Abstract
[Pb(Hmptrz)4(NO3)2] complex (1) and [Pb(μ-mptrz)2(H2O)]n complex (2) (Hmptrz is 4-methyl-1,2,4-triazole-3-thiol) were prepared from the reaction of 4-methyl-1,2,4-triazole-3-thiol with Pb(NO3)2 and Pb(OAc)2·3H2O in CH3OH/H2O, respectively. Both complexes were characterized by elemental analysis, IR, UV–Vis, 1H NMR, 13C{1H}NMR, and luminescence spectroscopy and their structures were studied by single-crystal X-ray crystallography. The thermal stabilities of the title complexes were studied by thermogravimetric and differential thermal analyses. Complex 1 is a discrete and complex 2 is a polymer; both structures are without precedent in the literature.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
4-Methyl-1,2,4-triazole-3-thiol (Hmptrz), with three potential donor atoms (one S atom and two N atoms), is a triazole-based heterocyclic thioamide. Its electron-donating thiol and methyl groups can obviously enhance the conjugation degree of the five membered aromatic heterocycle [1]. The reaction of the Hmptrz ligand with metal salts is expected to isolate different sorts of coordination polymers under variable synthetic conditions such as [Cu(μ4-mptrz)]n, [Cu(μ-Hmptrz)(μ-I)]n, [Cu12(μ4-mptrz)4(μ4-I)3(μ3-I)4(μ-I)]n), and [Cu(μ-Hmptrz)(μ-DmptrzSS)I]n (DmptrzSS = 4,4′-dimethyl-3,3′-dithiodi-1,2,4-triazole) [1], [Cd(mptrz)2]n, [Cd(mptrz)X]n (X = I and Br), [Cd(mptrz)(μ-X)]n (X = Cl and Br) and [Cd3(μ3-OH)2(mptrz)4]n [2], [Ag2(mptrz)(μ3-X)]n (X = I and Br) and [Pb4(μ4-O)(mptrz)4(μ-X)2] (X = I and Cl) [3] and [Me3Sn(mptrz)]n and [Ph3Sn(mptrz)]n [4], which have been synthesized and characterized by single-crystal X-ray diffraction methods. We report herein the synthesis, characterization and crystal structure of complexes [Pb(Hmptrz)4(NO3)2] (1) and [Pb(μ-mptrz)2(H2O)]n (2). It is notable that complex 1 is the first discrete complex containing the Hmptrz ligand. On the other hand, two-dimensional polymeric complexes of Pb(II) containing Hmptrz ligand have been previously reported, but here, complex 2 is the first example of a one-dimensional coordination polymer of Pb(II) and Hmptrz ligand [3].
2 Experimental
2.1 Materials and Physical Methods
All chemicals were purchased from Merck and Aldrich. Infrared spectra (4,000–250 cm−1) of solid samples were taken as 1 % dispersion in CsI pellets using a Shimadzu-470 spectrometer. 1H NMR spectra were recorded on a Bruker AC-300 spectrometer for protons at 300.13 MHz and for 13C{1H} NMR at 75.45 MHz in DMSO-d 6. Melting points were obtained on a Kofler Heizbank Rechart type 7841 melting point apparatus. Elemental analysis was performed using a Heraeus CHN–O Rapid analyzer. Thermal behavior was measured with a STA 503 Bähr apparatus. UV–Vis spectra were recorded on a Shimadzu 2100 spectrometer using a 1 cm path length cell in DMSO at room temperature, and luminescence spectra were recorded on a Perkin Elmer LS 45 using a 1 cm path length cell.
2.2 Synthesis of [Pb(Hmptrz)4(NO3)2] (1)
4-Methyl-1,2,4-triazole-3-thiol (0.37 g, 3.16 mmol) in CH3OH (20 ml) was added to a solution of Pb(NO3)2 (0.27 g, 0.79 mmol) in H2O (5 ml) and the resulting colorless solution was stirred at 45 °C for 20 min. After 1 week, colorless prismatic crystals of 1 were isolated (yield 0.48 g, 76.7 %, m.p. 200 °C). IR (CsI, cm−1): 3125w 3078w, 2985w, 2909w, 1532s, 1515s, 1456s, 1357s, 1294m, 1265s, 1232s, 1168m, 1080s, 999s, 969w, 931m, 873s, 723m, 644s, 501m, 482m, 423w, 397w, 349s, 307m, 290 m. UV–Vis: λ max (DMSO, nm), 262. 1H NMR (DMSO-d 6, ppm): 2.50 (s, 3H), 8.39 (s, 1H) and 13.65 (s, 1H).13C{1H} NMR (DMSO-d 6, ppm): 31.7(s), 143.1(s) and 166.7(s). Anal. Calcd. C, 18.20; H, 2.53; N, 24.75. Found: C, 18.03; H, 2.51; N, 24.56.
2.3 Synthesis of [Pb(μ-mptrz)2(H2O)]n (2)
4-Methyl-1,2,4-triazole-3-thiol (0.37 g, 3.16 mmol) in CH3OH (10 ml) was added to a solution of Pb(OAc)2·3H2O (0.31 g, 0.79 mmol) in H2O (15 ml) and the resulting colorless solution was stirred at 45 °C for 20 min. Suitable crystals for X-ray diffraction measurement were obtained by methanol diffusion to a colorless solution of 2 in DMSO over 2 weeks (yield 0.27 g, 75.4 %, m.p. 277 °C). IR (CsI, cm−1): 3440m, 3055w, 2980w, 2901w, 1631m, 1516s, 1466s, 1409s, 1382m, 1350s, 1208m, 1164s, 1059w, 1035m, 963m, 863w, 839m, 694s, 645s, 511m, 420m, 352m, 326m. UV–Vis: λ max (DMSO, nm), 266. 1H NMR (DMSO-d 6, ppm): 2.48 (s, 3H), 3.52 (s, 2H) and 8.20 (s, 1H). 13C{1H} NMR (DMSO-d 6, ppm): 31.4(s), 142.1(s) and 165.4(s). Anal. Calcd. C, 15.89; H, 2.20; N, 18.52. Found: C, 15.74; H, 2.19; N, 18.35.
2.4 X-ray Structure Analysis
The X-ray diffraction measurements were made on a Bruker APEX II CCD area detector diffractometer at 298 K (Mo-Kα radiation, graphite monochromator, λ = 0.71073 Å). The structures of 1 and 2 were solved by SHELX-97 and absorption corrections were done using the SADABS programs [5, 6]. Softwares including Bruker APEX II (data collection and cell refinement) [7], Bruker SHELXTL (data reduction) [8], and WinGX (publication material) [9] were properly used. The molecular graphics programs used, were ORTEP-3 for windows [10], PLATON and MERCURY [11].
3 Results and Discussion
3.1 Synthesis of 1 and 2
Compound 1 was obtained from the reaction of one equivalent of Pb(NO3)2 with four equivalents of 4-methyl-1,2,4-triazole-3-thiol in a mixture of CH3OH/H2O at 45 °C after 20 min, isolated in 76.7 % yield. Compound 2 was synthesized with the same procedure with Pb(OAc)2·3H2O and the crude product was recrystallized in a DMSO/methanol solution to obtain suitable crystals for X-ray studies in 75.4 % yield. The synthetic routes of these complexes are shown in Scheme 1.
3.2 Spectroscopic Characterization of 1 and 2
IR absorptions of 1 and 2 are listed in the experimental section. The vibrational bands present at 3,125 and 3,440 cm−1 are assigned to ν(N–H) and ν(O–H) for 1 and 2, respectively. The weak absorption bands present around 3,060 and 2,900 cm−1 are due to the C–H stretching of the triazole ring and methyl group, respectively. The explicit feature in the IR spectra of the two complexes 1 and 2 is the absence of the band in the region of 2,550–2,430 cm−1, which appears in the free ligand as the –SH stretching vibration, thus indicating metal–ligand bond formation through this site [4]. The bands observed in the range 1,530–1,200 cm−1 are assigned to C=N stretching vibrations and/or N–H deformation vibrations. The medium to strong vibrations in the region 1,170–600 cm−1 are assigned to the C=S stretching and deformation vibrations [1, 2, 12, 13]. The ν(NO3) vibrations are found at 1,455 and 1,294 cm−1 for 1 [14, 15]. Far infrared spectra of 1 and 2 were recorded between 500 and 250 cm−1. The Pb–O stretching vibrations are found at 482 and 349 cm−1 for 1 and 511 cm−1 for 2. The Pb–S stretching vibrations are found at 307 and 290 cm−1 for 1 and 420 and 352 cm−1 for 2, and the Pb–N stretching vibration for 2 is found at 326 cm−1 [14–18].
The UV–Vis spectra of the DMSO solutions of 1 and 2 have bands at 262 and 266 nm, respectively, which can be assigned to π→π * transitions.
The 1H NMR spectra of 1 and 2 exhibited a singlet around 2.5 ppm for the methyl group and a singlet around 8.3 ppm for the =C–H of triazole ring. The 1H NMR spectrum of 1 also exhibited a singlet at 13.65 ppm for N–H. The signal at 13.65 ppm is absent in the 1H NMR spectrum of 2 and exhibited mptrz− ligand coordinated to Pb(II) cation from N and S atoms. In addition, the new signal at 3.52 in the 1H NMR spectrum of 2 is assigned to non coordinated H2O. It seems DMSO is coordinated from the opposite site of H2O in complex 2 and H2O is left. 1H NMR data showed that the signal of the –SH proton (1.63 ppm) in the spectrum of the ligand is absent in the spectra of 1 and 2, indicating the removal of the –SH proton and the formation of Pb–S bonds [4]. The 13C{1H}NMR spectra of 1 and 2 showed a singlet at around 31 ppm for CH3 groups and two singlets at 142–166 ppm for the triazole ring.
The luminescence emission spectra of Hmptrz, 1 and 2 were obtained in DMSO at room temperature and the results are displayed in Fig. 1. As shown in Fig. 1a (λex = 262 nm), Hmptrz exhibits a broad luminescent emission centered at 322 nm and 1 displays a broad luminescent emission at 324 nm. The luminescent emission of complex 1 is weaker than that of the free Hmptrz ligand. As shown in Fig. 1b (λex = 266 nm), the maximum emission of Hmptrz appeared at 323 nm and complex 2 displays a luminescent emission broad band at 324 nm. This band in complex 2 is stronger than the band in the free Hmptrz ligand. The shapes of the luminescence emission spectra for Hmptrz, 1 and 2 are similar, so the emission properties of these compounds are believed to have originated from π* → π or π* → n transitions in Hmptrz [19–22].
3.3 Thermal Studies of 1 and 2
The thermal stability of [Pb(Hmptrz)4(NO3)2] (1) and [Pb(μ-mptrz)2(H2O)]n (2) have been determined on single-crystalline samples between 30 and 790 °C in an air atmosphere with a heating rate of 10 °C min−1 by thermogravimetric (TG) and differential thermal analyses (DTA) (Fig. 2). For complex 1 (Fig. 2a), TGA shows that chemical decomposition starts at about 180 °C and ends at 540 °C with the weight loss of 70.15 % corresponds to the removing of two nitrate ions and four Hmptrz molecules (calcd.: 71.74 %). The remaining weight of 29.16 % is corresponded to the PbO (calcd. 28.19 %). The DTA curve of 1 displays three distinct endothermic peaks at 222, 278 and 435 °C and six distinct exothermic peaks at 201, 247, 299, 451, 488 and 503 °C. The TGA curve of 2 (Fig. 2b) exhibits three distinct weight-loss steps. The first step between 165 and 200 °C with a mass loss of 3.82 % corresponds to the loss of one coordinated water molecule (calcd. 3.97 %). In the last two steps between 290 and 590 °C the two mptrz− ligands are lost and the framework decomposes (45.22 %, calcd.: 46.78 %). The final residual weight is 50.96 % corresponding to PbO (calcd. 49.25 %). The DTA curve of 2 displays five distinct endothermic peaks at 141, 177, 280, 329 and 467 °C and four distinct exothermic peaks at 316, 519, 551 and 585 °C.
3.4 Description of the Molecular Structure of 1 and 2
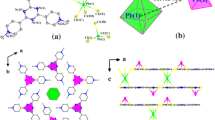
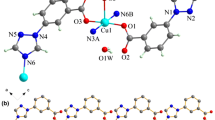
Crystallographic data for 1 and 2 are given in Table 1 and selected bond lengths and angles are presented in Table 2. The crystal structure of 1 is given in Fig. 3. The asymmetric unit of 1 contains one Pb(II) atom, one Hmptrz ligand and one nitrate anion. Each Pb(II) center is eight-coordinated by four O atoms from two nitrate anions and four S atoms from four Hmptrz ligands. The Pb–O distances is 2.720(5) Å, and the Pb–S distances is 3.0191(16) Å (Table 2). The bond distances of Pb–O and Pb–S are in the normal, and are comparable with the values in related lead(II) complexes [3, 23, 24].
In the crystal structure of 1, Fig. 4a, b, π···π interaction between the triazole rings, Cg1···Cg1i [distance = 3.701(3) Å, symmetry code: (i) 2 − x, −y, z, where Cg1 is centroids of the ring (N1/N2/C2/N3/C1)], intermolecular C–H···O and N–H···N hydrogen bonds (Table 3) and short O···O interaction [O1···O1i = 3.015(8) Å (i = x, y, z−1)] are effective in the stabilization of the crystal structure and formation of the 3-D supramolecular assembly.
The ORTEP view with the numbering scheme for complex 2 is shown in Fig. 5. Complex 2 is a one-dimensional polymeric chain structure. The asymmetry unit of 2 contains one Pb(II) atom, one mptrz− anion and one water molecule. Each Pb(II) center is seven-coordinated by four μ3-S atoms from four mptrz− anions, two N atoms from two mptrz− anions and one O atom from one water molecule. The mptrz− ligand exhibits a bidentate bridging coordination mode through its one monodentate nitrogen atom and the μ3-S atom. The Pb–O, Pb–N and Pb–S bond distances are 2.570(11), 2.635(9) and 3.024(5) Å, respectively (Table 2). The bond dimensions involving lead are normal, and are comparable with the values in related lead(II) complexes [23, 25, 26]. As depicted in Fig. 5, the Pb ions are linked by the μ-mptrz− ligands to produce a one-dimensional chain alone the b axis. Two mptrz− ligands link two neighboring lead atoms to form a 4-membered [Pb2S2] ring. The Pb1···Pb1 interatomic separation in the polymeric units of 2 is 4.647 Å, which is quite longer than the upper limit for Pb–Pb bonds in diplumbene dimers (3.527 Å) [27, 28].
In the crystal structures of 2, Fig. 6, there is no π···π interaction between the triazole rings, and only intermolecular O–H···N hydrogen bonds (Table 3) are effective in the stabilization of the crystal structure and formation of the 2-D supramolecular assembly.
4 Conclusion
A new coordination monomer, 1, and polymer, 2, of Pb(II) have been synthesized by the reaction of the Hmptrz ligand and Pb(NO3)2 and Pb(OAc)2·3H2O, respectively. Both complexes were fully characterized. Complex 1 is the first discrete complex containing the Hmptrz ligand and complex 2 is the first example of a one-dimensional coordination polymer of Pb(II) and Hmptrz ligand.
5 Supplementary material
CCDC Nos. 928566 and 928567 contain the supplementary crystallographic data for 1 and 3, respectively. These data can be obtained free of charge via http://www.ccdc.cam.ac.uk/conts/retrieving.html, or from the Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK; Fax: (44) 1223 336 033; or E-mail: deposit@ccdc.cam.ac.uk.
References
Y.L. Wang, N. Zhang, Q.Y. Liu, Z.M. Shan, R. Cao, M.S. Wang, J.J. Luo, E.L. Yang, Cryst. Growth Des. 11, 130 (2011)
Y.L. Jiang, Y.L. Wang, J.X. Lin, Q.Y. Liu, Z.H. Lu, N. Zhang, J.J. Wei, L.Q. Li, Cryst. Eng. Comm. 13, 1697 (2011)
Y.L. Wang, Y.L. Jiang, Q.Y. Liu, J.J. Wei, L.Q. Li, Aust. J. Chem. 65, 50 (2012)
C. Ma, G. Tian, R. Zhang, J. Inorg. Organomet. Polym Mater. 16, 139 (2006)
G.M. Sheldrick, SADABS (Bruker AXS, Madison, 1998)
Bruker, APEX2 Software Package (Version 2.0–1) (Bruker AXS Inc., Madison, 2005)
G.M. Sheldrick, SHELXTL (Version 5.1), Structure Determination Software Suite (Bruker AXS, Madison, 1998)
G.M. Sheldrick, Acta Crystallogr. A 64, 112 (2008)
L.J. Farrugia, J. Appl. Crystallogr. 32, 837 (1999)
L.J. Farrugia, J. Appl. Crystallogr. 30, 565 (1997)
Mercury 1.4.1, Copyright Cambridge Crystallographic Data Center, 12 Union Road, Cambridge CB2 1EZ, UK, (2001–2005)
J. Jolley, W.I. Cross, R.G. Pritchard, C.A. McAuliffe, K.B. Nolan, Inorg. Chim. Acta 315, 36 (2001)
L.J. Bellamy, The Infrared Spectra of Complex Molecules, 3rd edn. (Chapman and Hall, London, 1975)
K. Nakamoto, Infrared and Raman Spectra of Inorganic and Coordination Compound Part B: Application in Coordination, Organometallic and Bioinorganic Chemistry (Wiley, New York, 2009)
R. Alizadeh, V. Amani, Struct. Chem. 22, 1153 (2011)
Y.Y. Zhang, S.X. Liu, K.K. Du, M.X. Xue, Inorg. Chem. Commun. 13, 641 (2010)
K. Lyczko, W. Starosta, I. Persson, Inorg. Chem. 46, 4402 (2007)
H. Yin, S.X. Liu, Inorg. Chem. Commun. 12, 187 (2009)
A. Abedi, N. Safari, V. Amani, H.R. Khavasi, J. Coord. Chem. 65, 325 (2012)
A. Abedi, V. Amani, N. Safari, Monatsh. Chem. 143, 589 (2012)
A. Abedi, V. Amani, N. Safari, Main Group Chem. 11, 223 (2012)
C.K. Modi, D.H. Jani, H.S. Patel, H.M. Pandya, Spectrochim. Acta A 75, 1321 (2010)
M.S. Bharara, C.H. Kim, S. Parkin, D.A. Atwood, Polyhedron 24, 865 (2005)
R. Ahmadi, K. Kalateh, R. Alizadeh, Z. Khoshtarkib, V. Amani, Acta Crystallogr. E 65, m1169 (2009)
E. Block, G.O. Okai, H. Kang, J. Wu, J. Zubieta, Inorg. Chim. Acta 190, 5 (1991)
H. Sadeghzadeh, A. Morsali, Cryst. Eng. Comm. 12, 370 (2010)
A. Morsali, A.R. Mahjoub, Solid State Sci. 7, 1429 (2005)
M. Stürmann, W. Saak, H. Marsmann, M. Weidenbruch, Angew. Chem. 111, 166 (1999)
Acknowledgments
We would like to thank the National University of Tajikistan and Islamic Azad University, Shahre-Rey Branch, for financial support.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Paqhaleh, D.M.S., Aminjanov, A.A. & Amani, V. Discrete and Polymeric Lead(II) Complexes Containing 4-Methyl-1,2,4-triazole-3-thiol Ligand: X-ray Studies, Spectroscopic Characterization, and Thermal Analyses. J Inorg Organomet Polym 24, 340–346 (2014). https://doi.org/10.1007/s10904-013-9961-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10904-013-9961-3