Abstract
The reaction of K3[Cu(CN)4] and ethyl isonicotinate (EIN) in the presence of Me3SnCl in H2O/acetonitrile medium at room temperature affords the 3D-supramolecular coordination polymer (SCP), ∞ 3[CuCN·(EIN)], 1. The structure of 1 consists of 1D-zig-zag chains, which extend via the EIN ligands through hydrogen bonds organized in AB 2D-layers. The infinite AB···AB layers are further extended to form a 3D-network via hydrogen bonds and π–π stacking interactions, thus creating wide channels. The emission spectrum of 1 in the solid state reveals a set of high energy and low energy distinct peaks in the visible region at 400–580 nm upon excitation at 280 nm. The luminescence excitation in 1 could be caused by different possible transitions including metal-to-ligand charge transfer (MLCT) or single-metal-centered (MC) transitions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The construction of metal–organic frameworks (MOF) via self assembly of appropriate metal ions with predesigned ligands is a subject of currently active research work because of their intriguing topological and special functional properties such as luminescence, redox activity, catalysis and gas storage [1–10]. Many MOF are microporous materials, which are functionally related to zeolites. The incorporation of CuCN in an MOF is of great interest in view of their appealing structural and photo-luminescent properties, which should lead to interesting new functional materials [11–13]. Pyridine derivatives can be considered, in most cases, as polydentate ligands, which allow a systematic probing of the effect of modifications to the ligand backbone through which the micro architectures of the arrays could be controlled [12–21]. In addition, MOF-containing ligand-based hydrogen bonds (with donor–acceptor capabilities) in the structure allow the connection of MOF via H-bonds thereby increasing dimensionality. These networks may present advantages in the design of new materials due to the fact that H-bonds are more flexible than coordination bonds [22]. In fact hydrothermal processes have been widely used in the preparation of new MOF-containing pyridine derivatives [14, 18, 20]. Furthermore, R3Sn+ cations have a good capability of bridging CuCN fragments to produce a limited number of 3D-frameworks [23]. Within these constraints, we have selected ethyl isonicotinate (EIN), which can act as a multi-dentate ligand with versatile binding and coordination modes, and cyanide as ligands with the Me3Sn+ cation to create multi-dimensional hetero bimetallic MOF. Thus, the present study deals with synthesis and characterization of the product of the reaction of K3[Cu(CN)4], Me3SnCl and EIN, which is expected to exhibit strong luminescence for potential use as a luminescent probe.
2 Experimental Section
All chemicals and solvents used in this study were of analytical grade supplied by Aldrich or Merck and used as received except K3[Cu(CN)4], which was prepared in the laboratory according to the literature [24]. Microanalyses (C, H, N) were carried out with a Perkin Elmer 2400 automatic elemental analyzer. The IR spectra were recorded on Perkin Elmer 1430 Ratio Recording Infrared Spectrophotometer as KBr discs. Thermogravimetric analysis was carried out on a Shimadzu AT 50 thermal analyzer (under N2 atmosphere). Electronic absorption spectra as solid matrices and in ethanol in presence and absence of HCl were measured on a Shimadzu (UV-3101 PC) spectrometer. Fluorescence spectra of solids were measured with a Perkin Elmer (LS 50 B) spectrometer.
2.1 Synthesis of [CuCN·EIN] (1)
A solution of 90 mg (0.3 mmol) of K3[Cu(CN)4] in 30 mL H2O was added with gentle stirring to a solution of 189 mg (0.95 mmol) of Me3SnCl in 10 mL H2O and 0.15 mL (1 mmol) of ethyl isonicotinate (EIN) in 30 mL acetonitrile. After 1 week yellow needle crystals started to grow from the initially clear solution. After filtration, washing with small quantity of cold H2O and acetonitrile and drying over night, 46 mg (60.8% based on K3[Cu(CN)4] of yellow crystals were obtained. All attempts to synthesize 1 at room temperature in absence of Me3SnCl have thus far been unsuccessful.
Anal. Calc. for 1 (C9H9O2N2Cu), %: C, 44.9; H, 3.7; N, 11.6. Found: C, 44.4; H, 3.91; N, 11.8.
2.2 Single Crystal Structure Determination
Structural measurement for 1 was performed on a Kappa CCd Enraf–Nonius FR 90 four-circle goniometer with graphite monochromatic MoKα radiation {[λMoKα] = 0.71073 Å} at 25 ± 2 °C. The structure was solved using direct methods and all of the non-hydrogen atoms were located from the initial solution or from subsequent electron density difference maps during the initial stages of the refinement. After locating all the non-hydrogen atoms in each structure, the models were refined against F2 first using isotropic and finally using anisotropic thermal displacement parameters. The positions of the hydrogen atoms were then calculated and refined isotropically, and the final cycle of refinements was performed.
3 Results and Discussion
The room temperature reaction of K3[Cu(CN)4], Me3SnCl and EIN affords yellow crystals of tin free [CuCN·EIN] (1). There are two abnormal features in this reaction; (a) it is formed at room temperature to produce significant amounts of single-phase product and (b) the resulting product is a tin free assembly, 1, in spite of the presence of Me3SnCl. It was expected that this reaction should produce a zeolitic-type SCP containing the Me3Sn cation. Interestingly, replacing EIN by 4-cyanopyridine (cpy), using the same compounds, gave the distorted diamondoid structure ∞3 [CuCN·Me3SnCN·cpy] (1′), which exhibits four equivalent and independent, interlocking frameworks [23]. Single crystals of 1′ were obtained by a very slow transfer of Me3SnCl from the gas phase into a beaker containing an aqueous solution of K3[Cu(CN)4] and cpy that was dissolved in a small quantity of MeCN. From the filtrate of this reaction, orange crystals (2) resulted after several days. The structure of 2 was identified by X-ray crystallography [23], which indicates that 2 is isostructural to the earlier reported 3D-assembly, [CuCN·cpy] ( ∞3 [Cu(μ-CN)(μ-cpy)], 2 [25]. Also, an identical product of 1′ was obtained as a polycrystalline precipitate [23] using the same route used to obtain 1. All attempts to obtain the organotin assembly containing EIN were unsuccessful.
3.1 Crystal structure of [CuCN·(C9H9NO2)] = [CuCN·EIN], 1
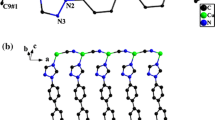
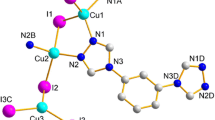
The asymmetric unit of 1 contains one copper atom, one cyanide group and one EIN ligand (Fig. 1). Crystallographic data for 1 are summarized in Table 1. Selected bond distances and bond angles are given in Table 2. The copper center forms a trigonal planar geometry via coordination to two cyanide groups and one EIN ligand. The extended structure of 1 along the a-axis exhibits a 1D-zigzag chain with trans EIN ligands that are present in two planes with an angle of 9.93°. In contrast, the EIN ligands seem to be in cis positions along the b-axis (Fig. 2). The structure of 1 extends through the EIN ligand to form 2D-layers. Each layer consists of infinite AB parallel CuCN chains along the b-axis; the chains are connected by hydrogen bonds between the ethyl groups with C10-H10 = 3.076 Ǻ and C17-H17A = 2.712 Ǻ (Table 3; Fig. 3). The distance between two copper atoms attached to two facing EIN ligands forming hydrogen bonds is 21.965 Ǻ. The hydrogen bonds connecting the chains form interwoven wide hexagonal [Cu6(CN)4(EIN)4] rings (Fig. 4). Each 2D-layer is further stabilized by extensive hydrogen bonds (2.711–3.076 Ǻ) and π–π stacking (3.337–3.833 Ǻ) (Tables 3, 4). There is no copper–copper interaction in the layers since the nearest distance between Cu1–Cu1 is 3.913 Ǻ. The structure of 1 is further extended to form 3D-infinite AB–AB layers via interlayer hydrogen bonds; (O18···H8 = 2.177 Ǻ, O18···H9 = 3.071 Ǻ, N2···H16 = 3.191 Ǻ) and π–π stacking (3.034–3.833 Ǻ) along the b- and c-axes (Tables 3, 4). The separation distances between the AB···AB layers are 11.722 Ǻ (C8–C8) and 13.493 Ǻ (Cu1–Cu1) creating wide channels (Figs. 3, 5). Thus, the network structure of 1 may be considered as a good example of a coordination polymer that extends its structure to a 3D open-framework via hydrogen bonds and π–π stacking. The bond lengths around the Cu1 atom are in the normal range reported for prototype compounds [12, 13, 15–17, 19] (Table 5). The N2-Cu1-C3 angle is 136.85°, which causes the zigzag shape of the CuCN chains. Also, the Cu1CN angle (177.89°) deviates slightly from linearity.
Comparing the structure of 1 with 2 ( 3∞ [Cu2(CN)(μ-CN)(3-Acpy)2]), 3 ( 3∞ [Cu2(CN)(μ-CN)(4-Acpy)2]), 4 (Acpy = acetylpyridine) [13] and 5 ( 3∞ [Cu2(CN)(IN)(HIN)] n (IN = isonicotinate) [19]) indicates that the structures 1, 3, 4 and 5 contains three-coordinate Cu(1) while 2 contains tetrahedral Cu(1). The structures of 3 and 4 consist of Cu2CN building blocks, which are connected by CN groups to form 1D-zig-zag chains. Each chain in 3 and 4 is bonded to another by hydrogen bonds into a 2D layer. In 3, however, there is further stacking into an interwoven mode by hydrogen bonds and π–π stacking as well as Cu···Cu interactions to create a 3D-network structure [13]. On the other hand, the structure of 2 consists of 1D CuCN chains connected by the cpy ligand to create a 3D network [25]. The structure of 5, however, is based on closed [Cu6(CN)2(C6H4NO2)2-(C6H4NO2H)2] distorted fused hexagons while the structure of 1 consists of hydrogen bonded [Cu6(CN)4(EIN)4] fused hexagons. Each closed hexagon unit in 5 is interpenetrated by two others, which extend to a three-fold interpenetrating 2D-network. Each independent 2D-network is hydrogen bonded to only one of the three independent networks of the adjacent layer to give a 3D-supramolecular architecture. In contrast, 1 is constructed of interwoven 2D-layers consisting of hydrogen-bonded hexagons connected by hydrogen bonds to create a 3D-network. Thus, 1 consists of an open non-interpenetrating 3D-framework with wide voids and channels. It is worth mentioning that in the case of pyridine and its methyl derivatives (3-methylpyridine (3-mpy) and 2,4,6-trimethylpyridine (tmpy)) 2D-polymers are formed with the general formula 2∞ [CuCN.0.5 (L)] [12]. Hence, the high dimensional topologies of 1, 3–5 result mainly from extensive hydrogen bonding due to the presence of the acetyl or the carboethoxy group while the presence of the cyanide group in cpy favors the formation of tetrahedral geometry around the Cu(1) site via the bridging capability of the cpy ligand. Such non-interpenetrating networks that contain MOF connected by unconventional H-bonds to build flexible networks should provide a concrete route to porous materials.
3.2 Infrared Spectrum of 1
The IR spectrum of 1 exhibits bands characteristic of the (CuCN) n fragment and the EIN ligand (Table 6). The IR spectrum of 1 displays the bands of the EIN ligand at 3035 cm−1 (υCH(arom)), 2989, 2916 cm−1 (υCH(aliph.)), 1410 cm−1 (δCH) and 790 and 770 cm−1(γCH). These bands are shifted to lower wave numbers from the vibrational frequencies of the free ligand due to the formation of hydrogen bonds between the hydrogen atoms of the ligands and the donor sites of the (CuCN) n fragment [26]. In contrast, the band in 1 at 1608 cm−1 corresponds to νC=N while those at 1559, 1467 and 1410 cm−1 are attributed to νC=C of the EIN ligands. These bands exhibit small shifts to lower wave numbers (6, 4 and 5 cm−1, respectively) from those of the EIN ligand, hence supporting coordination of EIN to Cu(I).
Also, the IR spectrum of 1 shows a strong band at 1727 cm−1 due to νC=O, which shifts to lower wave number relative to the carbonyl group of the free ligand (1738 cm−1) due to the formation of hydrogen bonds.
It is noteworthy that the νC≡N band of 1 (2121 cm−1) is different from that in K3[Cu(CN)4] (2081 cm−1), which supports the presence of one type of cyanide group. In addition, the νCu–C band at 431 cm−1 confirms the presence of the (CuCN) n fragment.
3.3 Thermogravimetric Analysis
The thermogram of 1 shows three degradation steps. The first and the second steps are at 100–200 °C and at 450–540 °C, respectively, and correspond to the release of the EIN ligand (M.W = 63%). The first step involves removal of the carboethoxy group (COOC2H5); Δm% obser. (calc.) 30.5 (30.3). The second step is due to the release of the pyridyl moiety of the EIN ligand; Δm% obser. (calc.) 32.5 (32.4). At 850–900 °C the weight loss is due to the decomposition of the cyanide group; Δm% obser. (calc.) 10.5 (10.8). The molecular weight of the residue obtained after complete thermolysis of 1 under nitrogen atmosphere is coincident with metallic copper, Δm% obser. (calc.) 26.8 (26.4).
The thermogravimetric analysis of 1 indicates that the formula of 1 contains stable CuCN chains up to 850 °C while the EIN ligands evolve at relatively high temperatures indicating that the EIN molecules are strongly tied in the 3D-network via hydrogen bonds and strong coordination to the Cu(1) atoms as was previously supported by the crystal structure.
3.4 Electronic Absorption and Emission Spectra of 1
The electronic absorption spectra of EIN as a liquid in absence and presence of HCl reveal three and two bands, respectively, between 220 and 320 nm (Table 7). The high intensity band at 220 nm corresponds to 1La ← 1A transition while that at 275 nm is due to 1Lb ← 1A transition [27]. These bands resemble those of benzene, which exhibit a red shift upon substitution [27]. The long wavelength band at 320 nm may be assigned to a n-π* transition, which disappears in presence of HCl (Fig. 6).
The electronic absorption spectra of 1 in ethanol in presence and absence of HCl show four absorption bands between 210–325 nm (Table 7; Fig. 6). The 1La band at 210 nm suffers a blue shift compared to EIN due to coordination to the copper site and the formation of different types of hydrogen bonds. However, the 1Lb band appears near the same position at 275 nm. Two new bands appear in the spectra of 1 at 225–235 nm and at 315–325 nm due to MLCT and LMCT, respectively; and, the band due to n-π* transition disappears due to coordination of EIN to the copper site.
Solid-state luminescent materials currently have potential applications in device displays, mainly as molecular sensing systems [28]. Thus, the luminescence of 1, CuCN and EIN were studied in solid state and in solution. The emission spectrum of EIN has no bands in ethanolic solution on excitation at 280 nm. This finding is expected since pyridine and its derivatives are, generally, not luminescent materials [29]. In contrast, the emission spectrum of 1 in the solid state reveals distinct high and low energ peaks in the visible region (390–530 nm) upon excitation at 280 nm (Fig. 7; Table 7). In this case, the luminescence may be discussed based on a number of possible photophysical transitions due to the CuCN chains, which are affected by coordination to EIN molecules. The emission spectrum of CuCN in solid-state shows broad bands at 400–545 nm on excitation at 280 nm.
Until recently, the luminescence of CuCN was unstudied. Based on TD-DFT calculations, the emission spectrum was assumed to arise from transitions between the lowest excited triplet state and the ground state singlet [30]. The So-T gap for the bent triplet chains is consistent with emission wavelength for bulk CuCN [29]. Since the emission spectrum of 1 resembles that of CuCN, it is apparent that the luminescence of 1 is caused by transitions within the CuCN chains, while the observed red shift and relatively high intensity relative to that of CuCN is due to coordination to EIN to form a 3D-network [31]. The luminescence excitation in 1 could be caused by different possible transitions including metal-to-igand charge transfer (MLCT), metal cluster-centered (CC) transitions, single-metal-centered (MC) transitions and ligand-centered (LC) transitions. Since, cyanide and EIN have large band gaps, they are ruled out. The absence of close Cu···Cu contacts in 1, where the nearest Cu···Cu distance is 3.913 Å, argues against CC transitions. Elimination of the LC and CC phenomena leaves the MC transition and MLCT as possible photophysical sources of luminescence behavior in 1.
The emission spectrum of 1 is assumed to arise from transitions from the lowest excited-triplet state to the ground state. Lifetime studies of soluble Cu(I)-cyano species have suggested that emission is usually a phosphorescence phenomenon [32–35]. The red shift and the relatively high intensity of the emission bands of 1 relative to CuCN may be due to three-coordinate copper centers and the bent structure of the CuCN chains, which allow for the distortion that is obtained in the bent triplet structures, which produce multiple triplet states [30].
4 Appendix A. Supplementary Data
CCDC 767192 contains the supplementary crystallographic data for 1. These data can be obtained free of charge via http://www.ccdc.cam.ac.uk/conts/retrieving.html, or from the Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK; fax: (+44) 1223-336-033; or e-mail: deposit@ccdc.cam.ac.uk.
References
A.M. Peedikakkal, J.J. Vittal, Inorg. Chem. 49, 10 (2010)
R.J. Kuppler, D.J. Timmons, Q.-R. Fang, J.-R. Li, T.A. Makal, M.D. Young, D. Yuan, D. Zhao, W. Zhuang, H.-C. Zhou, Coord. Chem. Rev. 253, 3042 (2009)
B.H. Ye, M.L. Tang, X.M. Chen, Coord. Chem. Rev. 249, 545 (2005)
Z. Su, K. Cai, J. Fan, S.S. Chen, M.S. Chen, W.Y. Sun, Cryst. Eng. Comm. 12, 100 (2010)
L.F. Ma, L.Y. Wang, M. Du, S.R. Batton, Inorg. Chem. 49, 365 (2010)
J. Lee, O. K. Farha, J. Roberts, K. A. Scheidt, S. T. Nguyen, J. T. Hupp, Chem. Soc. Rev. 38, 1450 (2009)
M.D. Allendorf, C.A. Bauer, R.K. Bhakta, R.J.T. Houk, Chem. Soc. Rev. 38, 1330 (2009)
M. Sabo, A. Henschel, H. Froede, E. Klemm, S. Kaskel, J. Mater. Chem. 17, 3827 (2007)
Y.K. Hwang, D.-Y. Hong, J.-S. Chang, S.H. Jhung, Y.-K. Seo, J. Kim, A. Vimont, M. Daturi, C. Serre, G. Férey, Angew. Chem. Int. Ed. 47, 4144 (2008)
S. Ma, H.-C. Zhou, Chem. Commun. 46, 44 (2010)
Q.-G. Zhai, S.-N. Li, X. Gao, W.-J. Ji, Y.-C. Jiang, M.-C. Hu, Inorg. Chem. Commun. 13, 211 (2010)
S.E.H. Etaiw, S.A. Amer, M.M. El-bendary, J. Mater. Sci. 45, 1307 (2010)
S.E.H. Etaiw, S.A. Amer, M.M. El-bendary, Polyhedron 28, 2385 (2009)
U.S. Schubert, C. Eschbaumer, Angew. Chem. Int. Ed. 4, 2829 (2004)
G.A. Bowmaker, C. Pettinari, B.W. Skelton, N. Somers, N.A. Vigar, A.H. White, Z. Anorg. Allg. Chem. 633, 415 (2007)
G.A. Bowmaker, K.C. Lim, B.W. Skelton, A.H. White. Z. Naturforsch 59b, 1264 (2004)
J.C. Dyason, P.C. Healy, L.M. Engelhardt, C. Pakawatchai, V. A Patrick, A.H. White. J. Chem Soc. Dalton Trans., 839 (1985)
R. Sekiya, S. Nishikiori, K. Ogura, Inorg. Chem. 45, 9233 (2006)
P. Amo-Ochoa, P. J. S. Miguel, O. Castilloc, F. Zamora, Cryst. Eng. Comm 9, 987 (2007), and references therein
M.H. Yang, Acta Cryst. C 65, m59 (2009)
P.Y. Cheng, C.Y. Chen, H.M. Lee, Inorg. Chim. Acta 362, 1840 (2009)
A.M. Beatty, CrystEngComm 51, 1 (2001)
H.H.-Heidl, S.E. H. Etaiw, M. Sh. Ibrahim, A.S. B. El-din, R.D. Fischer, J. Organomet. Chem. 684, 329 (2003), and references therein
J.W. Eastes, W.M. Burgess. J. Am. Chem. Soc. 64, 1187 (1942)
D.T.Cromer, A.C. Larson, Acta Cryst. Sec. B 28, 105 (1972)
A. Spalleti, G. Cruciani, U. Mazzucato, J. Mol. Struct. 612, 339 (2002)
H. H. Jaffé, M. Orchin, Theory and Applications of Ultraviolet Spectroscopy, Fifth Printing (John Wily and Sons, Inc., 1970)
R. C. Ropp, Luminescence and the Solid State, 2nd edn (Elsevier, Amsterdam, 2004)
B. Valeur, Molecular Fluorescence Principles and Applications, (WILEY-VCH Verlag GmbH, 69469 Weinheim (Federal Republic of Germany, 2002), p. 59
C.A. Bayse, T.P. Brewster, R.D. Pike, Inorg. Chem. 48, 174 (2009)
M.J. Lim, C.A. Murray, T.A. Tronic, K.E. deKrafft, A.N. Ley, J.C. deButts, R.D. Pike, H. Lu, H.H. Patterson, Inorg. Chem. 47, 6931 (2008)
A. Horváth, K.L. Stevenson, Inorg. Chem. 32, 2225 (1993)
A. Horváth, C.E. Wood, K.L. Stevenson, Inorg. Chem. 33, 5351 (1994)
K.L. Stevenson, J.H. Jarboe, S.A. Langmeyer, T.W. Acra, Inorg. Chem. 42, 3559 (2003)
A. Horváth, Z. Zsilák, S. Papp, J. Photochem. Photobiol. A 50, 129 (1989)
Acknowledgment
This work was financially supported by Tanta University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Etaiw, S.Ed.H., El-bendary, M.M. Metal-Organic Framework Constructed by Copper(I) Cyanide and Ethyl Isonicotinate Through Hydrogen Bonding. J Inorg Organomet Polym 20, 739–745 (2010). https://doi.org/10.1007/s10904-010-9396-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10904-010-9396-z