Abstract
Gambling pathology has been associated with elevated levels of distress, depression and impulsivity. The present investigation assessed whether these behavioral features would be evident among problem gamblers as they are among pathological gamblers. As well, given that gambling has been associated with increased life stress, as an objective index of ongoing distress, elevations of morning cortisol levels were assessed in problem and pathological gamblers relative to recreational gamblers, and their relations to depressive symptoms and impulsivity were assessed. Recreational, problem, and pathological gamblers (N = 140) completed the Beck Depression Inventory and the Barratt Impulsiveness Scale-11, and provided saliva samples at awakening, 30 min, 3.5 h, and 5.5 h afterward. Consistent with the view that problem and pathological gambling are associated with elevated life stressors, the rise of morning cortisol from awakening to 30 min following awakening was greater than in recreational gamblers. Heightened impulsivity was evident among both problem and pathological gamblers, whereas depressive symptoms were only evident among pathological gamblers. In neither instance were these psychological indices related to the morning cortisol rise. Indeed, increased depressive symptoms were not evident among problem gamblers, despite the fact that elevated morning cortisol levels were evident. The elevated morning cortisol rise may be secondary to gambling problems or distress related to gambling problems. Furthermore, the sustained morning cortisol elevations may be indicative of allostatic overload, and could potentially be a harbinger for potential health risks among problematic gamblers.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Shadowing the growth of the gambling industry (Marshall and Wynne 2003), there has been an increase of research implicating gambling as a risk factor for poor psychological and physical well being (e.g., Messerlian et al. 2005). This research focus has been reinforced by, among other things, the endemically high rates of gambling pathology. Current estimates suggest that within North America about 1.6% of the general public exhibit gambling at pathological levels and an additional 3.85% indicated problems at what might be considered ‘sub-clinical’ levels (i.e., at-risk for developing severe difficulties) (Shaffer et al. 1999). Among young adults, these rates more than double and, in fact, a meta-analysis of North American prevalence studies revealed that among college students problem gambling (16.4%) exceeded that of older adults (6.1%) (Shaffer and Hall 2001).
Pathological gambling has been associated with elevated depressive and anxiety symptoms (Black and Moyer 1998; Goodyear-Smith et al. 2006; Kim et al. 2006; Scherrer et al. 2005), increased risk of suicidal ideation and attempts (Gupta and Derevensky 2000), poor general health (Potenza et al. 2002), elevated life stress, and a high incidence of life-time emotional, physical or sexual traumas (Black and Moyer 1998; Kausch et al. 2006). Although mood disturbances may be secondary to gambling pathology, it is equally possible that among some individuals gambling may actually serve as a way of coping with depression, or factors that would ordinarily promote depressive symptoms (e.g., loneliness). Owing to biases inherent in retrospective analyses, however, it is uncertain whether gamblers had actually experienced more or greater distressing events, or whether the reported history of stressful experiences was secondary to distress associated with gambling problems.
In addition to elevated stressor experiences, as well as altered coping styles or strategies (Bergevin et al. 2006; Getty et al. 2000; Wohl et al. 2006), gambling problems may be comorbid with impulsivity (Clarke 2004), which may itself contribute to gambling severity as well as to behavioral and psychological disturbances associated with gambling (Blaszczynski et al. 1997; Clarke 2004; Petry et al. 2005; Steel and Blaszczynski 1998). It is uncertain, however, whether impulsivity and stress-responses are related to one another, and whether their conjoint actions had particularly notable effects with respect to problematic gambling behavior. By example, impulsivity may be incongruent with problem-focused coping, and more aligned with emotion-based strategies, which together, represent ineffective methods of dealing with gambling-related distress.
Not surprisingly, when problem gamblers engaged in casino gambling, heart rate increased, as did circulating levels of stressor-sensitive hormones, norepinephrine and cortisol (Krueger et al. 2005; Meyer et al. 2000, 2004). Inasmuch as psychogenic and neurogenic stressors ordinarily promote hypothalamic–pituitary–adrenal (HPA) activation, culminating in cortisol release from the adrenal gland (Sapolsky et al. 2000), the reported cortisol elevations are in keeping with the view that gambling may be perceived as a stressor. Yet, HPA activation not only occurs in response to stressors, but may also be activated in response to positive (arousing) stimuli (Merali et al. 1998; Piazza and Le Moal 1997), and thus the elevated cortisol levels may be a reflection of non-stress related factors. Moreover, even if it were assumed that post-gambling elevations reflect a stress response, these likely reflect situation-specific occurrences, and are not necessarily indicative of the general distress that may be present among problem gamblers.
Beyond cortisol changes elicited by explicit stressors, cortisol variations may occur in relation to life stressors in general. Specifically, cortisol levels ordinarily rise over the first 30 min following awakening, and then decline over the day (Pruessner et al. 2003; Schmidt-Reinwald et al. 1999). The early morning cortisol rise appears to be sensitive to ongoing life stressors, being particularly pronounced among individuals with elevated life distress (Grossi et al. 2005; Kunz-Ebrecht et al. 2004; Melamed et al. 1999; Pruessner et al. 2003; Schlotz et al. 2004; Steptoe et al. 2000). Given that problem gamblers might be experiencing ongoing (or intermittent) distress, it was hypothesized that the early morning rise of cortisol would be particularly notable among problem and pathological gamblers relative to recreational gamblers. Moreover, given that distress associated with depression and impulsivity could be associated with cortisol variations, we assessed the relation between these factors and cortisol levels, with a focus on determining whether they accounted for the cortisol variations associated with gambling.
Methods
Participants
First year university students (N = 1584) were screened for gambling problems during mass testing using the DSM-IV checklist for pathological gambling (APA 1994). The DSM-IV checklist assesses symptoms of pathological gambling and formed the basis of the sample of participants used in the present study. Participants were asked to indicate ‘Yes’ if the statement applied to them or ‘No’ if it did not. For example, item 8 asked, “In the last year, have you committed any illegal acts such as forgery, fraud, theft, or embezzlement to get money to gamble or to pay gambling debts?” This format was used to identify recreational gamblers (0 items endorsed), problem gamblers (1–4 items endorsed), and pathological gamblers (5 + items endorsed) (for a discussion on DSM-IV categorization, see Cox et al. 2004).
On the basis of their scores, 140 participants were contacted, of which 125 agreed to participate. These comprised 66 recreational gamblers (31 males and 35 females), 51 problem gamblers (35 males and 16 females), and 8 pathological gamblers (7 males and 1 female). The age of participants did not differ as a function of sex or level of gambling pathology (F s < 1). The mean age of the male recreational and problem/pathological gamblers was 20.32 (SD = .40) and 20.27 (SD = .77) years, respectively, and that of female recreational and problematic gamblers was 19.70 (SD = .26) and 20.61 (SD = 1.57) years, respectively. Age was unrelated to gambling type or mood. Participants’ self-reported ethnicity was Caucasian (n = 92), Black (n = 4), Asian (n = 16), Middle Eastern (n = 4), Hispanic (n = 3), Native Canadian (n = 3), or other (n = 3). Of the participants, 67 did not hold a job (i.e., other than being a student), 42 held a part time job, 2 held full-time jobs, and 14 worked on a contract or seasonal basis.
The number of DSM-IV symptoms in problem and pathological gamblers ranged from 1 to 10 (M = 1.97, SD = 1.02 and M = 6.27, SD = 1.27, respectively), whereas recreational gamblers reported no symptoms. Participants were told that, if they agreed to take part in the study, they would complete a series of questionnaires, and provide saliva samples that would be used for neuroendocrine analyses. In return for their participation, they received $20, or if enrolled in an Introductory Psychology course, participants could opt to receive experimental course credit and $10.
Exclusion criteria included the use of drugs that could affect cortisol levels (e.g., anti-inflammatories, antihistamines) (n = 5 of the initial sample of 140). Several participants were excluded (n = 10) who did not take saliva samples within 10 min of awakening. Although it has been reported that oral contraceptives may influence the cortisol response to stressors (Kirschbaum et al. 1999), women taking oral contraceptives were not excluded. However, a separate analysis was conducted to assess the early morning salivary cortisol response as a function of the type of gambler and whether female participants were using oral contraception. Finally, although several participants reported using antidepressants (n = 5), they were included in the analyses as depression is a comorbid feature among problem gamblers.
Procedure
The study was conducted over a 2-month period between February and March in order to avoid both the mid-term and final exam periods as these periods generate considerable stress among students. Thus, all participants were relatively free of exam related stressors during the course of the current study.
Upon arrival at the laboratory, an experimenter explained that the purpose of the study was to investigate factors associated with gambling attitudes and behavior, and to assess biological characteristics detected in saliva, in relation to gambling behaviors. After written, informed consent was obtained, participants were provided with a set of tubes (Salivettes) that they were to use to collect their own saliva at various times of the day. Specifically, participants were instructed that they were to take four saliva samples over the course of a given day, including immediately upon awakening, 30 min later, approximately 3.5 and 5.5 h afterward. These samples were taken on a weekday (as opposed to a weekend) in order to avoid confounding that might be related to weekend activities or changes in sleep cycle. Saliva sampling comprised participants placing a piece of dental cotton in their cheek for a 2 min period, and once thoroughly wet, it was placed into a Salivette and refrigerated. Participants were instructed to take the morning saliva samples before breakfast or brushing their teeth, and to record, on a form provided, the time of their waking, and the time at which each saliva sample was taken. As well, they were to come to the laboratory at a pre-arranged time (between 1400 and 1600 h) to complete a series of questionnaires. If participants took either of the morning samples more than 10 min late, the data were not included in subsequent analyses.
When participants arrived at the laboratory on the second occasion, they returned the Salivettes, which were then stored at −80°C for subsequent assay. Participants then completed several questionnaires, including the Beck Depression Inventory (Beck et al. 1961), and the Barratt Impulsiveness Scale-11 (Patton et al. 1995). After the session, participants were verbally debriefed and presented with a take-home sheet explaining the rationale of the study. Included in the take home sheet were contact phone numbers for the local distress center hotline, as well as the Ontario Problem Gambling Helpline.
Salivary cortisol levels were subsequently determined, in duplicate, by means of a solid phase radio-immuno assay using 125I kits (ICN Biomedicals Inc., CA). The intra- and extra-assay variability was less than 8%.
Instruments
Depressive Affect
The 21-item Beck Depression Inventory (BDI) was used as a measure of depressive symptoms (Beck et al. 1961). Each item comprises 3–4 statements ranging in the extent to which they were symptomatic, from not at all (e.g. ‘I do not feel sad’) to highly symptomatic (e.g. ‘I am so sad or unhappy that I can’t stand it’). Participants marked which statement of each set most accurately reflected them. Responses were summed to provide an index of depressive symptomatology (α = .90).
Impulsivity
The 30-item Barratt Impulsiveness Scale-11 (Patton et al. 1995) assessed three dimensions of impulsivity using 4-point frequency scales, ranging from 1 (rarely/never) to 4 (almost always). The dimensions included behavioral impulsivity (motor, α = .60), and two components of cognitive impulsivity (non-planning, α = .64 and cognitive focus, α = .68). The internal reliabilities of the subscales were relatively low, even though the internal reliability for the total scale score (α = .78) was similar to those reported by Patton et al. (1995) for other normative samples. The correlations among the subscales were only moderate (r’s ranging from .21 to .44). Given the potential implications of distinguishing between behavioral versus cognitive impulsivity, analyses were conducted using the three subscale scores; however, given their reliability, analyses were also conducted using general impulsivity scores.
Statistical Analyses
Depression and impulsivity scores were analyzed by one-way analyses of variance (ANOVA) as a function of three gambling subtypes. As well, given the small number of female pathological gamblers, problem and pathological gamblers were combined to conduct 2 (recreational versus problem + pathological gamblers) × 2 (sex) ANOVAs on depression and impulsivity scores. Cortisol levels (nmol/l) were analyzed using a mixed measures ANOVA with three gambling subtypes as the between groups variable, and time of measurement as the within groups measure. Further analyses also assessed cortisol levels over time as a function of gambling subtype (pooling problem and pathological gamblers) × Sex. As well, a subsidiary mixed measures ANOVA was conducted to determine whether cortisol levels varied over time among women taking oral contraceptives and those who did not. Comparable outcomes were observed irrespective of whether cortisol data were analyzed using raw scores or as a percentage change from baseline (awakening). Thus, only the former are reported. However, for the purpose of correlational analyses (Pearson product) between cortisol changes and depression and impulsivity scores, percentage change of cortisol was used. Where appropriate, follow-up tests were conducted using t-tests with Bonferonni corrections to maintain family-wise α at .05.
Results
Depression scores varied as a function of the type of gambler, F(2, 123) = 6.17, p < .05. Follow-up tests indicated that the depression scores among pathological gamblers were considerably higher than among problem or recreational gamblers. Depression scores in the latter two conditions did not differ from one another (see Table 1). As only a single female pathological gambler was tested, an ANOVA with sex as a factor crossed with the three gambling subtypes could not be conducted. However, a separate analysis pooling problem and pathological gamblers indicated main effects for sex, F(1, 123) = 5.27, p < .05, and gambling subtype, F(1, 123) = 6.87, p < .01, and the interaction was not significant. Specifically, female gamblers (M = 11.65, SD = 9.15) indicated higher depression scores than did male gamblers (M = 7.70, SD = 5.73), and both female and male problem/pathological gamblers reported higher levels of depression than recreational gamblers (M = 7.32, SD = 5.57 and M = 5.97, SD = 5.98, respectively).
Impulsivity was categorized as falling into three types, namely behavioral impulsivity, cognitive impulsivity, and non-planning impulsivity, along with general or overall impulsivity. General impulsivity varied as a function of the subtype of gambler, F(2, 123) = 5.69, p < .01. This reflected variations in both the behavioral and cognitive dimensions, F(2, 123) = 4.93, 4.91, ps < .01, but the groups did not differ with respect to non-planning impulsivity, F(2, 123) = 1.78, ns. As seen in Table 1, follow-up comparisons indicated that general impulsivity among recreational gamblers was lower than among either the problem or pathological gamblers; problem and pathological gamblers did not differ from one another. As well, analyses of pooled problem and pathological gamblers versus recreational gamblers, indicated that there were no differences as a function of sex.
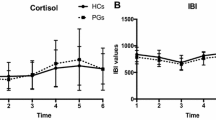
To evaluate cortisol levels over time following awakening, an initial ANOVA was conducted pooling males and females, revealing a significant Type of Gambler × Time interaction, F(6, 336) = 2.34, p < .05. Figure 1 shows the cortisol levels at awakening, 30 min later, and again approximately 3.5 and 5.5 h afterward. Follow-up comparisons indicated that cortisol levels rose from awakening to 30 min afterward, and then declined. However, the rise of cortisol was significantly greater among the problem and pathological gamblers than among the recreational gamblers. At the fourth sampling time cortisol levels had declined to the levels seen at awakening, and did not differ between groups. An additional analysis performed using percentage changes of cortisol from awakening yielded comparable differences as a function of the type of gambler.
To confirm that the outcome observed was not simply an artifact of an unequal number of male and female participants in each condition, an additional analysis was conducted comparing cortisol levels only among male participants. This analysis confirmed that among males the cortisol rise was greater in problem and pathological gamblers than in recreational gamblers, and then declined to comparable levels in each of the groups.
As cortisol levels did not differ between pathological and problem gamblers, the cortisol data for these groups were pooled for further analyses comparing males and females. This analysis indicated that cortisol levels increased significantly over the initial two samples, and that this effect was moderated by the type of gambler, F(3, 312) = 4.89, p < .001, being greater among problem than among recreational gamblers. Neither the effect of Sex, nor the Sex × Type of gambler interaction reached significance.
As indicated earlier, inasmuch as oral contraceptive use can influence cortisol levels, particularly among females that have been stressed (Krueger et al. 2005), a subsidiary analysis was conducted to assess the influence of this variable on cortisol levels. Of the female recreational gamblers, oral contraception was used by 18 (of 35) women, and among the problem gamblers, 8 (of 17) used oral contraceptives. The ANOVA revealed that cortisol levels varied as a function of the Type of Gambler × Oral contraceptive use × Time interaction, F(3, 144) = 4.30, p < .01. Follow-up comparisons indicated that among the problem gamblers, the morning rise of cortisol was greater among women not using oral contraceptives (increasing from 11.13 (SE = 2.04) at awakening to 28.38 (SE = 6.24) nmol/l 30 min later) than among those who were using oral contraceptives (from 11.76 (SE = 1.68) to 16.01 (SE = 1.99) nmol/l measured 30 min later), and their cortisol levels remained elevated at the third sampling time.
Despite the elevated morning cortisol levels associated with gambling pathology and the rise of morning cortisol, and the finding that gambling was associated with increased symptoms of depression as well as impulsivity, the percentage rise of cortisol from awakening to 30 min later was not significantly correlated to either BDI scores, r = .08, or general impulsivity, r = .04. This was the case among males and females, and irrespective of whether correlations were conducted using the entire sample or specifically among recreational or problem gamblers. Thus, neither depressive mood nor impulsivity could account for the relation between gambling and morning cortisol elevations.
Discussion
As described earlier, cortisol levels in the period following casino gambling were reported to be elevated among problem or pathological gamblers (Krueger et al. 2005; Meyer et al. 2000, 2004). Such findings suggest that gambling was either perceived as stressful, or that the situation was particularly arousing. However, cortisol levels following a challenge do not speak to whether problematic gambling represented a chronic stressor for these individuals. Thus, morning cortisol elevations were assessed, as this hormone rise may be indicative of the presence of excessive and persistent stressors. Indeed, in other contexts (e.g., job strain, psychosocial distress) chronic strain was associated with elevated morning salivary cortisol levels (Grossi et al. 2005; Kunz-Ebrecht et al. 2004; Melamed et al. 1999; Pruessner et al. 2003; Schlotz et al. 2004; Schmidt-Reinwald et al. 1999; Steptoe et al. 2000).
Consistent with the perspective that problematic gambling places a chronic strain on individuals, in the present investigation the morning rise of cortisol levels among pathological gamblers, as well as those with subclinical gambling symptoms, were more pronounced than they were among recreational gamblers. Symptoms of depression and impulsivity, as previously reported (Blaszczynski et al. 1997; Getty et al. 2000; Goodyear-Smith et al. 2006; Kim et al. 2006; Petry et al. 2005; Scherrer et al. 2005; Steel and Blaszczynski 1998), were also elevated in relation to gambling behaviors. However, the elevated morning cortisol rise was unrelated to either of these psychological factors. Importantly, although problem gamblers demonstrated an elevated morning cortisol rise comparable to that of the pathological gamblers, these sub-clinical gamblers did not show similarly high levels of depressive symptoms. Instead, among these individuals signs of depression were comparable to that of recreational gamblers. Thus, it does not appear that the elevated cortisol levels of gamblers were secondary to the presence of depressive symptoms.
In contrast to depressive symptoms, problem and pathological gamblers exhibited similar levels of impulsivity. In effect, problem gamblers, like pathological gamblers, appeared to be disposed to acting with little forethought, self-control, or regard for consequences. However, as the cortisol rise and impulsivity were not significantly correlated with one another, the morning cortisol rise was not secondary to impulsivity. At this point, it is important to underscore that as the high levels of impulsivity that normally characterize pathological gambling (DSM-IV) were also evident among those with subclinical gambling symptoms, and these latter individuals also exhibited the elevated morning cortisol levels, the presence of these factors might be indicative of increased risk of further escalation of gambling related disturbances.
The HPA neuroendocrine activation that ordinarily occurs in response to stressors is thought to be of adaptive significance by acting in a preparatory fashion, facilitating behavioral coping responses, providing energy stores to contend with environmental demands, and limiting excessive activation of systems (e.g., immune activation) that could engender adverse outcomes (Sapolsky et al. 2000). It will be recalled, however, that if a stressor is sufficiently intense, protracted, and unpredictable, then the wear and tear on biological systems may become excessive (allostatic overload), rendering the individual more vulnerable to pathological outcomes (McEwen 2000). The fact that morning cortisol levels were elevated in problem and pathological gamblers could potentially augment their ability to deal with ongoing strain. Yet, the sustained morning cortisol elevations among gamblers could potentially have the effect of promoting allostatic overload and hence might contribute to increased vulnerability to pathology through the breakdown of regulatory capacities.
The finding that unlike pathological gambling, problem gambling was not associated with increased levels of depressive symptoms might be taken to suggest that this level of gambling is, in fact, not of concern with respect to mood disorders. Indeed, it was suggested that the association between depression and gambling may be less robust than initially proposed (Cunningham-Williams et al. 2000). This said, as problem gamblers exhibited increased levels of the morning cortisol response, just as pathological gamblers did, it may be the case that both problematic categories of gambling are experiencing ongoing distress. The elevated cortisol levels could potentially be a harbinger of future adverse effects, such as depression. Of course, prospective analyses of problem gamblers are necessary to confirm this possibility.
It has been reported that problem and pathological gambling are associated with diminished health-related quality of life as well as a constellation of other stressful experiences coupled with poor coping strategies (Scherrer et al. 2005). Inasmuch as the cortisol elevations were simply related to gambling problems, it is inappropriate to conclude that the gambling disturbance was causally related to the salivary cortisol levels, although, of course, problems stemming from gambling may place considerable strain on individuals, hence leading to the hormonal variations. It could, however, be argued that wagering is, in fact, used by the gambler as a means of coping with life stressors (i.e. gambling as a distraction), and these behaviors, which are not secondary to gambling, are responsible for the elevated morning cortisol. Likewise, although depression was not related to cortisol levels, it remains possible that engaging in gambling may be secondary to depression, perhaps as an attempt to cope with low mood or related difficulties. Finally, as gambling disturbances may be related to other factors (e.g., increased alcohol use), the possibility cannot be dismissed that such factors contributed to the elevated morning cortisol response that was observed.
Although the present findings link gambling to ongoing distress, there is considerable evidence supporting the view that gambling pathology may stem from effects related to excessive functioning of biological systems, namely dopamine (DA), involved in reward processes (Goudriaan et al. 2004; Shizgal and Arvanitogiannis 2003). Indeed, problem gambling is provoked among Parkinsonian patients treated with l-DOPA, a DA precursor (Weintraub and Potenza 2006), and gambling pathology has also been associated with polymorphisms for DA receptors (Goudriaan et al. 2004; Ibanez et al. 2003). We do not take issue with the view that DA functioning may be involved in depression, but it nevertheless ought to be noted that elevated DA activity within mesocortical brain regions is not only associated with reward processes, but is a prototypical response to stressors (Anisman and Zacharko 1992; Deutch and Roth 1990). Thus, it is possible that altered DA reactivity associated with gambling may reflect responses to stressors rather than reward processes. To be sure, gambling pathology is likely a heterogeneous illness with multiple precipitating factors, including variations of reward processes, stressor reactivity, and coping disturbances. In fact, it can be imagined that gambling, in a subset of individuals, may initially represent a coping response to deal with stressors, and subsequently evolves into gambling pathology owing to disturbed DA functioning.
Although a fairly consistent pattern of results was observed, several limitations of the present investigation ought to be considered. Foremost among these is that the sample of pathological gamblers was small, and only a single female pathological gambler was recruited. To be sure, the incidence of female pathological gamblers is smaller than that of males, but psychiatric symptoms of (adolescent) female pathological gamblers tend to be more severe than in males (Desai et al. 2005). Ultimately, to define the processes associated with gambling, greater focus on female gamblers is important, especially as the processes related to gambling among females may differ from those that favor gambling in males (Ibanez et al. 2003; Nower et al. 2004).
Summarizing, pathological gambling was associated with signs of chronic strain, including both depressive symptoms and elevated morning cortisol levels. In addition, despite the fact that problem gambling was not associated with depressive symptoms, the presence of elevated morning cortisol levels may be indicative of ongoing distress. It remains to be established whether these signs of distress among problem gamblers represent a harbinger of greater risk for later disturbances, including more profound gambling problems.
Abbreviations
- HPA:
-
Hypothalamic–pituitary–adrenal
- BDI:
-
Beck Depression Inventory
- DA:
-
Dopamine
References
Anisman, H., & Zacharko, R. M. (1992). Depression as a consequence of inadequate neurochemical adaptation in response to stressors. British Journal of Psychiatry, 160, 36–43.
APA. (1994). Diagnostic and statistical manual of mental disorders (4th ed.). Washington, DC: American Psychiatric Association.
Beck, A. T., Ward, C. H., Mendelson, M., Mock, J., & Erbaugh, J. (1961). An inventory for measuring depression. Archives of General Psychiatry, 4, 561–569.
Bergevin, T., Gupta, R., Derevensky, J., & Kaufman, F. (2006). Adolescent gambling: Understanding the role of stress and coping. Journal of Gambling Studies, 22, 195–208.
Black, D. W., & Moyer, T. (1998). Clinical features and psychiatric comorbidity of subjects with pathological gambling behavior. Psychiatric Services, 49, 1434–1439.
Blaszczynski, A., Steel, Z., & McConaghy, N. (1997). Impulsivity in pathological gambling: The antisocial impulsivist. Addiction, 92, 75–87.
Clarke, D. (2004). Impulsiveness, locus of control, motivation and problem gambling. Journal of Gambling Studies, 20, 319–345.
Cox, B. J., Enns, M. W., & Michaud, V. (2004). Comparisons between the south oaks gambling screen and a DSM-IV-based interview in a community survey of problem gambling. Canadian Journal Psychiatry, 49, 258–264.
Cunningham-Williams, R., Cottler, L., Compton, W., Spitznagel, E., & Ben-Abdalla A. (2000). Problem gambling and comorbid psychiatric and substance use disorders among drug users recruited from drug treatment and community settings. Journal of Gambling Studies, 16, 347–376.
Desai, R. A., Maciejewski, P. K., Pantalon, M. V., & Potenza, M. N. (2005). Gender differences in adolescent gambling. Annals of Clinical Psychiatry, 17, 249–258.
Deutch, A. Y., & Roth, R. H. (1990). The determinants of stress-induced activation of the prefrontal cortical dopamine system. Progress in Brain Research, 85, 367–402.
Getty, H. A., Watson, J., & Frisch, G. R. (2000). A comparison of depression and styles of coping in male and female GA members and controls. Journal of Gambling Studies, 16, 377–391.
Goodyear-Smith, F., Arroll, B., Kerse, N., Sullivan, S., Coupe, N., Shepherd, R., et al. (2006), Primary care patients reporting concerns about their gambling frequently have other co-occurring lifestyle and mental health issues. BMC Family Practice, 7, 25.
Goudriaan, A. E., Oosterlaan, J., de Beurs, E., & Van den Brink, W. (2004). Pathological gambling: A comprehensive review of biobehavioral findings. Neuroscience Biobehavioral Review, 28, 123–141.
Grossi, G., Perski, A., Ekstedt, M., Johansson, T., Lindstrom, M., & Holm, K. (2005). The morning salivary cortisol response in burnout. Journal of Psychosomatic Research, 59, 103–111.
Gupta, R., & Derevensky, J. L. (2000). Adolescents with gambling problems: From research to treatment. Journal of Gambling Studies, 16, 315–342.
Ibanez, A., Blanco, C., de Castro, I. P., Fernandez-Piqueras, J., & Saiz-Ruiz, J. (2003). Genetics of pathological gambling. Journal of Gambling Studies, 19, 11–22.
Ibanez, A., Blanco, C., Moreryra, P., & Saiz-Ruiz, J. (2003). Gender differences in pathological gambling. Journal of Clinical Psychiatry, 64, 295–301.
Kausch, O., Rugle, L., & Rowland, D. Y. (2006). Lifetime histories of trauma among pathological gamblers. American Journal of Addiction, 15, 35–43.
Kim, S. W., Grant, J. E., Eckert, E. D., Faris, P. L., & Hartman, B. K. (2006). Pathological gambling and mood disorders: Clinical associations and treatment implications. Journal of Affective Disorders, 92, 109–116.
Kirschbaum, C., Kudielka, B. M., Gaab, J., Schommer, N. C., & Hellhammer, D. H. (1999). Impact of gender, menstrual cycle phase, and oral contraceptives on the activity of the hypothalamus–pituitary–adrenal axis. Psychosomic Medicine, 61, 154–162.
Krueger, T. H., Schedlowski, M., & Meyer, G. (2005). Cortisol and heart rate measures during casino gambling in relation to impulsivity. Neuropsychobiology, 52, 206–211.
Kunz-Ebrecht, S. R., Kirschbaum, C., & Steptoe, A. (2004). Work stress, socioeconomic status and neuroendocrine activation over the working day. Social Science Medicine, 58, 1523–1530.
Marshall, K., & Wynn, H. (2003). Fighting the odds. Perspectives, Statistics Canada, catalogue no. 75-001-X1E.
McEwen, B. S. (2000). Allostasis and allostatic load: Implications for neuropsychopharmacology. Neuropsychopharmacology, 22, 108–124.
Melamed, S., Ugarten, U., Shirom, A., Kahana, L., Lerman, Y., & Froom, P. (1999). Chronic burnout, somatic arousal an elevated salivary cortisol levels. Journal of Psychosomatic Research, 46, 591-598.
Merali, Z., McIntosh, J., Kent, P., Michaud, D., & Anisman, H. (1998). Aversive as well as appetitive events evoke the release of corticotropin releasing hormone and bombesin-like peptides at the central nucleus of the amygdala. Journal of Neuroscience, 18, 4758–4766.
Messerlian, C., Derevensky, J., & Gupta, R. (2005). Youth gambling problems: A public health framework. Health Promotion International, 20, 69–79.
Meyer, G., Hauffa, B. P., Schedlowski, M., Pawlak, C., Stadler, M. A., & Exton, M. S. (2000). Casino gambling increases heart rate and salivary cortisol in regular gamblers. Biological Psychiatry, 48, 948–953.
Meyer, G., Schwertfeger, J., Exton, M. S., Janssen, O. E., Knapp, W., Stadler. M. A., et al. (2004). Neuroendocrine response to casino gambling in problem gamblers. Psychoneuroendocrinology, 29, 1272–1280.
Nower, L., Derevensky, J., & Gupta, R. (2004). The relationship of impulsivity, sensation seeking, coping, and substance abuse in youth gamblers. Psychology of Addictive Behavior, 18, 49–55.
Patton, J. H., Stanford, M. S., & Barratt, E. S. (1995). Factor structure of the Barratt impulsiveness scale. Journal of Clinical Psychology, 51, 768–774.
Petry, N. M., Stinson, F. S., & Grant, B. F. (2005). Comorbidity of DSM-IV pathological gambling and other psychiatric disorders: Results from the National Epidemiologic Survey on Alcohol and Related Conditions. Journal of Clinical Psychiatry, 66, 564–574.
Piazza, P. V., & Le Moal, M. (1997). Glucocorticoids as a biological substrate of reward: Physiological and pathophysiological implications. Brain Research. Brain Research Reviews, 25, 359–372.
Potenza, M. N., Fiellin, D. A., Heninger, G. R., Rounsaville, B. J., & Mazure, C. M. (2002). Gambling: An addictive behavior with health and primary care implications. Journal of General Internal Medicine, 17, 721–732.
Pruessner, M., Hellhammer, D. H., Pruessner, J. C., & Lupien, S. J. (2003). Self-reported depressive symptoms and stress levels in healthy young men: Associations with the cortisol response to awakening. Psychosomatic Medicine, 65, 92–99.
Sapolsky, R. M., Romero, L. M., & Munck, A. U. (2000). How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocrinology Review, 21, 55–89.
Scherrer, J. F., Xian, H., Shah, K. R., Volberg, R., Slutske, W., & Eisen, S. A. (2005). Effects of genes, environment, and lifetime co-occurring disorders on health-related quality of life in problem and pathological gamblers. Archives of General Psychiatry, 62, 677–683.
Schlotz, W., Hellhammer, J., Schulz, P., & Stone, A. A. (2004). Perceived work overload and chronic worrying predict weekend–weekday differences in the cortisol awakening response. Psychosomatic Medicine, 66, 207–214.
Schmidt-Reinwald, A., Pruessner, J. C., Hellhammer, D. H., Federenko, I., Rohleder, N., Schurmerer, T. H., et al. (1999). The cortisol response to awakening in relation to different challenge tests and a 12-h cortisol rhythm. Life Sciences, 64, 1653–1660.
Shaffer, H. J., & Hall, M. N. (2001). Updating and refining prevalence estimates of disordered gambling behavior in the United States and Canada. Canadian Journal of Pubic Health, 92, 168–172.
Shaffer, H. J., Hall, M. N., & Vander Bilt, J. (1999). Estimating the prevalence ofdisordered gambling behavior in the United States and Canada: A research synthesis. American Journal of Public Health, 89, 1369–1376.
Shizgal, P., & Arvanitogiannis, A. (2003). Neuroscience: Gambling on dopamine. Science, 299, 1856–1858.
Steel, Z., & Blaszczynski, A. (1998). Impulsivity, personality disorders and pathological gambling severity. Addiction, 93, 895–905.
Steptoe, A., Cropley, M., Griffith, J., & Kirschbaum, C. (2000). Job strain and anger expression predict early morning elevations in salivary cortisol. Psychosomatic Medicine, 62, 286–292.
Weintraub, D., & Potenza, M. N. (2006). Impulse control disorders in Parkinson’s disease. Current Neurology and Neuroscience Reports, 6, 302–306.
Wohl, M. J. A., Young, M. M., Matheson, K., & Anisman, H. (2006). Outcome expectancies, subjective appraisals, and treatment readiness: The role of perceived personal luck in pathological gambling behavior 2006. Paper presented at the 13th International Conference on Gambling and Risk Taking, Lake Tahoe, CA.
Acknowledgments
This research was supported by a research grant from the Ontario Problem Gambling Research Centre (#2212) to Wohl, Anisman, and Matheson.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wohl, M.J.A., Matheson, K., Young, M.M. et al. Cortisol Rise Following Awakening Among Problem Gamblers: Dissociation from Comorbid Symptoms of Depression and Impulsivity. J Gambl Stud 24, 79–90 (2008). https://doi.org/10.1007/s10899-007-9080-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10899-007-9080-6