendrimer-poly (ethylene glycol) conjugates with disulfide linkages for efficient intracellular drug release. Colloids Surf B Biointerfaces 123:254–263. doi:10.1016/j.colsurfb.2014.09.024
Jain K, Kesharwani P, Gupta U, Jain NK (2010) Dendrimer toxicity: Let’s meet the challenge. Int J Pharm 394:122–142. doi:10.1016/j.ijpharm.2010.04.027
Jevprasesphant R, Penny J, Jalal R, Attwood D, McKeown NB, D'Emanuele A (2003) The influence of surface modification on the cytotoxicity of PAMAM dendrimers. Int J Pharm 252:263–266
Kim TI, Baek JU, Zhe Bai C, Park JS (2007) Arginine-conjugated polypropylenimine dendrimer as a non-toxic and efficient gene delivery carrier. Biomaterials 28:2061–2067
Kloeckner J, Bruzzano S, Ogris M, Wagner E (2006) Gene carriers based on hexanediol diacrylate linked oligoethylenimine: effect of chemical structure of polymer on biological properties. Bioconjug Chem 17:1339–1345
Kunath K, von Harpe A, Fischer D, Petersen H, Bickel U, Voigt K, Kissel T (2003) Low-molecular-weight polyethylenimine as a non-viral vector for DNA delivery: comparison of physicochemical properties, transfection efficiency and in vivo distribution with high-molecular-weight polyethylenimine. J Control Release 89:113–125
Lee CC, MacKay JA, Frechet JM (2005) Szoka FC. Designing dendrimers for biological applications Nat Biotechnol 23:1517–1526
Lim Y et al (2001) Cationic hyperbranched poly(amino ester): a novel class of DNA condensing molecule with cationic surface, biodegradable three-dimensional structure, and tertiary amine groups in the interior. J Am Chem Soc 123:2460–2461
Liu Q, Muruve DA (2003) Molecular basis of the inflammatory response to adenovirus vectors. Gene Ther 10:935–940
Liu H, Wang Y, Wang M, Xiao J, Cheng Y (2014) Fluorinated poly(propylenimine) dendrimers as gene vectors. Biomaterials 35:5407–5413. doi:10.1016/j.biomaterials.2014.03.040
Mahmoudi A, Oskuee RK, Ramezani M, Malaekeh-Nikoue B (2014) Preparation and in-vitro transfection efficiency evaluation of modified cationic liposome-polyethyleneimine-plasmid nanocomplexes as a novel gene carrier. Current Drug Delivery 11:636–642
Malaekeh-Nikouei B, Malaekeh-Nikouei M, Oskuee RK, Ramezani M (2009) Preparation, characterization, transfection efficiency, and cytotoxicity of liposomes containing oligoamine-modified cholesterols as nanocarriers to Neuro2A cells. Nanomedicine: Nanotechnology, Biology, and Medicine 5:457–462. doi:10.1016/j.nano.2009.02.001
Martinho N, Florindo H, Silva L, Brocchini S, Zloh M, Barata T (2014) Molecular modeling to study dendrimers for biomedical applications. Molecules 19:20424–20467. doi:10.3390/molecules191220424
Nimesh S, Aggarwal A, Kumar P, Singh Y, Gupta KC, Chandra R (2007) Influence of acyl chain length on transfection mediated by acylated PEI nanoparticles. Int J Pharm 337:265–274
Oskuee RK, Dehshahri A, Shier WT, Ramezani M (2009) Alkylcarboxylate grafting to polyethylenimine: a simple approach to producing a DNA nanocarrier with low toxicity. J Gene Med 11:921–932
Oskuee RK, Mohtashami E, Golami L, Malaekeh-Nikouei B (2014) Cationic liposomes-polyallylamine-plasmid nanocomplexes for gene delivery. J Exp Nanosci 9:1026–1034. doi:10.1080/17458080.2013.771245
Oskuee RK, Dosti F, Gholami L, Malaekeh-Nikouei B (2015) A simple approach for producing highly efficient DNA carriers with reduced toxicity based on modified polyallylamine. Mater Sci Eng C 49:290–296. doi:10.1016/j.msec.2015.01.016
Paleos CM, Tsiourvas D, Sideratou Z (2007) Molecular engineering of dendritic polymers and their application as drug and gene delivery systems. Mol Pharm 4:169–188. doi:10.1021/mp060076n
Parhiz H, Hashemi M, Hatefi A, Shier WT, Amel Farzad S, Ramezani M (2013) Arginine-rich hydrophobic polyethylenimine: potent agent with simple components for nucleic acid delivery. Int J Biol Macromol 60:18–27. doi:10.1016/j.ijbiomac.2013.05.001
Park SC, Nam JP, Kim YM, Kim JH, Nah JW, Jang MK (2013) Branched polyethylenimine-grafted-carboxymethyl chitosan copolymer enhances the delivery of pDNA or siRNA in vitro and in vivo. Int J Nanomedicine 8:3663–3677. doi:10.2147/IJN.S50911
Paul A, Eun C-J, Song JM (2014) Cytotoxicity mechanism of non-viral carriers polyethylenimine and poly-l-lysine using real time high-content cellular assay. Polymer 55:5178–5188. doi:10.1016/j.polymer.2014.08.043
Pednekar PP, Jadhav KR, Kadam VJ (2012) Aptamer-dendrimer bioconjugate: a nanotool for therapeutics, diagnosis, and imaging. Expert Opinion on Drug Delivery 9:1273–1288. doi:10.1517/17425247.2012.716421
Rekha MR, Sharma CP (2011) Hemocompatible pullulan-polyethyleneimine conjugates for liver cell gene delivery: in vitro evaluation of cellular uptake, intracellular trafficking and transfection efficiency. Acta Biomater 7:370–379. doi:10.1016/j.actbio.2010.07.027
Russ V, Gunther M, Halama A, Ogris M, Wagner E (2008) Oligoethylenimine-grafted polypropylenimine dendrimers as degradable and biocompatible synthetic vectors for gene delivery. J Control Release 132:131–140. doi:10.1016/j.jconrel.2008.09.003
Schatzlein AG et al (2005) Preferential liver gene expression with polypropylenimine dendrimers. J Control Release 101:247–258
Sun JY, Anand-Jawa V, Chatterjee S, Wong KK (2003) Immune responses to adeno-associated virus and its recombinant vectors. Gene Ther 10:964–976. doi:10.1038/sj.gt.3302039
Svenson S, Tomalia DA (2005) Dendrimers in biomedical applications—reflections on the field. Adv Drug Deliv Rev 57:2106–2129
Tack F et al (2006) Modified poly(propylene imine) dendrimers as effective transfection agents for catalytic DNA enzymes (DNAzymes). J Drug Target 14:69–86
Takahashi T, Kono K, Itoh T, Emi N, Takagishi T (2003) Synthesis of novel cationic lipids having polyamidoamine dendrons and their transfection activity. Bioconjug Chem 14:764–773
Taratula O et al (2009) Surface-engineered targeted PPI dendrimer for efficient intracellular and intratumoral siRNA delivery. J Control Release 140:284–293. doi:10.1016/j.jconrel.2009.06.019
Tziveleka LA, Psarra AM, Tsiourvas D, Paleos CM (2007) Synthesis and characterization of guanidinylated poly(propylene imine) dendrimers as gene transfection agents. J Control Release 117:137–146. doi:10.1016/j.jconrel.2006.10.019
Verma IM, Somia N (1997) Gene therapy—promises, problems and prospects. Nature 389:239–242
Yoo H, Juliano RL (2000) Enhanced delivery of antisense oligonucleotides with fluorophore-conjugated PAMAM dendrimers. Nucleic Acids Res 28:4225–4231
Zinselmeyer BH, Mackay SP, Schatzlein AG, Uchegbu IF (2002) The lower-generation polypropylenimine dendrimers are effective gene-transfer agents. Pharm Res 19:960–967
Acknowledgements
This work was founded by the National Science Foundation (INSF) (Grant number 91002289). The financial support by Mashhad University of Medical Sciences is also acknowledged. This study was a part of the MSc thesis of S. J. Alavi.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
ESM 1
(DOCX 951 kb)
Rights and permissions
About this article
Cite this article
Alavi, S.J., Gholami, L., Askarian, S. et al. Hyperbranched–dendrimer architectural copolymer gene delivery using hyperbranched PEI conjugated to poly(propyleneimine) dendrimers: synthesis, characterization, and evaluation of transfection efficiency. J Nanopart Res 19, 49 (2017). https://doi.org/10.1007/s11051-017-3739-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-017-3739-4