Abstract
In this paper reports synthesis of a new kind of Schiff base-polyurethane containing azomethine linkage and uses of this poly(azomethine-urethane) as fluorescence probe for determination of transition metal ions in aqueous medium. The spectroscopic behavior of poly(azomethine-urethane) toward metal ions has been investigated by the fluorescence method and photoluminescence (PL) characteristics of compounds were investigated in different polarity solvents including MeOH, tetrahydrofurane (THF) and dimethylformamide (DMF). PL measurements showed that both Schiff base and polymer have higher emission intensity and Stoke’s shift value (ΔλST) in THF solution than DMF and MeOH. The proposed sensor was found to show good selectivity to Zn2+ ion over other metal ions in THF/deionized water solution (1:2, v:v). Notably, the proposed sensor could clearly distinguish Zn2+ from Cd2+. Detection limit of the proposed sensor was also determined as 3.06 × 10−4 mol L−1.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Along with the various kinds of chemical sensors, fluorescent sensors have many advantages such as high sensitive, simple manipulation, facile visualization, low cost, ease of handling and real-time monitoring with fast response time due to photoluminescence (PL) measurements [1–3]. Because of these advantages, fluorescent sensors or probes for biologically active metal ions have been considerable interest in recent years and they are a lot of applications in the field of material, biological and environmental sciences [4, 5].
Among the various kinds of transition metal ions, zinc is the second most abundant transition metals in human body after iron [6]. Zn2+ plays a significant role in various physiological and pathological processes such as gene expression, enzyme regulation, neurotransmission, apoptosis regulation [7], DNA binding or recognition [8], neural signal transmission [9] and brain function [10]. Additionally, Zn2+ exists in various biological systems including pancreas, prostate, retina and brain [11, 12]. This metal ion is also known to have a role in neurological disorders, such as Alzheimer’s disease, cerebral ischemia, epilepsy, amyotrophic lateral sclerosis (ALS), Parkinson’s disease and hypoxia ischemia [13, 14]. On the other hand, excessive quantities of Zn2+ intake may lead to both acute and chronic toxicity, and excessive concentration of this metal ion may reduce the soil microbial activity resulting in phytotoxic effects [15, 16]. Moreover, zinc ion (Zn2+) has similar coordination properties with cadmium ion (Cd2+) and they are difficult to distinguish due to they are both in the IIB group elements of the periodic table [17]. Because of these properties, Zn2+ and Cd2+ ions often respond together with similar spectral changes. Therefore, there is a great importance to developing fluorescencent Zn2+ selective sensor or probe that distinguish Zn2+ from other transition metal ions especially Cd2+ in the biological system, material and environmental science.
In this paper, we have designed and prepared a Schiff base-polymeric fluorescent probe for detection of transition metal ions including Cd2+, Co2+, Cr3+ Cu2+, Mn2+, Ni2+, Pb2+, Zn2+ and Cr3+. Polymeric probe was prepared in two steps. In the first step, Schiff base was synthesized by condensation reaction of 2,2′-dithio-dianiline with 4-(diethylamino)salicylaldehyde. In the second step, this preformed Schiff base was converted to polyurethane derivative by step-polymerization using toluene diisocyanate. As known, Schiff base or azomethine (–N=CH) ligand exhibits a strong affinity for transition metal ions due to nitrogen atom in the structure and they have special coordination ability with transition metal ions [18]. The prepared compounds were characterized by FT-IR, 1H, 13C-NMR and SEC techniques. Photophysical behaviors of Schiff base and its poly(azomethine-urethane) derivative were studied in different polarity solvents such as dimethylformamide (DMF), MeOH or tetrahydrofurane (THF), and in presence of different metal ions, focusing the attention on their emission properties using fluorescence spectroscopy. To the best of our knowledge, there are only a few paper in literature about poly(azomethine-urethane)-based fluorescent sensor or probe for determination of transition metal ion in aqueous solution.
2 Experimental
2.1 Materials
2,2′-Dithio-dianiline (TDA), 4-(diethylamino)salicylaldehyde (SAL), toluene diisocyanate (TDI), acetone, acetonitrile (MeCN), carbon tetrachloride (CCl4), chloroform (CHCl3), DMF, ethanol (EtOH), ethyl acetate, methanol (MeOH), n-hexane, THF, toluene, Mn(CH3COO)2·H2O and Pb(CH3COO)2·3H2O were supplied from Merck Chemical Co. (Germany), Cd(CH3COO)2·2H2O, Co(CH3COO)2·4H2O, Cu(CH3COO)2·H2O, Ni(CH3COO)2·4H2O and Zn(CH3COO)2·2H2O were from Fluka, CrCl3 and ZrCl4 were from Riedel–Dehaen, and all the reagents were used without further purification.
2.2 Synthesis of 6,6′-((1Z,1′Z)-((disulfanediylbis(2,1-phenylene))bis(azanylylidene)) bis(methanylylidene))bis(3-(diethylamino)phenol) (SPAMEP)
SPAMEP was prepared by the condensation of 2,2′-dithio-dianiline (TDA, 2.484 g, 1 × 10−2 mol) with 4-(diethylamino)salicylaldehyde (SAL, 3.865 g, 2 × 10−2 mol) in MeOH (30 mL) by stirring the mixture under reflux for 3 h, and then cooling at room temperature (Scheme 1). The Schiff base was washed with MeCN (2 × 50 mL) to remove the unreacted components and dried in a vacuum desiccator at 75 °C [19].
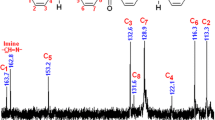
Yield: 88 %. 1H-NMR (DMSO-d 6 , δpmp): 10.06 (–OH), 8.78 (–imine (–N=CH)), 7.75, 7.54, 7.50, 7.43, 7.39, 6.41 and 6.13 (aromatic protons), 3.45 (–CH 2–CH3) and 1.19 (–CH2–CH 3). 13C-NMR (DMSO-d 6 , δpmp): 162.55 (–C–OH), 159.50 ((–imine (–N=CH)), 156.47, 153.80, 140.12, 136.44, 132.73, 128.84, 125.50, 117.60, 111.82, 109.35, 104.48 (aromatic carbons), 47.60 (–CH2–CH3) and 13.40 (–CH2–CH3).
2.3 Synthesis of (5-(diethylamino)-2-((Z)-((2-((2-((Z)-(4-(diethylamino)-2-methoxybenzylidene)amino)cyclohexa-1,3-dien-1-yl)disulfanyl)phenyl)imino)methyl)phenyl (5-acetamido-2-methylphenyl)carbamate) (P-SPAMEP)
P-SPAMEP was prepared by the step polymerization reaction of the preformed Schiff base (1.197 g, 2 × 10−3 mol) in DMF/THF (1/3) mixture (60 mL) with TDI (0.348 g, 2 × 10−3 mol) in THF (30 mL) by stirring the mixture. Reaction was maintained for 6 h under argon atmosphere and, then mixture cooled at the room temperature and kept for 24 h (Scheme 1). P-SPAMEP was washed with MeOH (2 × 50 mL) and MeCN (2 × 50 mL) to remove the unreacted components and dried in a vacuum oven at 75 °C for 24 h [20].
Yield: 79 %. 1H-NMR (DMSO-d 6 , δpmp): 9.72 (urethane–NH) 8.93 (–imine (–N=CH)), 7.98, 7.68, 7.46, 7.39, 7.34, 7.30, 7.25, 7.21, 6.68 and 6.49 (aromatic protons) 3.48 (–CH 2–CH3), 2.18 (–CH 3) and 1.24 (–CH2–CH 3), 13C-NMR (DMSO-d 6 , δpmp): 168.84 (urethane–C=O), 160.26 (imine (–N=CH)), 157.65, 154.24, 151.82, 138.58, 136.43, 134.78, 132.85, 130.7, 129.13, 127.84, 126.95, 124.87, 118.33, 117.97, 114.58, 113.20, 111.72, 108.14 (aromatic carbons), 47.63 (–CH2–CH3), 18.16 (–CH3), and 13.36 (–CH2–CH3), SEC: Mn: 21,600 g mol−1, Mw: 39,800 g mol−1, PDI:1.843.
2.4 Preparation of the Stock Solutions
A 1.00 × 10−3 M solution of P-SPAMEP (4.01 × 10−2 g, 5.00 × 10−5 mol) was prepared in 50 mL DMF, MeOH or THF, respectively. Solutions of Cd2+, Co2+, Cu2+, Mn2+, Ni2+, Pb2+ and Zn2+ were prepared from their acetate salts while solutions of Cr3+ and Zr2+ prepared from their chloride salts. Concentrations of these solutions were adjusted as 1.00 × 10−1 M in deionized water. These prepared stock solutions were also used in all measurements.
2.5 Characterization Techniques
The infrared spectra were obtained by Perkin Elmer FT-IR Spectrum one using the universal ATR sampling accessory (4000–550 cm−1). 1H and 13C-NMR spectra (Bruker AC FT-NMR spectrometer operating at 400 and 100.6 MHz, respectively) were recorded in deuterated DMSO-d6 at 25 °C. Tetramethylsilane (TMS) was used as internal standard. The number-average molecular weights of poly(azomethine-urethane) were determined by size exclusion chromatography (SEC) technique of Shimadzu Co. For SEC investigations, an SGX (100 Å and 7 nm diameter loading material) 3.3 mm i.d. × 300 mm columns was used; eluent: DMF (0.4 ml min−1), polystyrene standards were used. A refractive index detector (RID) was used to analyze the products at 25 °C.
2.6 Fluorescence Measurements
A Shimadzu RF-5301PC spectrofluorophotometer was used for fluorescence measurements. PL measurements of SPAMEP and P-SPAMEP were carried out in MeOH, THF and DMF solutions. Emission spectra of these compounds were obtained MeOH, THF and DMF with the concentration of 1 × 10−3 M SPAMEP and P-SPAMEP due to determine the optimal emission and excitation wavelengths in each solvent. The effects of transition metal ions on quenching-growing of the emission spectra were investigated in 1:2 (v:v) THF/deionized water solutions each of which containing 1 × 10−3 M of P-SPAMEP and 1 × 10−1 M metal ion. The changes of fluorescence intensities depending on the concentration of Zn2+ ions were determined using a series of different concentrated metal solutions in THF/deionized water mixtures (1:2, v:v). Excitation and emission slit width was adjusted as 5 nm in the mentioned experiments. Another series of Zn2+ containing P-SPAMEP solutions were prepared to determine the detection limit. For this purpose several solutions with different concentrated Zn2+ in the range 1.000 × 10−3 to 3.125 × 10−5 M were used. In detection limit experiments excitation slit width was adjusted as 10 nm while the emission slit was kept as 5 nm. This slit change made the intensity differences more detectable due to the increasing separation of emission peaks from each other. Fluorescence quantum yield (ΦF) is calculated by comparative methods using rhodamine 6G solution in ethanol as in the literature [21].
2.7 Morphological Properties
Atomic force microscopy (AFM) topography and 3D images of P-SPAMEP were recorded using WITec Alpha 300A AC mode (cantilever 42 N/m 285 kHz) AFM instrument. The measurements were carried out at room temperature. The system is covered with an acoustic chamber to prevent electromagnetic impacts, which may disturb the measurements.
3 Results and Discussion
3.1 Solubility and Characterization
The prepared SPAMEP and P-SPAMEP are red and dark-red colored powder compounds, respectively. According to the solubility tests results, P-SPAMEP is soluble in THF and DMF partly soluble in MeOH and EtOH whereas it is insoluble in n-hexane, benzene, toluene, acetone, MeCN, CHCl3, CCl4 and ethyl acetate.
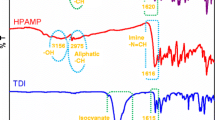
The chemical structures of SPAMEP and P-SPAMEP were verified by FT-IR, NMR and SEC analyses. FT-IR spectra of SAL, TDA, SPAMEP, TDI and P-SPAMEP were shown in Fig. 1. According to FT-IR spectra of SAL, hydroxyl (–OH), aldehyde (–CHO) and aliphatic –CH stretch vibrations are observed at 3288 and 1620 cm−1, respectively. Amine (–NH2) and –C–S stretch vibrations of TDA are observed at 3376 and 745 cm−1, respectively [22]. After the condensation reaction of these two compounds stretch vibrations (aldehyde and amine) disappear and the new stretch vibration appears at 1635 cm−1 indicating imine (–N=CH) stretch vibration. Also, hydroxyl (–OH), aliphatic –CH and –C–S stretch vibrations of SPAMEP are observed at 3298, 2972 and 753 cm−1, respectively. As can be seen in Fig. 1, characteristic isocyanate stretch vibrations (–N=C and –C=O) of TDI are observed at 1615 and 2230 cm−1, respectively [23]. After the step-polymerization reaction of SPAMEP and TDI, isocyanate stretch vibrations of TDI and hydroxyl (–OH) stretch vibration of SPAMEP disappear due to urethane formation. According to FT-IR spectrum of P-SPAMEP, urethane –NH and –C=O, imine (–N=CH), –C–S and aliphatic –CH stretch vibrations are observed at 3282, 1714, 1605, 2968 and 757 cm−1, respectively. These spectral data clearly confirm the formation of proposed SPAMEP and P-SPAMEP in Scheme 1. Additionally, NMR spectral data of SPAMEP and P-SPAMEP were given in experimental section.
3.2 PL Characteristics of SPAMEP and P-SPAMEP
To investigate of the solvent effect on SPAMEP and P-SPAMEP, the excitation and emission spectra of these compounds (1.00 × 10−3 M) were recorded in different polarity solvents such as MeOH, THF or DMF using PL spectrophotometer (Fig. 2). The fluorescence spectral data of SPAMEP and P-SPAMEP were also summarized in Table 1. Emission intensities of SPAMEP and P-SPAMEP are determined as 21 and 70 in MeOH, 37 and 122 in THF and 16 and 8 in DMF, respectively. Stoke’s shift values of SPAMEP and P-SPAMEP are also found as 71 and 75 nm in MeOH, 80 and 102 nm in THF, 60 and 62 nm in DMF, respectively. These obtained results shown that, SPAMEP and P-SPAMEP have higher emission intensity and Stoke shift value in THF solution than the other solvents. This can be probably polarity of solvents (polarity order of solvents: DMF > MeOH > THF) [24, 25]. Furthermore, Stoke’s shift is very important parameter for fluorescence sensor or probe due to the higher Stoke’s shift value supplies very low background signals and resultantly allows the usage of the material in construction of a fluorescence sensor [26]. Also, the quantum yield (ΦF) of P-SPAMEP-Zn2+ solution and in the absence of Zn2+ ions was found to be 4.6 and <0.04 %, respectively.
3.3 Selective of P-SPAMEP Fluorescence Response by Zn(II)
1.00 × 10−1 M solutions of Cd3+, Co2+, Cr2+, Cu2+, Mn2+, Ni2+, Pb2+, Zn2+ and Zr4+ were used to investigate the metal ion selection of P-SPAMEP, as shown in Fig. 3. In these selectivity studies, polyurethane concentration adjusted as 1.00 × 10−3 M due to minimize the PL intensity of metal-free polymer solution in working range. The obtained results were also summarized in Table 2. It can be seen that the emission peak of Cd2+, Zr4+, Ni2+ and Zn2+ is increased at 487 nm while this peak of Pb2+, Mn2+, Co2+, Cr3+ and Cu2+ is decreased at the same wavelength. According to Table 2, the emission intensities of Cu2+, Cr3+, Co2+, Mn2+, Pb2+, Cd2+, Ni2+, Zr4+ and Zn2+ are found as 55, 78, 112, 126, 134, 150, 165, 213 and 966 at 487 nm respectively. According to these results, Zn2+ has about 4.5 and 17.5 times more emission intensity than Zr4+ and Cu2+, respectively. These results indicatates that P-SPAMEP could be used as very selective Zn2+ sensor in aqueous solution.
3.4 Concentration Effect of Zn(II) on P-SPAMEP
The fluorescence property of P-SPAMEP and its responses of Zn2+ ion concentration were investigated in THF/deionized water (1:2, v:v) at 487 nm (Fig. 4a). As can be seen in Fig. 4a, with the decreases of Zn2+ ion concentration the emission intensity continuously decreased. Also, 3D images of emission intensity changes of Zn2+ ion concentration was shown in Fig. 4b.
To calculate regression equations and coefficients, relative emission intensities (I − Io/Io) at 487 nm were obtained from Fig. 4. Then, these intensities were plotters vs. Zn2+ ion concentration in the range 1.000 × 10−3 to 3.125 × 10−5 M, as shown in Fig. 5. Also, the obtained equations were given in Eqs. 1 and 2.
where I is the emission intensity of tested metal ion at 487 nm and I0 is the emission intensity of metal free polyurethane solution. According to these results, a good linearity relationship is obtained with regression coefficient R1 = 0.9415 and R2 = 0.9996. These calculated regression equations and coefficients could be assumed that the novel fluorescent poly(azomethine-urethane) had potential prospects as a selective detector of Zn2+ ion in aqueous environment.
The detection limit of sensor is another important parameter in sensor applications. The detection limit of Zn2+ sensor was calculated as in the literature [27] and the detection limit of sensor was calculated as 3.06 × 10−4 mol L−1.
3.5 Interference Study
To investigate interference and anti-interference effect of the other transition metal ions, interference study of the proposed sensor was carried out using 1 × 10−1 M metal ion and 1 × 10−3 M stock polyurethane solution at 487 nm (Fig. 6). The obtained results shown that P-SPAMEP and Zn2+ solution mixture have the highest relative intensity (5.69). Also, this mixture have about 12, 38 and 142 times more relative intensity than Zr4+ (0.48), Ni2+ (0.15) and Zr4+ (0.04), respectively.
On the other hand, according to the electronic configuration of these transitional metal ions, Cd2+ and Zn2+ ions have similar electronic configuration in last orbits. Both Cd2+ and Zn2+ ions have d 10 electronic structure in last orbits. Due to this property, Zn2+ fluorescent probe can also respond to Cd2+ [28]. In this study, the proposed Zn2+ fluorescent probe was not responded to Cd2+ and it could clearly distinguish Zn2+ from Cd2+.
3.6 Quenching Ion Effect
Quenching ion effect on the proposed fluorescent sensor were investigated using 1 × 10−3 M P-SPAMEP, 1 × 10−1 M Zn2+ in THF/deionized water (2:1, v:v) and 1 mg metal ion such as Cd2+, Co2+, Cr3+, Cu2+, Mn2+, Ni2+, Pb2+ or Zr4+ as shown in Fig. 7. As can be seen in Fig. 7, the presence of some transitional metal ions such as Cd2+, Co2+, Cr3+, Cu2+, Mn2+, Ni2+, Pb2+ or Zr4+ caused only slight changes in the emission spectra of the proposed sensor.
3.7 Binding Model and Responsive Mechanism
To determine binding model between P-SPAMEP and Zn2+, molecular orbital charge density and charge of atom or groups in P-SPAMEP was calculated using Huckel calculation method [27]. Figure 8a shows the molecular orbital charge density of P-SPAMEP. As can be seen in Scheme 1, –S–S, imine (–N=CH), urethane (–NH), (–C=O) and (–O–) groups or atoms composed a conjugated chromophore moiety in the proposed sensor. According to Fig. 8a, imine nitrogens (–N=CH) and –S–S groups have higher charge density than urethane group. This indicates that the possible complexation could be mainly carried out between these groups.
The calculated charge of atom or groups in structure of poly(azomethine-urethane) is shown in Fig. 8b. As can be seen in Fig. 8b, charge of sulfur atom in –S–S bond, nitrogen atom in imine group (–N=CH), nitrogen (–NH), oxygen (–O) and carbon atoms (–C=O) in urethane bonding are calculated as −0.1015, −0.629, −0.547, −0.2325 and −0.57, respectively. According to these results, imine nitrogens have quite higher negative charges than the other heteroatoms.
The possible structure of Zn2+ with P-SPAMEP is given in Fig. 8c. As known, polymeric Schiff base contain one or more imine (–N=CH) linkages in the structures and they readily form stable complexes with most of the metal ions [29]. According to these explanations, calculated molecular orbital charge diagram and charges of atoms or groups in the structure of poly(azmethine-urethane)s, the possible complexation could be mainly carried out with the imine nitrogens and –S–S bond.
3.8 The Time-Resolved Curve
The time resolved PL spectrum of P-SPAMEP (1 × 10−3 M) and Zn2+ ion (1 × 10−1 M) complex in THF/deionized water solution (1:2, v:v) is shown in Fig. 9 and results are also summarized in Table 3. As can be seen in Fig. 9 and Table 3, the fluorescence lifetime data of the proposed sensor were found as 0.62, 1.97, 3.73, 6.10, 8.28, 10.97, 14.08, 16.46, 18.22 and 20.08 % for 60, 120, 180, 240, 300, 360, 420, 480, 540 and 600 s, respectively. These obtained results shown that P-SPAMEP and Zn2+ complex is quite stable.
3.9 Morphological Properties
Morphological properties of poly(azomethine-urethane)s were investigeted by using AFM, as shown in Fig. 10. This technique was used in order to evaluate the evolution of topography and the phase roughness of P-SPAMEP. According to AFM images of the proposed polymeric probe, the surface of this compound seems to be rough and dense with non-uniform dispersion.
4 Conclusion
A novel Schiff base-polyurethane containing azomethine linkage was successfully synthesized and designed as fluorescent sensor for detection of transition metal ions in aqueous solutions. The spectroscopic behavior of poly(azomethine-urethane) was investigated using fluorescence measurements in different polarity solvents (DMF, MeOH and THF). Fluorescence measurements shown that the proposed probe have higher emission intensity and Stoke’s shift value (ΔλST) in THF solution than the other solvents. The proposed sensor was found to show good selectivity to Zn2+ ion over other metal ions in THF/de-ionized water solution (1:2, v:v). Notably, the proposed sensor could clearly distinguish Zn2+ from Cd2+ and possible interference and quenching effect of the other tested transition metal ions were found too low. Detection limit of the proposed sensor was also determined as 3.06 × 10−4 mol L−1.
References
D. Zhang, J.R. Cochrane, A. Martinez, G. Gao, RSC Adv. 4, 29735–29749 (2014)
T. Hong, H. Song, X. Li, W. Zhang, Y. Xie, RSC Adv. 4, 6133–6140 (2014)
S. Anbu, R. Ravishankaran, M.F.C. Guedes da Silva, A.A. Karande, A.J.L. Pombeiro, Inorg. Chem. 53, 6655–6664 (2014)
K. Tayade, S.K. Sahoo, B. Bondhopadhyay, V.K. Bhardwaj, N. Singh, A. Basu, R. Bendre, A. Kuwar, Biosens. Bioelectron. 61, 429–433 (2014)
L. Wang, W. Qin, W. Liu, Anal. Methods 6, 1167–1173 (2014)
H.M. Liu, P. Venkatesan, S.P. Wu, Sensor. Actuat. B-Chem. 203, 719–725 (2014)
L. Wang, W. Qin, W. Liu, Inorg. Chem. Commun. 13, 1122–1125 (2010)
J. Sun, T. Yu, H. Yu, M. Sun, H. Li, Z. Zhang, H. Jiang, S. Wang, Anal. Methods 6, 6768–6773 (2014)
E.L. Que, D.W. Domaille, C.J. Chang, Chem. Rev. 108, 1517–1549 (2008)
E. Mocchegiani, C. Bertoni-Freddari, F. Marcellini, M. Malavolta, Prog. Neurobiol. 75, 367–390 (2005)
A. Takeda, Biometals 14, 343–351 (2001)
C.J. Frederickson, L.J. Giblin III, B. Rengarajan, R. Masalha, C.J. Frederickson, Y. Zeng, E.V. Lopez, J.Y. Koh, U. Chorin, L. Besser, M. Hershfinkel, Y. Li, R.B. Thompson, A. Krezer, J. Neurosci. Methods 154, 19–29 (2006)
N. Roy, H.A.R. Pramanik, P.C. Paul, S.T. Singh, J. Fluoresc. 24, 1099–1106 (2014)
L. Li, F. Liu, H.W. Li, Spectrochim. Acta A-M. 79, 1688–1692 (2011)
M. Iniya, D. Jeyanthi, K. Krishnaveni, A. Mahesh, D. Chellappa, Spectrochim. Acta A-M. 120, 40–46 (2014)
A. Voegelin, S. Pfister, A.C. Scheinost, M.A. Marcus, R. Kretzschmar, Changes in Zinc Speciation in Field Soil after Contamination with Zinc Oxide. Environ. Sci. Technol. 39, 6616–6623 (2005)
J.T. Hou, B.Y. Liu, K. Li, K.K. Yu, M.B. Wu, X.Q. Yu, Talanta 116, 434–440 (2013)
L. Salmon, P. Thuéry, E. Rivière, M. Ephritikhine, Inorg. Chem. 45, 83–93 (2006)
M. Kamacı, I. Kaya, J. Macromol. Sci. A 51, 805–819 (2014)
M. Kamacı, I. Kaya, J. Inorg. Organomet. Polym. 24, 803–818 (2014)
C. Ben-nan, H. Qin, H. Yan, J. Chun-man, Z. Qi, Chem. Res. Chin. Univ. 29(3), 419–423 (2013)
V. Somerset, J. Leaner, R. Mason, E. Iwuoha, A. Morrin, Electrochim. Acta 55, 4240–4246 (2010)
I. Kaya, M. Kamacı, Prog. Org. Coat. 74, 204–214 (2012)
D.A. Yushchenko, V.V. Shvadchak, M.D. Bilokin, A.S. Klymchenko, G. Duportail, Y. Mely, V.G. Pivovarenko, Photochem. Photobiol. Sci 5, 1038–1044 (2006)
X. Yang, H. Lyu, K. Chen, X. Zhu, S. Zhang, J. Chen, Bio. Resour. 9(3), 5219–5233 (2014)
I. Kaya, M. Yıldırım, M. Kamacı, Synth. Met. 161, 2036–2040 (2011)
I. Kaya, M. Kamacı, J. Fluoresc. 23, 115–121 (2013)
N. Saleh, A.M.M. Rawashdeh, Y.A. Yousef, Y.A. Al-Soud, Spectrochim. Acta A-M. 68, 728–733 (2007)
N.S. Abdel-Kader, R.R. Mohamed, J. Therm. Anal. Calorim. 114, 603–611 (2013)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kamacı, M., Kaya, İ. The Novel Poly(azomethine-urethane): Synthesis, Morphological Properties and Application as a Fluorescent Probe for Detection of Zn2+ Ions. J Inorg Organomet Polym 25, 1250–1259 (2015). https://doi.org/10.1007/s10904-015-0234-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10904-015-0234-1