Abstract
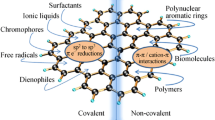
Graphene, as one of the most promising new materials, has a wide range of applications in biosensors, super-capacitors and catalysts. Herein, we focus on the covalent and noncovalent modification of both graphene and graphene oxide recently reported, and review the interesting properties, e.g., wider electrical band gap and higher dispersibility. We cover covalent derivatization of graphene and graphene oxide with various species, such as nitrenes, carbenes, aryl intermediates, polymers, biomaterials, carbon materials (fullerenes and carbon nanotubes), and organic molecules. As regards, noncovalent functionalization, we consider π–π interactions, van der Waals forces, ionic interactions, and hydrogen bonding. This review also covers some efforts to achieve tailored functionalization for applications. Finally, we assess the future prospects of covalently and noncovalently modified graphene and graphene oxide (Fig. 1).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Graphene, that is, one-atom thick, two-dimensional sp2-hybridized carbon arranged in a honey-comb crystal lattice, has received great attention because of its unrivalled thermal and electrical conductivity. The electronic properties of graphene were first studied experimentally in 2004 [1]. By virtue of its zero band-gap energy and the quantum hall effect under ambient conditions, graphene exhibits superior electronic, mechanical, optical and transport properties, resulting the applications in solar cells [2], biosensors [3], transparent conductive films [4], supercapacitors [5], contaminant removal [6] and flexible electronics [7].
Lots of methods have been used to prepare graphene, such as micromechanical or chemical exfoliation of graphite [8], chemical vapor deposition (CVD) growth [9, 10], and chemical, electrochemical, thermal, or photocatalytic reduction of graphene oxide (GO) [11].
However, there are still two shortcomings that researchers are striving to overcome for real applications of graphene. Firstly, because of the zero band-gap energy, graphene transistors have a small on–off ratio [12]. Secondly, exfoliated graphene cannot be dissolved and dispersed in most organic solvents [13].
Chemical functionalization of graphene offers obvious solution to the problems associated with graphene. Electron-donating or -withdrawing groups can be bonded to the graphene network by synthetic chemistry methods, which could contribute to the band-gap widening and good dispersibility in common organic solvents. Furthermore, these new functional groups might provide some additional capabilities. Conversely, modification through noncovalent interactions, such as π–π interactions, van der Waals forces, ionic interactions, and hydrogen bonding, can avoid major alteration of the structure and electronic properties of graphene. In addition, graphene oxide, which is more amenable to the chemical modification than pristine graphene, is also considered in this review. Its covalent and noncovalent functionalizations are also discussed. Finally, the applications of functionalized graphene and graphene oxide are discussed (Fig. 1).
2 Covalent Functionalization of Graphene
Multiple strategies have been utilized for the covalent modification of graphene. However, the use of radical species, including nitrene, carbene and aryl intermediates, is most prominent in forming covalent bonds with graphene.
2.1 Diazonium Salts
Using a diazonium salt to directly graft an aryl group on the sp2 carbon network of graphene has been widely applied to form covalently functionalized conducting or semiconducting materials [14]. In neutral or basic environments, a dinitrogen molecule is eliminated, and then, an electron is transferred from graphene to the diazonium salt to form an aryl radical.
Modification of graphene sheets with diazonium salts was first presented by Haddon’s group [15]. In their study, epitaxial graphene grown on SiC wafers was modified with aryl groups by treatment with (p-nitrophenyl)diazonium tetrafluoroborate. The reaction was facilitated by rapid electron transfer from the graphene layer to the diazonium salt. The authors also determined that there were ca. 1 × 1015 molecules/cm2 on the functionalized graphene surface and the resistance was enhanced. A further study involving the append of nitrophenyl groups onto graphene was reported by Strano et al. in 2010 [16]. They concluded that a single layer was far more reactive than a bi-or multi-layer according to Raman spectra and that the reactivity at the edge was at least twice that at the center of the single-layer graphene. In exploring the conductivity of modified graphene, Gao’s group estimated that a highly modified graphene material would have a higher charge carrier density because of the prominent charge-transfer effect imparted by the nitrophenyl groups compared with the scattering effect (Fig. 2), and thus the conductivity would be significantly enhanced [17].
Illustration of charge transfer effect brought by the nitrophenyl groups functionalized to graphene. a Fewer holes, as the charge carriers lead to the fewer electrons transferring and the scattering effect of nitrophenyl groups is more predominant. b More holes are present to top the scattering effect and result in the heightened conductivity of graphene material [17].
In order to control the surface potential of graphene, Stark et al. also investigated the modification with diazonium salts [18]. They utilized a photoresist, beneath which highly oriented pyrolytic graphite (HOPG) was preserved, and the exposed portion was then modified by highly diluted diazonium reagents (Fig. 3a). The surface potential (ΔΨ) of p-nitrophenyl-functionalized HOPG was found to be changed by −74 ± 16 mV compared with that of HOPG, which was observed in dependence on the Hammett substituent parameters (σp) (Fig. 3b).
a Modification of an HOPG surface with diazonium salts. b Change in surface potential and the Hammett substitution constant on the graphene stack [18].
Recent years further modifications of graphene with diazonium salts have emerged. Daasbjerg et al. used cyclic voltammetry to electrochemically reduce aryldiazonium salts and provided a versatile approach to functionalizing multi-layered graphene with a homogeneous distribution of defects on Ni [19]. Yang and his group employed a potentiodynamic technique to conduct electrochemical modification of graphene nanoplatelets with aryl diazonium salts [20]. By applying different types and amounts of reactive terminal groups (–Cl, –NO2 or –NH2), the interfacial properties of layers could be modified correspondingly. The resulting functionalized graphene nanoplatelets were then used to detect ions, such as Pb2+, NO2 − and SO3 2−.
As regards the application of graphene functionalized with groups from diazonium cations, Shervedani recently reported that a GC–GNs–Th electrode (glassy carbon (GC) electrode covalently modified with thionine (Th) diazonium cation through graphene nanosheets (GNs)) could be further functionalized to construct sensors for detecting glucose and nitrite [21].
2.2 Peroxides
Besides diazonium salts, phenyl radicals can also be generated from benzoyl peroxide. Brus et al. studied the photochemical reaction between benzoyl peroxide and graphene [22]. Under an Ar ion laser beam, single-layer graphene grown on the silicon substrate was immersed in a 5 mM solution, leading to a decrease in the electrical conductivity of the graphene flake and an increase in the hole doping level. Under irradiation, benzoate radicals were generated from benzoyl peroxides, and then decomposed to phenyl radicals by the elimination of CO2. The as prepared phenyl radicals played a vital role in the functionalization of the graphene sheet (Fig. 4).
Mechanism of generating the phenyl radical and then functionalizing to the graphene sheet [22].
Recently, researchers have found that by changing the twist angle (θ), the Van Hove singularities (VHSs) in twisted bilayer graphene (tBLG) can be tuned, which modifies the physical properties. Liu et al. explored the chemical reactivity of tBLG with VHSs through the photochemical reaction between graphene and benzoyl peroxide [23]. When the incident energy was equal to the energy gap of VHSs, the chemical reactivity of the tBLG could be greatly enhanced.
2.3 Nitrenes
Phenyl nitrene, as a highly reactive intermediate, is typically generated from azide groups through thermolysis or irradiation [24]. Researchers have utilized phenyl nitrene to chemically modify epitaxial graphene [25] through its [2 + 1] cycloaddition to the graphene sheets. At room temperature, benzazirine can be obtained by the isomerization of phenyl nitrene and then cyclized to the aromatic ketenimine in solution. Importantly, the obtained cyclic ketenimine can react with the raw material to form a polymer in the absence of amine. This reaction can also be induced by a photosensitizer, which would lead to PhN dimerization.
Based on this mechanism, Kim’s group used azidotrimethylsilane (ATS) to modify epitaxial graphene in vacuum. The band gap of the resulting modified graphene was 0.66 eV, larger than that of epitaxial graphene [26]. This functionalized graphene proved to be stable at temperatures up to 200 °C though nonmetallic, in contrast to the epitaxial graphene. The author stated that the band gap of epitaxial graphene was widened by the addition of one nitrene radical per 53 carbon atoms. Conversely, Iribarne’s group concluded that band-gap widening was not observed even for the adsorption of one NH group per 32 carbons atoms [27].
Perfluorophenyl azides (PFPAs) are known as highly efficient reagents suitable for covalently functionalizing carbohydrates [28], e.g., C60 [29] and graphene [30]. Phenyl azide can release N2 to form phenyl nitrene under photocatalysis, and then the phenyl nitrene can react with numerous organic molecules, resulting in different types of products [31]. In 2009, Yan’s group prepared derivatives of PFPAs and offered a simple mean of covalently modifying graphene with PFPAs. They heated mixtures of pristine graphene and PFPAs in the o-dichlorobenzene (DCB) as solvent. The functionalized graphene exhibited some new chemical functionalities because the PFPAs groups imparted solubility in both water or organic solvents [32].
Recent technological developments in X-ray photoelectron spectroscopy (XPS) allow its application to study nitrogen in PFPA-functionalized graphene sheets [33].
2.4 Carbenes
Carbenes are well-known reactive intermediates and have also been used to modify graphene sheets. There are two kinds of carbene precursors, namely chloroform and diazirines. In an alkaline environment, such as a concentrated sodium hydroxide solution, dichlorocarbene can be generated through thermal elimination from chloroform [34]. Diazirines, consisting of a carbon atom bound to two mutually double-bonded nitrogen atoms, feature a cyclopropene-like ring. Decomposition of diazirines by heating or irradiation can generate carbenes and diazo intermediates [35]. The reactivity of these carbenes is high because organic functional groups can react through C–H, N–H and O–H bonds, making it useful in the modification of carbon materials, such as carbon nanotubes [36], diamond [37], fullerene [38, 39], and reduced graphene oxide [40, 41]. In some respects, diazirines resemble azides, being stable in the absence of light, but immediately forming carbenes upon irradiation. However, the synthesis of diazirines is inefficient and time-consuming. Workentin et al. used 3-aryl-3-(trifluoromethyl)diazirines to prepare carbene-modified gold nanoparticles (AuNPs). Functionalized with both 1-decanethiol and a thiol-derivatized diazirine, AuNPs released N2 under photocatalysis to conditions to form a carbene. The product could react with reduced graphene oxide in THF as solvent to provide graphene–AuNPs (Fig. 5), and the resulting product could be used for detecting Pb2+ [40].
Process of making graphene–AuNPs from the 3-aryl-3-(trifluoromethyl)diazirine [40].
3 Noncovalent Functionalization of Graphene
The noncovalent modification of graphene is mostly based on van der Waals forces, electrostatic interactions, or π–π interactions with organic molecules or polymers. Compared with covalent functionalization, noncovalent functionalization would not disrupt the extended π system of graphene nanostructures, and thus not affect important properties of graphene, such as electrical conductivity and mechanical strength. This review covers the noncovalent interactions of graphene with aromatic species, organic molecules, other carbon nanostructures, and inorganic species.
3.1 π–π Interactions
Aromatic π–π interaction is regarded as one of the most interesting noncovalent interactions. Single-layer graphene interacts preferentially with small aromatic molecules, which can benefit the exfoliation of graphite and the stable dispersion of graphene derivatives, due to the extended aromatic system with almost planar geometry. Such interactions can also widen the band gap of graphene. Kuo found that the adsorption of aromatic molecules onto graphene, e.g., borazine (B3N3H6), triazine (C3N3H3) and benzene (C6H6), resulted in the widen of band gap to as much as 62.9 meV [42]. Zhou’s group used single-pyridine-linked (PDL) molecules sandwiched between two zigzag-edged graphene nanoribbons to study spin-dependent electron transport [43]. Prior to this research, Shin et al. had used density functional theory to study the effect of pyridine adsorption and an applied electric field on the band structure and metallicity of zigzag graphene nanoribbons (ZGNRs) [44]. They found that adsorption of any electron-accepting organic molecule, such as pyridine, on ZGNRs should provide a simple and useful way to widen the band gap. Loh used Pyridine-functionalized graphene as a metal-organic framework (MOF) to react with iron porphyrin, which formed a graphene–metalloporphyrin MOF [45]. As shown in Fig. 6, the authors used 5,10,15,20-tetrakis(4-carboxyl)-21H,23H-porphyrin (TCPP) and FeCl3 to form the MOF, designated as (Fe–P)n MOF. They used a pyridinium analogue as a donor–π–acceptor dye to functionalize r-GO sheets, and the resulted composite was designated as G-dye. Finally, (G-dye–FeP)n MOF was obtained by combining the two composites. Patra et al. [46] reported similar work employing TCPP and RGO to form a nanostructure. They found that the photocurrent of this composite system under visible light illumination was increased due to electron transfer from TCPP NR to rGO, suggesting potential application in solar light harvesting.
Formation and application of the (G-dye–FeP)n MOF [45].
Green et al. demonstrated a facile technique which used a triphenylene as the stabilizer to noncovalently functionalize the pristine few-layer graphene (FLG) in water without micelle formation [47]. The dispersion of the functionalized FLG was improved a lot and the stability against heat and lyophilization was also largely enhanced.
Mi et al. proposed a new method, whereby pyrene was strongly bounded on the surface of graphene quantum dots (GQDs) by π–π stacking interactions and was used to detect microRNAs (miRNAs) [48]. They designed the pyrene-functionalized molecular beacon probes (py-MBs) with 5′-modification of pyrene and 3′-modification of selected fluorescent dyes (Cy3 or Cy5) appended on the GQDs, making the sequence of the loop structures completely complementary to the miRNA targets. The pyrene unit served to shorten the distance between py-MBs and GQDs and to generate an increased fluorescence signal from dyes appended on the probes. When hybridized with the target miRNAs, the hairpin structure of py-MBs opened and formed more precise duplex structures. The distance between the GQDs and fluorescent dyes was thereby increased, which resulted in suppression of FRET and a decrease in the fluorescence signals of the dyes (Fig. 7).
Pyrene functionalized graphene application in the field of fluorescence [48].
Geng and Jung devised a new method for the reduction of GO through treatment with hydrazine hydrate to synthesize graphene functionalized with porphyrin dissolved in water [49]. A membrane made of the resulting functionalized graphene showed a low sheet resistance of was ca. 5 KΩ sq−1 with 80% transparency at 550 nm.
Besides simple aromatic molecules, polymers with repetitive aryl structural units can also be used to functionalize graphene. Compared with neat polymer materials, polymer nanocomposites can show superior thermal, mechanical, electrical and/or optical properties. Kim et al. [50] functionalized graphene with PEDOT through strong π–π interactions, and the resulting rGO showed improved colloidal stability.
3.2 Hydrogen Bonding
Tyagi et al. used poly(ethylene glycol) 200 (PEG 200) to functionalize reduced graphene oxide by γ-radiolysis [51]. Hydrogen bonding occurred between the hydroxyl groups of rGO and the oxygen atoms of PEG 200 molecules, resulting in an increase in the spacing of the graphene sheets and a decrease in the defect density of the carbon network in rGO.
3.3 Ionic Interactions
Ionic interactions can also occur between rGO and end-functionalized polymers, such as amine-terminated polystyrene, by which hydrophilic rGO might be transformed into a lipophilic rGO/polymer composite dispersible in organic solvents [52] (Fig. 8).
The transformation of the hydrophilic rGO into a lipophilic rGO/polymer composite [52].
4 Functionalization of Graphene Oxide
Graphene oxide (GO) consists of monolayer graphene sheets randomly functionalized with oxygen-containing functional groups, such as hydroxyl, carbonyl, carboxyl and epoxy. Inexpensive graphite can be chemically oxidized and ultrasonically exfoliated to generate GO [53]. Being hydrophilic with the presence of oxygen groups, GO can be easily covalently functionalized with organic groups.
4.1 Addition of Polymers
Polymer composites containing carbon nanoparticles have recently attracted much attention because of their favorable electronic, mechanical and gas barrier properties. In particular, polymer nanocomposites containing functionalized GO are among the most promising new materials for electrochemical applications. In this context, GO functionalized with polymers can be better dispersed.
Dai’s group functionalized graphene with amine-octaphenylsilsesquioxane (POSS). Amido groups derived from the amine-POSS could react with carboxyl groups on GO to complete the acylation reaction. The obtained product dissolved readily in various organic solvents, which was important for further applications [54]. With dicyclohexylcarbodiimide (DCC) as solvent, GO could be readily functionalized with amine-POSS (Fig. 9). The functionalization of GO with POSS converted it from hydrophilic to lipophilic (Fig. 9b, c). Moreover, the interlayer spacing of POSS–GO was increased compared with that of GO. POSS–graphene could be concentrated in THF of 30 mg/mL, and the solution proved to be stable without any precipitation even after 24 h under ambient conditions. Otherwise, rich amino compounds functionalized GO can enhance the adsorbability of heavy metal ions because of the chelation [55].
a Scheme of functionalizating the graphene oxide with POSS, b transformation of GO and POSS–GO from the water phase into (c) the CHCl3 phase, and d different concentrations of POSS–graphene in the solution of THF [54].
Geng’s group modified GO with polystyrene (PS) and poly(styrene–isoprene) (PSI). GO itself was utilized as a cationic initiator to homopolymerize styrene and to co-polymerize styrene and isoprene [56]. The resulting products showed improved dispersibility in common organic solvents (Fig. 10).
Optical images of a GO as a powder, b a powder of GO-g-PS, c a powder of GO-g-PSI (10:1), d a suspension of GO in THF, e a suspension of GO-g-PS in THF (0.2 mg mL−1), and f a suspension of GO-g-PSI (10:1) in THF (0.2 mg mL−1). The images d–f of the suspensions had been standing for 6 weeks [56].
Lim et al. used poly(9,9-di-n-octylfluorenyl-2,7-diyl) nanoparticles to enwrap GO nanoplatelets [57]. They started with a two-phase system consisting of water and the solution of polymer and GO in chloroform. This two-phase system was then emulsified and chloroform was removed. Photographs showed the emulsified solution with and without GO nanoplatelets (Fig. 11).
a Process of the preparation of GO-PFO NPs. b Photograph exhibited the emulsified solution with or without GO nanoplatelets under the UV light at 365 nm [57].
There are some disadvantages in the above methods more or less, such as the need for anhydrous conditions and harsh reagents. Pentzer et al. proposed a new method for the covalent functionalization of GO in acidic aqueous suspensions under ambient conditions, using the Pinner reaction between hydroxyl groups on rGO and nitriles [58].
4.2 Addition of Biomaterials
The numerous oxygen-containing functional groups distributed on GO nanosheets provide an ideal environment for the modification of biomolecules, and so are important in bio-related applications. In addition, GO is so-called “superquencher” that can quench the fluorescence of various dyes through energy transfer. Ye et al. proposed a new method employing peptides as probe biomolecules to establish a biosensor based on GO [59]. The resulting biosensor based on FRET between GO and dye-labeled peptides could be applied to monitor protease activity in real time. Peptides were adsorbed on GO through π–π Interactions. Similarly, biomolecules such as glucose oxidase [60], dopamine [61], DNA probes [62], and so on can be functionalized through π–π interactions with GO, allowing their application in sensors.
4.3 Addition of Carbon Nanoallotropes
Recently, researchers have identified a remarkable noncovalent interaction between carbon nanotubes (CNTs) and GO nanosheets that could lead to stable superstructures of these carbon hybrids, offering possible applications in environmental and sustainable energy fields. For example, 1D CNTs can be placed between 2D graphene oxide layers resulting in a versatile 3D graphene–CNT hybrid network. The resulting product precludes the stacking of graphene, so that electrical conductivity and mechanical stability would not be reduced. Chen et al. prepared a hybrid of GO functionalized with multiwalled carbon nanotubes (MWNTs), the π–π stacking interaction between which was probed by UV/Vis absorption spectroscopy [63]. This modified hybrid material exhibited good electro-catalytic properties in the determination of glucose and excellent analytical performance. Such hybrid materials may also be used for batteries, capacitors and so on. For example, single-walled carbon nanotubes (SWCNTs) combined with rGO gives a high capacitance [64]. Oh et al. synthesized G–CNT–Fe nanostructure, in which carbon nanotubes grew on graphene sheets by microwave irradiation as the anode material in lithium-ion batteries [65].
4.4 Addition of Organic Molecules
Functionalization of GO with organic molecules has attracted a great deal of attention continuously, especially with regard to improving the solubility in both water and organic solvents. GO functionalized with small organic molecules shows outstanding properties, such as photoactivity or electroactivity.
Mai et al. first presented the functionalization of GO with octadecylamine (ODA), which reacted with carboxylic groups on GO to form the amide bonds [66]. In 2013, Ryu et al. synthesized GO derivative with alkylamines of different chain lengths to form membranes [67]. Specifically, they used hexylamine (A6), decylamine (A10), hexadecylamine (A16) and octadecylamine (A18) to assess the influence of chain length on the hydrophobicity of the functionalized GO. As shown in Fig. 12, with increasing alkylamine chain length, the hydrophobicity of the product was increased.
Photograph of the contact angle of pristine and alkylamine-functionalized GO samples [67].
GO functionalized with amides provides intermediates that can be used to synthesize many compounds. Schaefer et al. prepared a nanocomposite through the functionalization of GO with octadecylamine (ODA) and polybutadiene (PBD) by solution mixing [68]. The toughness and elongation at break of this composite were improved by 332 and 191%, respectively, in comparison with those of pure PBD. Dai et al. used amine-functionalized GO to synthesize polyamide-6 (PA6)–graphene nanocomposites [69]. Kumar et al. used amide-functionalized GO to prepare thin films for hydrogen sulfide gas sensing [70]. The applications of amide-functionalized GO area thus demonstrably wide.
Other small molecules, such as imidazolium derivatives, are also worthy of mention. In 2010, Tagmatarchis et al. prepared hybrid materials of GO functionalized with imidazole and imidazolium bromide moieties to facilitate anion-exchange reactions [71]. In this case, carboxylic groups on GO reacted with 1-(3-aminopropyl)imidazole to form amide bonds. The functionalized GO was then treated with N-butyl bromide for N-alkylation of the imidazole (Fig. 13).
Process of preparing the imidazolium-modified graphene-oxide hybrid materials and anion exchange reactions [71].
Recently, Wang et al. first proposed distillation-precipitation polymerization and subsequent quaternization to synthesize imidazolium-functionalized GO (ImGO) nanosheets [72]. GO nanosheets were then functionalized with propyltrimethoxysilane (MPS) to introduce reactive vinyl groups, and polymerization was achieved through distillation-precipitation of the polymeric layer [poly(EGDMAco-VI)]. Cationic quaternary ammonium imidazolium chloride was synthesized through Menshutkin reaction and then grafted onto the GO sheets (ImGO) (Fig. 14).
Preparation of ImGO [72].
Stalikas described the functionalization of GO with 1-butyl-3-aminopropyl-imidazolium chloride. The product was used to adsorb anabolic steroids and β-blockers, which is important for environmental protection [73].
Besides the above organic molecules, porphyrins [74], aromatic dyes [75], pyrene [76] and other compounds have been used to covalently or noncovalently modify GO. For example, Xu et al. reported a new type of GO modified with a porphyrin derivative, namely 1-methyl-pyridinium-4-yl porphyrin (TMPyP) to form GOLMs [77]. The size of channels in the resulting GOLMs was around 1 nm, enabling high salt rejection performance. As illustrated in Fig. 15, GO and TMPyP was mixed together and then directly filtered to prepare the cross-linked GOLMs on porous substrates. In this case, GOLMs with different degrees of cross-linking could be fabricated by controlling the ratio of TMPyP to GO in the assembly process.
a Assembly of GO with TMPyP; fabrication of GOLMs Intercalated b without or c with TMPyP via a vacuum filtration process [77].
5 Conclusion and Prospects
In summary, since the electrical conductivity of graphene was first reported by Novoselov, attention from scientific community has increased exponentially. Over the past few years, researchers’ interests in this new material have continued to increase due to its extraordinary properties, which can be exploited in many fields, such as biology, electronics and catalysis. Herein, we have summarized various methods and common molecules that have hitherto been used to chemically modify graphene and graphene oxide, offering different ways to widen the electrical band gap and increase dispersibility. Although, there are still hindrances to overcome, graphene and its derivatives set to be applied flexible screens for mobile phones, graphene batteries and graphene-made body armor. Recently, many efforts have been spent into the water permeation of graphene and then new exploitations of this property is proceeding, for example, desalination, and water purification.
However, for any such future applications, reliable synthetic procedures must first be established. Indeed, using “less-than-perfect graphene” (e.g., rGO) is an easy method for various applications. However, one cannot accurately answer are the two rGO samples chemically similar if they were obtained from the same GO sample but were reduced? Thus it can be seen, the rGO will arrive its bottleneck in a short time. So, we need to seek a new method to synthesize a high quality monolayer graphene.
Finally, the uncertainty of toxicology of graphene is also a cause that hurdles the application in biosome.
References
K.S. Novoselov, A.K. Geim, S.V. Morozov, D. Jiang, Y. Zhang, S.V. Dubonos, I.V. Grigorieva, A.A. Firsov, Science 306, 666 (2004)
A. Reina, X.T. Jia, J. Ho, D. Nezich, H.B. Son, V. Bulovic, M.S. Dresselhaus, J. Kong, Nano Lett. 9, 3087 (2009)
R. Van Noorden, Nature 469, 14 (2011)
T. Tomasevic-Ilic, J. Pesic, I. Milosevic, J. Vujin, A. Matkovic, M. Spasenovic, R. Gajic, Opt. Quantum Electron. 48, 7 (2016)
V. Chandra, J. Park, Y. Chun, J.W. Lee, I.C. Hwang, K.S. Kim, ACS Nano 4, 3979 (2010)
W.H. Lee, J. Park, Y. Kim, K.S. Kim, B.H. Hong, K. Cho, Adv. Mater. 23, 3460 (2011)
S. Huh, J. Park, K.S. Kim, B.H. Hong, S.B. Kim, ACS Nano 5, 3639 (2011)
M. Lotya, Y. Hernandez, P.J. King, R.J. Smith, V. Nicolosi, L.S. Karlsson, F.M. Blighe, S. De, Z.M. Wang, I.T. McGovern, G.S. Duesberg, J.N. Coleman, J. Am. Chem. Soc. 131, 3611 (2009)
Z.Z. Sun, Z. Yan, J. Yao, E. Beitler, Y. Zhu, J.M. Tour, Nature 468, 549 (2010)
K.S. Kim, Y. Zhao, H. Jang, S.Y. Lee, J.M. Kim, K.S. Kim, J.H. Ahn, P. Kim, J.Y. Choi, B.H. Hong, Nature 457, 706 (2009)
Y.W. Zhu, S. Murali, M.D. Stoller, K.J. Ganesh, W.W. Cai, P.J. Ferreira, A. Pirkle, R.M. Wallace, K.A. Cychosz, M. Thommes, D. Su, E.A. Stach, R.S. Ruoff, Science 332, 1537 (2011)
Y.Q. Wu, Y.M. Lin, A.A. Bol, K.A. Jenkins, F.N. Xia, D.B. Farmer, Y. Zhu, P. Avouris, Nature 472, 74 (2011)
Y. Hernandez, V. Nicolosi, M. Lotya, F.M. Blighe, Z.Y. Sun, S. De, I.T. McGovern, B. Holland, M. Byrne, Y.K. Gun’ko, J.J. Boland, P. Niraj, G. Duesberg, S. Krishnamurthy, R. Goodhue, J. Hutchison, V. Scardaci, A.C. Ferrari, J.N. Coleman, Nat. Nanotechnol. 3, 563 (2008)
A. Sinitskii, A. Dimiev, D.A. Corley, A.A. Fursina, D.V. Kosynkin, J.M. Tour, ACS Nano 4, 1949 (2010)
E. Bekyarova, M.E. Itkis, P. Ramesh, C. Berger, M. Sprinkle, W.A. de Heer, R.C. Haddon, J. Am. Chem. Soc. 131, 1336 (2009)
R. Sharma, J.H. Baik, C.J. Perera, M.S. Strano, Nano Lett. 10, 398 (2010)
P. Huang, H.R. Zhu, L. Jing, Y.L. Zhao, X.Y. Gao, ACS Nano 5, 7945 (2011)
F.M. Koehler, N.A. Luechinger, D. Ziegler, E.K. Athanassiou, R.N. Grass, A. Rossi, C. Hierold, A. Stemmer, W.J. Stark, Angew. Chem. Int. Ed. 48, 224 (2009)
M. Lillethorup, M. Kongsfelt, M. Ceccato, B.B.E. Jensen, B. Jorgensen, S.U. Pedersen, K. Daasbjerg, Small 10, 922 (2014)
Z. Qiu, J. Yu, P. Yan, Z. Wang, Q. Wan, N. Yang, ACS Appl. Mater. Interfaces 8, 28291 (2016)
R.K. Shervedani, A. Amini, N. Sadeghi, Biosens. Bioelectron. 77, 478 (2016)
H.T. Liu, S.M. Ryu, Z.Y. Chen, M.L. Steigerwald, C. Nuckolls, L.E. Brus, J. Am. Chem. Soc. 131, 17099 (2009)
L. Liao, H. Wang, H. Peng, J.B. Yin, A.L. Koh, Y.L. Chen, Q. Xie, H.L. Peng, Z.F. Liu, Nano Lett. 15, 5585 (2015)
W.T. Borden, N.P. Gritsan, C.M. Hadad, W.L. Karney, C.R. Kemnitz, M.S. Platz, Acc. Chem. Res. 33, 765 (2000)
H.K. He, C. Gao, Chem. Mater. 22, 5054 (2010)
J. Choi, K.J. Kim, B. Kim, H. Lee, S. Kim, J. Phys. Chem. C 113, 9433 (2009)
P.A. Denis, F. Iribarne, J. Phys. Chem. C 115, 195 (2011)
L.H. Liu, H. Dietsch, P. Schurtenberger, M.D. Yan, Bioconjug. Chem. 20, 1349 (2009)
M.D. Yan, S.X. Cai, J.F.W. Keana, J. Org. Chem. 59, 5951 (1994)
L.H. Liu, M.D. Yan, Nano Lett. 9, 3375 (2009)
R. Poe, K. Schnapp, M.J.T. Young, J. Grayzar, M.S. Platz, J. Am. Chem. Soc. 114, 5054 (1992)
L.H. Liu, M.D. Yan, J. Mater. Chem. 21, 3273 (2011)
G. Zorn, L.H. Liu, L. Arnadottir, H. Wang, L.J. Gamble, D.G. Castner, M.D. Yan, J. Phys. Chem. C 118, 376 (2014)
J. Hine, J. Am. Chem. Soc. 72, 2438 (1950)
J. Brunner, H. Senn, F.M. Richards, J. Biol. Chem. 255, 3313 (1980)
E.J. Lawrence, G.G. Wildgoose, L. Aldous, Y.M.A. Wu, J.H. Warner, R.G. Compton, P.D. McNaughter, Chem. Mater. 23, 3740 (2011)
H. Ismaili, M.S. Workentin, Chem. Commun. 47, 7788 (2011)
Y. Maeda, Y. Matsunaga, T. Wakahara, S. Takahashi, T. Tsuchiya, M.O. Ishitsuka, T. Hasegawa, T. Akasaka, M.T.H. Liu, K. Kokura, E. Horn, K. Yoza, T. Kato, S. Okubo, K. Kobayashi, S. Nagase, K. Yamamoto, J. Am. Chem. Soc. 126, 6858 (2004)
T. Akasaka, M.T.H. Liu, Y. Niino, Y. Maeda, T. Wakahara, M. Okamura, K. Kobayashi, S. Nagase, J. Am. Chem. Soc. 122, 7134 (2000)
H. Ismaili, D.S. Geng, A.X.L. Sun, T.T. Kantzas, M.S. Workentin, Langmuir 27, 13261 (2011)
X.Y. Huang, T. Lan, B.C. Zhang, J.C. Ren, Analyst 137, 3659 (2012)
C.H. Chang, X.F. Fan, L.J. Li, J.L. Kuo, J. Phys. Chem. C 116, 13788 (2012)
X.B. Li, L.M. Cao, H.L. Li, H.Q. Wan, G.H. Zhou, J. Phys. Chem. C 120, 3010 (2016)
H. Park, J.Y. Lee, S. Shin. J. Phys. Chem. C 116, 20054 (2012)
M. Jahan, Q.L. Bao, K.P. Loh, J. Am. Chem. Soc. 134, 6707 (2012)
R. Bera, S. Mandal, B. Mondal, B. Jana, S.K. Nayak, A. Patra, ACS Sustain. Chem. Eng. 4, 1562 (2016)
S. Das, F. Irin, H.S.T. Ahmed, A.B. Cortinas, A.S. Wajid, D. Parviz, A.F. Jankowski, M. Kato, M.J. Green, Polymer 53, 2485 (2012)
H. Zhang, Y.S. Wang, D.W. Zhao, D.D. Zeng, J.Y. Xia, A. Aldalbahi, C. Wang, L.L. San, C.H. Fan, X.L. Zuo, X.Q. Mi, ACS Appl. Mater. Interfaces 7, 16152 (2015)
J.X. Geng, H. Jung, J. Phys. Chem. C 114, 8227 (2010)
K. Jo, T. Lee, H.J. Choi, J.H. Park, D.J. Lee, D.W. Lee, B.S. Kim, Langmuir 27, 2014 (2011)
B. Gupta, N. Kumar, K. Panda, A.A. Melvin, S. Joshi, S. Dash, A.K. Tyagi, J. Phys. Chem. C 120, 2139 (2016)
E.Y. Choi, T.H. Han, J.H. Hong, J.E. Kim, S.H. Lee, H.W. Kim, S.O. Kim, J. Mater. Chem. 20, 1907 (2010)
D.R. Dreyer, S. Park, C.W. Bielawski, R.S. Ruoff, Chem. Soc. Rev. 39, 228 (2010)
Y.H. Xue, Y. Liu, F. Lu, J. Qu, H. Chen, L.M. Dai, J. Phys. Chem. Lett. 3, 1607 (2012)
Y. Yuan, G.H. Zhang, Y. Li, G.L. Zhang, F.B. Zhang, X.B. Fan, Polym. Chem. 4, 2164 (2013)
B.P. Li, W.P. Hou, J.H. Sun, S.D. Jiang, L.L. Xu, G.X. Li, M.A. Memon, J.H. Cao, Y. Huang, C.W. Bielawski, J.X. Geng, Macromolecules 48, 994 (2015)
D.Y. Yoo, N.D.K. Tu, S.L. Lee, E. Lee, S.R. Jeon, S. Hwang, H.S. Lim, J.K. Kim, B.K. Ju, H. Kim, J.A. Lim, ACS Nano 8, 4248 (2014)
B.T. McGrail, B.J. Rodier, E. Pentzer, Chem. Mater. 26, 5806 (2014)
M. Zhang, B.C. Yin, X.F. Wang, B.C. Ye, Chem. Commun. 47, 2399 (2011)
X.T. Tian, S. Lian, L.M. Zhao, X.M. Chen, Z.Y. Huang, X. Chen, J. Solid State Electrochem. 18, 2375 (2014)
I. Kaminska, M.R. Das, Y. Coffinier, J. Niedziolka-Jonsson, J. Sobczak, P. Woisel, J. Lyskawa, M. Opallo, R. Boukherroub, S. Szunerits, ACS Appl. Mater. Interfaces 4, 1016 (2012)
J. Zhou, Q. Lu, Y. Tong, W. Wei, S.Q. Liu, Talanta 99, 625 (2012)
V. Mani, B. Devadas, S.M. Chen, Biosens. Bioelectron. 41, 309 (2013)
N. Jha, P. Ramesh, E. Bekyarova, M.E. Itkis, R.C. Haddon, Adv. Energy Mater. 2, 438 (2012)
S.-H. Lee, V. Sridhar, J.-H. Jung, K. Karthikeyan, Y.-S. Lee, R. Mukherjee, N. Koratkar, I.-K. Oh, ACS Nano 7, 4242 (2013)
W.J. Li, X.Z. Tang, H.B. Zhang, Z.G. Jiang, Z.Z. Yu, X.S. Du, Y.W. Mai, Carbon 49, 4724 (2011)
A.M. Shanmugharaj, J.H. Yoon, W.J. Yang, S.H. Ryu, J. Colloid Interface Sci. 401, 148 (2013)
Y. Zhang, J.E. Mark, Y.W. Zhu, R.S. Ruoff, D.W. Schaefer, Polymer 55, 5389 (2014)
W.J. Hou, B.Q. Tang, L.L. Lu, J. Sun, J.J. Wang, C.X. Qin, L.X. Dai, RSC Adv. 4, 4848 (2014)
S. Rani, M. Kumar, R. Garg, S. Sharma, D. Kumar, IEEE Sens. J. 16, 2929 (2016)
N. Karousis, S.P. Economopoulos, E. Sarantopoulou, N. Tagmatarchis, Carbon 48, 854 (2010)
H.L. Chen, J.S. Wang, H.J. Bai, J. Sun, Y.F. Li, Y. Liu, J.T. Wang, RSC Adv. 5, 88736 (2015)
M. Serrano, T. Chatzimitakos, M. Gallego, C.D. Stalikas, J. Chromatogr. A 1436, 9 (2016)
Y.F. Xu, Z.B. Liu, X.L. Zhang, Y. Wang, J.G. Tian, Y. Huang, Y.F. Ma, X.Y. Zhang, Y.S. Chen, Adv. Mater. 21, 1275 (2009)
H. Liu, J. Gao, M.Q. Xue, N. Zhu, M.N. Zhang, T.B. Cao, Langmuir 25, 12006 (2009)
Q. Su, S.P. Pang, V. Alijani, C. Li, X.L. Feng, K. Mullen, Adv. Mater. 21, 3191 (2009)
X.L. Xu, F.W. Lin, Y. Du, X. Zhang, J. Wu, Z.K. Xu, ACS Appl. Mater. Interfaces 8, 12588 (2016)
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (21375051, 21605062, 51504242), Natural Science Foundation of Jiangsu Province (BK20140191), and the Natural Science Foundation of Jiangsu Normal University (15XLR011), the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD), and the Brand Major of Universities in Jiangsu Province, the Top-notch Academic Programs Project of Jiangsu Higher Education Institution (TAPP).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Yang, Gh., Bao, Dd., Liu, H. et al. Functionalization of Graphene and Applications of the Derivatives. J Inorg Organomet Polym 27, 1129–1141 (2017). https://doi.org/10.1007/s10904-017-0597-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10904-017-0597-6