Abstract
A series of poly(azomethine)s (PAMs) were synthesized from N1-(4-aminophenyl)-N1-(4-phenoxyphenyl)benzene-1,4-diamine (DA) and various dialdehydes to investigate the influence of structure of polymer chain and triphenylamine-based phenoxy pendant group on the optoelectronic properties. The structural characterization of the resulting poly(azomethine)s was carried out by solubility test, gel permeation chromatography, viscosity measurement, fourier transform infrared (FTIR) spectral and CHN elemental analysis. The photophysical and electrochemical properties of the materials were scrutinized by UV–vis, photoluminescence, time correlation photon counting spectral analysis (TCSP) and cyclic voltammetry. The thermal stability of the poly(azomethine)s was assessed by differential scanning calorimetry and thermogravimetric analysis found to be stable upto 300 °C. These polymers exhibit moderate inherent viscosity range from 0.99 to 1.15 g dL− 1 and appreciable organosolubility. The presence of triphenylamine and azomethine (CH = N) linkage in our synthesized materials rendered them fluorescent, emitting green light upon excitation at 375 nm with quantum efficiencies of 3.9–8.5%. The pendant phenoxy group at para-position in new poly(azomethine)s has also lowered the onset oxidation potentials and elevated the HOMO levels. Additionally, the presence of conjugation increases the fluorescence time of the excited state in conjugated polymers which was found in the range 9.22–11.17 ns, sufficient to be use in future optoelectronic applications.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Aromatic poly(azomethine)s are distinguished as high performance materials with excellent thermal stability, good mechanical strengths and photophysical properties. Recently, conjugated aromatic poly(azomethine)s (PAMs) magnetized modern researchers because of their applications in optoelectronics such as polymer light-emitting diodes, pH sensors and photovoltaics [1,2,3]. However, their rigid rod like aromatic polymer backbone is responsible for their low organosolubility/processability and performance in optical applications [4]. Thus their utility in various fields is restricted. To enhance the solubility of polymers, various efforts have been made such as incorporation of bulky disruptive units and flexible ether linkage in main polymer chain, or bulky pendent groups at polymer backbone are various approaches to improve the solubility of the poly(azomethine)s without compromise on their thermal stability. The introduction of propeller shaped, 3-dimensional triphenylamine (TPA) unit in main polymer chain has potential to form an amorphous structure, enhancing its solubility and film-forming capability [5]. It is reported that the TPA based poly(azomethine)s are self-focusing materials for non-linear optics [6].
The photophysical properties of the polymer light-emitting layers are of special interest for the optoelectronic applications. Polymers based on (TPA) constructing unit, are of special interest of the day due to their interesting photophysical properties and electrochemical properties [7]. The TPA based poly(azomethine)s have found their applications in organic electronics because of excellent hole-transport properties. Small Schiff base molecules with TPA units are promising materials for electrochromic applications [8]. Sanchez et al., have investigated the potential applications of polytriphenylamine in photovoltaic applications [9]. Palewicz and his co-workers [10] have demonstrated that the spin-coated processable poly(azomethine)s (PAMs) are effective as one of the layers in organic solar cells. Tuning of photophysical properties can be achieved by tailoring the structure of PAMs for its potential use in various applications. The study of optical parameters, light-emission of polymer chain, fluorescence time of exciting species and its electrochemical stability prove its effectiveness for use in future optoelectronic applications.
The present work is focused on the synthesis of TPA based PAMs as the materials with good optoelectronic properties and to achieve high thermal stability with good organosolubility and excellent photophysical properties. For this purpose, a series of triphenylamine based conjugated and non-conjugated aromatic poly(azomethine)s (PAMs A–D) from N1-(4-aminophenyl)-N1-(4-phenoxyphenyl)benzene-1,4-diamine (DA) [11] and some dialdehydes (Diald A–D) via piperidine catalyzed high temperature polycondensation reaction were synthesized. The dialdehydes (diald A–D) were selected to introduce flexible methylene spacers and ether linkage in main polymer backbone to study the structure–property relationship. The photophysical properties of the synthesized PAMs revealed shallow HOMO levels (hence low oxidation potentials), low HOMO–LUMO gaps and emission of green light. The synthesized materials also exhibited good thermal and electrochemical stability along with good fluorescence time. The observed behavior and tuned optoelectronic properties of the synthesized materials suggest their future use as promising materials for various applications.
Experimental
Materials
Dialdehydes: 4,4′-[1,4-butandiyl bis (oxy)] dibenzaldehyde (Diald-A), 4,4′-[1,12-dodecandiyl bis (oxy)] dibenzaldehyde (Diald-B) were purchased from Syntechem Co. Ltd, China. Diamine monomer N1- (4-aminophenyl)-N1-(4-phenoxyphenyl) benzene-1,4-diamine (DA) was synthesized as described in literature [11]. Terephthaladehyde (Diald-C), 4,4′-diformyl-triphenylamine (Diald-D), piperidine and absolute ethanol were obtained from Merck, Germany.
Measurements
The CHNS analyzer, Flash 2000 series was used for elemental analysis and Bruker α-Alpha-P model was used to collect FTIR spectra by ATR method. The NMR measurement were conducted on Bruker spectrometer, operating at 300 MHz in deuterated CDCl3 containing TMS as internal reference. The GPC measurements of poly(azomethine)s solution in NMP were carried out on GPC instrument (PL-GPC 220, high temperature) to estimate PDI, weight and number average molecular weight distribution. NETZSCH TGA analyzer, model TG209 F3 was used to evaluate thermal stability of the polymers in air at rate of 10 °C. Netzsch DSC-404C (selb, Germany) differential scanning calorimeter was used to determine glass transition temperatures (Tg). UV–vis and PL spectra were recorded on Cary, 100 Conc UV–vis and PC1 photon counter spectrophotometers respectively. CV measurements were conducted on BAS-100B electrochemical analyzer using three-electrode system with carbon, platinum wire and Ag/AgCl as working, auxiliary and reference electrode, respectively. The time-correlation photon-counting FL920, (Edinburgh instrument) was used to determine fluorescence lifetime. The compact picosecond pulse diode laser (375 nm), containing triple excitation monochromator TMS 300-X and red sensitive high-speed photon multiplier tube was used with detector response width of 250 ps and spectral range 200–850 nm.
Synthesis of Poly(azomethine)s (A–D)
The poly(azomethine)s (A–D) were synthesized by the reaction of diamine (DA) and dialdehyde (Diald A–D) as outlined in synthetic scheme, Fig. 1. Dialdehydes (DialdA–Diald D, 2.0 mmol), piperidine (0.3 mL) and absolute ethanol (25 mL) was taken in pre-dried round bottom flask. Stochiometric amount of diamine DA in10 mL ethanol solution was added dropwise with vigorous stirring. The reaction mixture was allowed to reflux for 30 min. The PAMs (A–D) were filtered and washed sequentially with warm water, ethanol and acetone to remove any low molecular weight oligomers [3]. The synthesized PAMs were vacuum desiccated at 50 °C for 12 h.
PAM A Yield: 74%. Colour:Yellow, FTIR: ῡ (cm− 1): 1638 (CH = N stretch, azomethine), 1360 (C–N stretch, TPA), 1221 (C–O–C stretch, ether), 685 (C-H stretch, azomethine). 1H-NMR: (CDCl3-TMS 25 °C) δ, ppm 8.45 (1H, s, azomethine), 7.84–7.87 (4H, dd, J = 6, 2.7 Hz. 1), 7.34–7.39 (6H, m, 2,6), 7.11–7.19 (7H, m, 3,7), 6.93–7.1 (4H, m, 4,5), 4.28 (4H, t, J = 6.3 Hz, 8) and 2.35 (4H, t, J = 6.9 Hz, 9). Anal. Calcd for C45H43N3O3 C, 80.21; H, 6.43; N, 6.24. found: C, 80.19; H, 6.42; N, 6.22.
PAM B Yield: 79%. Colour:Yellow, FTIR: ῡ (cm− 1): 1638 (CH = N stretch, azomethine), 1358 (C–N stretch, TPA), 1240 (C–O–C stretch, ether), 688 (C-H stretch, azomethine). 1H-NMR: (CDCl3-TMS 25 °C) δ, ppm 8.44 (1H, s, azomethine), 7.84 (4H, d, J = 7.8 Hz. 1,1′), 7.30–7.34 (10H, m, 2,3,7), 7.14–7.2 (8H, m, 4,8), 6.94–6.99 (4H, m, 5,6), 4.28 (4H, t, J = 6.3 Hz, 9), 2.35 (4H, t, J = 6.9 Hz, 10), 1.6 (20H, m, 11). Anal. Calcd for C53H59N3O3 C, 80.98; H, 7.57; N, 5.35. found: C, 80.96; H, 7.55; N, 5.33.
PAM C Yield: 76%. Colour:orange, FTIR: ῡ (cm− 1): 1639 (CH = N stretch, azomethine), 1365 (C–N stretch, TPA), 1227 (C–O–C stretch, ether), 699 (C-H stretch, azomethine). Anal. Calcd for C35H31N3O C, 82.48; H, 6.13; N, 8.25. found: C, 82.46; H, 6.12; N, 8.23.
PAM D Yield: 75%. Colour:Yellow, FTIR: ῡ (cm− 1): 1633 (CH = N stretch, azomethine), 1375 (C–N stretch, TPA), 1226 (C–O–C stretch, ether), 686 (C-H stretch, azomethine). 1H-NMR: (CDCl3-TMS, 25 °C) δ, ppm 8.48 (1H, s, azomethine), 7.74–7.86 (4H, m, 1), 7.34–7.39 (13H, m, 2,4,6), 6.65–6.73 (11H, m, 3,5,7,10) and 7.05–7.24 (2H, m, 9) Anal. Calcd for C47H40N4O C 83.40; H, 5.96; N, 8.28. found: C, 83.38; H, 5.95; N, 8.26.
Results and Discussion
Synthesis and Charaterization of PAMs A–D
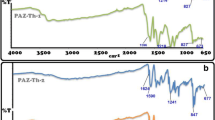
The polycondensation reaction of dialdehydes (Diald A–D) and diamine (DA) at 78 °C was used to synthesize PAMs (A–D). The structure of the PAMs was investigated by FTIR and 1H-NMR spectral analysis. In the FTIR spectra, Fig. 2. appearance of absorption peak in the 1600–1610 cm− 1 region corresponds to the stretching vibrations of azomethine (CH = N) functional group. Moreover, the disappearance of the two absorption bands in the 3300–3500 cm− 1 region is another convincing evidence for the complete conversion of primary amino group to azomethine [12].
The 1H-NMR spectral data of all PAMs (A,B and D) is presented in Table 1 and 1H-NMR spectrum of the polyazomethine A is depicted in Fig. 3. The resonance peak at 8.45 ppm was assigned to azomethine proton, confirming complete polymerization [13]. A triplet at 4.26 ppm, indicated the methylene proton (H8) of aliphatic segments directly attached to oxygen atom. In the same way, a triplet at 2.35 ppm was assigned to methylene protons (H9). The rest of the resonance signals in aromatic region are in good agreement with the targeted structure of PAM A. The 1H-NMR spectral data of all the synthesized PAMs A,B and D confirmed their successful synthesis.
Organosolubility of PAMs A–D
The organosolubility of synthesized PAMs A–D was determined at concentration of 10 mg/ mL and data is summarized in Table 2 from which it is obvious that PAMs A–D are appreciably soluble in most of the polar organic solvents like NMP and DMAc. In protonating solvents, like m-cresol, the polymer solution turned blue. This may be attributed to the protonation of the azomethine functional group. Among the series, the PAM C was found to exhibit lower solubility than PAM A, B and D. It is obvious from its rigid rod like structure. Increase in TPA units in D has enhanced its organosolubility and hence its processability. However, in comparison to analogous reported polyazomethine(s) without TPA (E), PAM C is appreciable soluble and processable. This may be attributed to the TPA linked phenoxy pendant, increasing the free volume among polymer chain, allowing solvent penetration easily [14].
Inherent Viscosity and GPC of the Synthesized PAMs A–D
The inherent viscosity, ɳinh of the PAMs A–D solutions in NMP at concentration of 0.5 g/ dL was determined and is listed in Table 3. The ɳinh values were found in the range 0.99–1.15 g/dL. The GPC was used to evaluate the molecular weight distribution and data is collected in Table 3. The number average and weight average molecular weight distribution were found in the range 6532–13,240 and 11,534–22,243 g mol− 1, respectively. The PDI values varied from 1.22 to 1.77. The inherent viscosity and GPC data validate the achievement of appreciable molecular weight of PAMs (A–D) and good polymerization activity of the diamine (DA).
Thermal Stability of PAMs A–D
The thermal stability of PAMs (A–D) was examined by thermogravimetric analysis (TGA) in air at rate of 10 °C min− 1. Thermal parameters are listed in Table 4 and TGA curves are displayed in Fig. 4. From the thermal data it is obvious that the synthesized PAMs are thermally stable upto 300 °C. Comparatively high thermal stability of the PAMs (C and D) is evident from their TGA curves, which is attributed to the wholly aromatic segments in polymer chain. The temperature at which 5% weight loss (Td) occurred varies from 340 to 394 °C. Ten percent weight loss occurred at 371–440 °C. Tmax, temperature at which maximum weight loss occurred varies from 547 to 630. All the thermal parameters (T5 and Tmaximum) for the synthesized materials revealed their appreciable thermal stability, suggestive of their future use in optoelectronic applications.
The differential scanning calorimetry (DSC) was used to determine the glass transition temperature (Tg) of the synthesized polyazomethine(s). DSC data as depicted in Table 4 showed range of Tg from 76 to 139 °C without any melting peak, indicating amorphous glassy nature of the synthesized materials. The lowest Tg for the PAM B is obvious from long aliphatic segments in the polymer chain. All aromatic PAMs (C and D), showed higher Tg as compared to the PAMs A and B. This is attributed to the rigid rod like aromatic segments in main polymer chain.
Photophysics of PAMs A–D
The photophysical properties of 10 µM NMP solution of PAMs (A–D) were explored by using UV–vis and photoluminescence (PL) spectral analysis. The photophysical data is summarized in Table 5. The UV–vis spectra of the PAMs (A–D), as shown in Fig. 5 indicated double absorbance, one at 295–303 nm and another at 390–450 nm for PAMs A to C. The wavelength maxima at 295, 301, 303 nm represents the π-π* transition of aromatic system of PAMs A, B and C respectively whereas the second absorption band at 392, 394 and 452 nm are attributed to the n-π* transition of CH = N unsaturated double bond in conjugation with TPA unit. The PAM (D) showed only one absorption maxima at 425 nm. This may be attributed to the greater number of TPA units in conjugation with CH = N bond.
The HOMO–LUMO energy gaps (Eg) were calculated from the onset wavelength λonset using the following equation [15].
The values of Eg were found in the range 2.40–2.79 eV, such value of Eg is sufficient to absorb significant portion of the solar radiations. The lower Eg values of PAM C (2.40 eV) and D (2.57 eV) is consistent with their conjugated polymer chain. The optical parameters (Extinction co-efficient, ε and energy of singlet state, E s ) of the PAMs (A–D) were calculated using Eqs. 2 & 3 given below:
Where,
- A:
-
absorbance at maximum wavelength
- c:
-
Concentration of the analyte in NMP and l is the path length.
The values of ε1 and ε2 of the PAMs solution in NMP are found in the range (2.49–9.2) 10 5 and (2.35–7.9) 10 5 M− 1 cm− 1 respectively. The molar absorptivity coefficients of the PAMs containing methylene flexible groups are higher than those of the PAMs containing aromatic rigid moieties.
The photoluminescence spectral analysis of the synthesized poly(azomethine)s were performed in NMP (10 µ M) at λexc = 375 nm and spectra are displayed in Fig. 6 while the PL data is collected in Table 6. PL spectra of all poly(azomethine)s (Fig. 6) revealed the emission of light in 490–500 nm region, assigned as green light [16]. The Stokes shifts are listed in Table 6. PL quantum efficiencies of all PAMs were calculated from Eq. 4 using quinine sulfate (% ϕ, 54.6) as standard as shown below.
where φstd is the quantum yield of quinine sulfate, φunk fluorescence quantum yield of PAMs, Iunk, Istd,ηunkand ηstd are the emission intensities and refractive indices of the PAMs solution and standard, respectively. The values of PL quantum efficiency were found 4.5, 4.6, 3.9 and 8.5 for PAMs A,B,C and D, respectively. The PAM D showed the highest quantum yield. This may be attributed to large number of TPA units in conjugation with CH = N in PAM D.
The life time decays of the synthesized poly(azomethine)s were measured by performing time correlation single photon counting. The excitation source was compact picosecond pulse diode laser (375 nm). For PAM A and B life time was not detectable. However, exponential fitting of fluorescent decays of PAM C and D were accomplished by using bi (Eq. 5) and tri (Eq. 6) exponential functions yielding life time in nanosecond (ns) listed in Table 6 and displayed in Fig. 7. From this table the τ1 is in the range 0.6456–3.0542 ns in NMP solution. This is attributed to the non-radiative decay [4]. The values of τ2 are 2.859 and 9.022 ns for PAM C and D, respectively. The value of τ3 is 11.17 ns for PAM C only. It is clear from TCSP data that in an optoelectronic device an appreciable number of excitons may reach the interface of acceptor layer. Our synthesized poly(azomethine)s has life time decay values in good agreement with the materials reported as potential candidate for the optoelectronic applications [17]. The detailed study of these PAMs also showed better photophysical properties than reported previously with same molecular backbone without any pendent. It is attributed to the electron donating phenoxy pendent at para position of the TPA.
Electrochemical Properties of PAMs A–D
The electrochemical stability of the synthesized materials PAMs A–D were evaluated by cyclic voltammetry carried out in three electrode cell comprised of carbon, platinum wire, and Ag/AgCl as working, supporting and reference electrodes, respectively, under argon atmosphere. The cyclic voltammograms of the PAMs A–D are depicted in Fig. 8 and data is summed up in Table 7. The onset of oxidation (Eonset) was determined from CV curves and values are found to be 0.4244, 0.442, 0.317 and 0.305 V for A, B, C and D, respectively. The lower onset of oxidation for PAM C and D are consistent with the extended conjugation in main polymer backbone. The energies of HOMO levels were calculated from E0 using Eq. 7 and energies of LUMO were obtained using Eq. 8. HOMO energy values were found in the range from − 4.7 to − 4.84 eV.
Where, Eg is the HOMO–LUMO gap obtained from Eq. 1. The high-lying HOMO levels of PAM C and D are consistent with the conjugated structure. The lower oxidation potentials and electrochemical stability of the PAMs is attributed to the para-blocked TPA unit. It is well reported in literature that such type of shallow HOMO levels and lower oxidation potentials of the materials are basic requirement for their application in optoelectronics [17, 18]. When compared with analogous conjugated TPA based poly(azomethine)s without substituent at para position, as reported previously (Eonset = 0.82 V, HOMO = −5.62 eV) [19] and (−5.26 eV),[3] PAMs A–D showed lower oxidation potential (0.3–0.44 V) and high lying HOMO levels (4.7–4.84 eV). This may be attributed to the electron donating–OPh substituent at para position of the TPA on main molecular chain, elevating its HOMO level. Thus it may be inferred that our synthesized materials are promising materials for future use in electro-optic applications.
Conclusion
A new series of TPA-based poly(azomethine)s are synthesized as promising materials for future applications in optoelectronics. The current results demonstrated that the incorporation of p- phenoxy pendant on TPA units of the main polymer backbone showed good PL property and not only maintained the electrochemical stability but also lowered the HOMO–LUMO gap (Eg) by elevating the HOMO levels. The present study reflects the effect of extended conjugation (as in PAM C and D) and molecular spacers (A and B) on the photophysical properties of the poly(azomethine)s. The PAMs C and D with aromatic rigid rod like polymer chain and greater number of disruptive TPA units have exhibited shallower HOMO levels and lower oxidation potentials. The lifetime decays of the synthesized materials revealed the stability of excited states showing radiative emissions. The observed characteristics of the synthesized materials demonstrated how photophysical properties of the poly (azomethine)s could be tuned by pendant-groups and polymer backbone which could be important for future use in optoelectronic applications.
References
Liou GS et al (2007) Synthesis and characterization of wholly aromatic poly (azomethine) s containing donor–acceptor triphenylamine moieties. J Polym Sci Polym Chem 45(21):4921–4932
Barik S, Bishop S, Skene W (2011) Spectroelectrochemical and electrochemical investigation of a highly conjugated all-thiophene polyazomethine. Mater Chem Phys 129(1):529–533
Yen H-J and Liou G-S (2010) Novel blue and red electrochromic poly (azomethine ether) s based on electroactive triphenylamine moieties. Org Electron 11(2):299–310
Hindson JC et al (2010) All-aromatic liquid crystal triphenylamine-based poly (azomethine) s as hole transport materials for opto-electronic applications. J Mater Chem 20(5):937–944
Sek D et al (2008) Hole transport triphenylamine–azomethine conjugated system: synthesis and optical, photoluminescence, and electrochemical properties. Macromolecules 41(18):6653–6663
Sek D et al (2009) Characterization and optical properties of oligoazomethines with triphenylamine moieties exhibiting blue, blue-green and green light. Spectrochim Acta A Mol Biomol Spectrosc 72(1):1–10
Leclerc N, Pasareanu M-C, Attias A-J (2005) Synthesis and photophysical properties of polymers containing a novel class of light emitters. Macromolecules 38(5):1531–1534
Petrus ML et al (2015) Device performance of small-molecule azomethine-based bulk heterojunction solar cells. Chem Mater 27(8):2990–2997
Sánchez C et al (2014) Schiff base polymer based on triphenylamine moieties in the main chain. characterization and studies in solar cells. Thin Solid Films 562:495–500
Palewicz M et al (2011) Optical and structural study of thin film of polyazomethine with triphenylamine unit prepared via spin-coating method. Polymer Bull 66(1):65–76
Wang Y et al (2015) High coloration efficiency and fast switching speed of poly (amic acid-imide) s containing triphenylamine in acidic electrolyte. RSC Adv 5(15):11071–11076
Niu H et al (2004) Study on crystallization, thermal stability and hole transport properties of conjugated polyazomethine materials containing 4, 4′-bisamine-triphenylamine. Mater Chem Phys 86(1):33–37
Suh SC, Shim SC (2000) Synthesis and properties of a novel polyazomethine, the polymer with high photoconductivity and second-order optical nonlinearity. Synth Met 114(1):91–95
Weaver M, Bradley D (1996) Organic electroluminescence devices fabricated with chemical vapour deposited polyazomethine films. Synth Met 83(1):61–66
Yang C-J, Jenekhe SA (1995) Conjugated aromatic polyimines. 2. Synthesis, structure, and properties of new aromatic polyazomethines. Macromolecules 28(4):1180–1196
Irfan M, Belfield KD, Saeed A (2015) Carbazole/fluorene based conjugated small molecules: synthesis and comparative studies on the optical, thermal and electrochemical properties. RSC Adv 5(60):48760–48768
Aly KI, Khalaf AA (2000) New polymer syntheses. IX. Synthesis and properties of new conducting polyazomethine polymers containing main chain cycloalkanone and pyridine moieties. J Appl Polym Sci 77(6):1218–1229
Tsai F-C et al (2005) New thiophene-linked conjugated poly (azomethine) s: theoretical electronic structure, synthesis, and properties. Macromolecules 38(5):1958–1966
Iwan A et al (2014) New air-stable aromatic polyazomethines with triphenylamine or phenylenevinylene moieties towards photovoltaic application. Synth Met 195:341–349
Acknowledgements
Special thanks are due to both Prof. Dr Jaemin Lee and Dr Shahid ameen, Centre for Solar Energy Materials, Division of Advanced Materials, Korea Research Institute of Chemical Technology (KRICT) for providing guidance and cyclic voltammetry facilities for the completion of this work. Financial support of this project was provided by the Higher education Commission of Pakistan under Indigenous Scholarship Program (2PS-469).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Khalid, N., Iqbal, A., Siddiqi, H.M. et al. Synthesis and Photophysical Study of New Green Fluorescent TPA Based Poly(azomethine)s. J Fluoresc 27, 2177–2186 (2017). https://doi.org/10.1007/s10895-017-2157-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-017-2157-4