Abstract
Scientific advances have allowed the development of multiplex gene-panels to assess many genes simultaneously in women who have tested negative for BRCA1/2. We examined correlates of interest in testing for genes that confer modest and moderate breast cancer risk and risk communication preferences for women from BRCA negative families. Female first-degree relatives of breast cancer patients who tested negative for BRCA1/2 mutations (N = 149) completed a survey assessing multiplex genetic testing interest and risk communication preferences. Interest in testing was high (70 %) and even higher if results could guide risk-reducing behavior changes such as taking medications (79 %). Participants preferred to receive genomic risk communications from a variety of sources including: primary care physicians (83 %), genetic counselors (78 %), printed materials (71 %) and the web (60 %). Factors that were independently associated with testing interest were: perceived lifetime risk of developing cancer (odds ratio (OR) = 1.67: 95 % confidence interval (CI) 1.06–2.65) and high cancer worry (OR = 3.12: CI 1.28–7.60). Findings suggest that women from BRCA1/2 negative families are a unique population and may be primed for behavior change. Findings also provide guidance for clinicians who can help develop genomic risk communications, promote informed decision making and customize behavioral interventions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The translation of genetic information is at the forefront of cancer prevention and control and is especially relevant for women at high risk for Hereditary Breast and Ovarian Cancer (HBOC). When HBOC is suspected, genetic testing for mutations in the BRCA1/2 breast cancer susceptibility genes is an evidence-based strategy that informs medical management options (e.g., prophylactic mastectomy and/or oophorectomy, tamoxifen therapy), helps women to make informed decisions about cancer prevention, and has been shown to improve patient survival when testing leads to risk reducing strategies (Bradbury et al. 2015a). Genetic cancer information not only impacts the affected individual but also has clinical and psychosocial implications for family members. Without a prior family history of a BRCA mutation, a BRCA negative test result is “uninformative”, (other genetic mutations may contribute to cancer in the family), and the patient and their family members may have lingering concerns about their cancer risk, genetic testing options beyond BRCA1 or BRCA2, or cancer prevention options (Kotsopoulos et al. 2014). Thus, strategies to improve genetic risk communication for BRCA negative families is an important emerging issue.
Until recently, genetic testing for HBOC focused mainly on assessing for mutations in BRCA1/2, but technological advances in DNA sequencing have made it possible to test multiple, even hundreds, of genes simultaneously using multiplex gene-panels (Desmond et al. 2015). Recent studies of the clinical utility of gene-panel testing for hereditary breast and ovarian cancer have found that gene-panel testing can identify actionable variants that would have otherwise gone undetected with BRCA1/2 testing alone (Daly et al. 2016; Desmond et al. 2015; Lincoln et al. 2015). Newer research exploring the utilization of SNP panels (often in conjunction with other data including BRCA1/2 and other high/moderate risk genes or breast density measurements) for breast cancer risk assessment in the general population further highlight how genetic testing considerations may become increasingly pertinent for unaffected women (Evans et al. 2012; Li et al. 2016; Mavaddat et al. 2015).
There is a broad range in the number of people reporting interest in genetic testing (28 % to over 90 %) in the general population and in high-risk individuals (Bottorff et al. 2002; Graves et al. 2011; Henneman et al. 2013; Hoberg-Vetti et al. 2016; Meisel et al. 2015; Ramirez et al. 2015; Sussner et al. 2011; Vermeulen et al. 2014). Studies vary widely based on the questions asked, the context of the genetic testing, and on population characteristics such as income, age, education, and country. Most recently, intense research and media attention have elevated public interest in genetic testing for HBOC (Evans et al. 2014; Lebo et al. 2015). Family members may believe that gene-panel testing can provide more cancer risk information and cancer prevention options. In most cases, unaffected family members from BRCA negative families do not meet criteria for further cancer genetic testing (“National Comprehensive Cancer Network Guidelines Version 2.2016 Genetic/Familial High Risk Assessment: Breast and Ovarian,”) nor is it likely their medical insurance covers genetic testing in this context. However, family members may ultimately desire genetic counseling and genetic testing to cope with lingering concerns. Thus, it is important to understand why relatives seek genetic testing, what they hope to gain from testing, and to provide them with relevant information to understand their risks and benefits in the context of the genetic evaluation that has already occurred in their family.
The increased availability of complex genomic information poses challenges to clinicians as well as patients. Medical professionals, who are tasked with interpreting test results for patients or family members, have concerns about gene-panels that include variants of unknown significance (VUS) and genes that have low clinical validity (i.e., how accurately the test predicts disease risk) and clinical utility (i.e., how useful the test is for medical decision-making) (Easton et al. 2015). Testing multiple genes simultaneously can lead to unanticipated results by revealing risks for other cancer syndromes or diseases. Inappropriate referrals for genetic testing are not uncommon (McCarthy et al. 2013; Teng and Spigelman 2014; Trivers et al. 2011). Physicians report inappropriately referring average risk individuals for testing or failing to refer individuals that meet criteria for genetic testing (Trivers et al. 2011).
Multiple individual and system-level factors inform the decision to use gene-panel testing including clinic, socioeconomic, cognitive and emotional factors (Bradbury et al. 2015a; Cragun et al. 2015; Easton et al. 2015; Powers and Stopfer 2014). Given that the majority of women who undergo testing actually test negative for BRCA1/2 mutations, it is important to understand attitudes toward multiplex testing and risk communication preferences among BRCA1/2 negative family members. Although the individual with cancer is the most informative person to receive genetic testing in a high risk family, they may not be the first person in the family to become aware of additional genetic testing options. When the cause of cancer is unknown in a family, relatives without cancer may gravitate toward new tests to help clarify their own cancer risks and healthcare options. The importance of understanding individual attitudes and preferences in genetic testing is underscored by national initiatives, such as Precision Medicine (Collins and Varmus 2015), (which calls for assessing individual variability in genes, environment, and lifestyle to improve treatment and prevention healthcare decisions) and recommendations to incorporate genomic information into behavior change interventions (McBride et al. 2015). Precision medicine and health behavior change can potentially be realized without promoting unnecessary genetic testing by more effectively communicating genetic information to entire families.
Currently, we know very little about the perspectives of unaffected women from BRCA1/2 negative families toward gene-panel testing and the most effective ways to communicate information about genetic testing to promote health behaviors (McBride et al. 2015). We address this gap by examining interest in and preferences for receiving information about gene-panel testing for modest to moderate increases in breast cancer risk for members of BRCA negative families. Specifically, we examined the association of multiple potential psychological, behavioral, demographic and clinical factors with interest in genetic testing for members of BRCA1/2 negative families. Such information can help guide risk communication strategies for clinicians in speaking with patients about gene-panel testing by having a better understanding of what relatives are seeking through additional testing. With this insight, genetic counselors can provide a more tailored discussion on the potential risks and benefits of gene-panel testing and the importance of identifying the most informative person to test within the family. Targeted communication strategies can better help women make informed decisions about whether or not to undergo gene-panel testing, risk reduction strategies and screening to improve cancer outcomes.
Methods
Participants
Study participants were the sisters or daughters of female breast cancer patients who were enrolled in a large clinical trial, the Risk Education and Assessment for Cancer Heredity (REACH) Project (Kinney et al. 2014). The REACH Project was a cluster randomized trial that tested 1) the equivalency of BRCA1/2 testing uptake and 2) non-inferiority of changes in psychosocial and informed decision-making measures among women who received remote telephone genetic counseling or in-person genetic counseling. Women in the telephone counseling arm who chose to have BRCA1/2 testing received a genetic test kit and women in the in-person counseling group either gave a sample directly in the clinic or, if they preferred, received a genetic test kit. Detailed information about the study’s population-based recruitment strategy, interventions, theoretical rationale, and outcomes are published elsewhere (Kinney et al. 2014). For the current study, only REACH participants who tested negative for a BRCA1/2 gene mutation were mailed a letter, family contact form, and a postage-paid envelope asking their permission to invite their potentially eligible sisters and/or daughters (the participants of this study) to participate in a survey. Relatives of BRCA negative breast cancer patients were contacted by mail and/or telephone to screen for eligibility. Eligibility criteria for the current study included: 1) age 40–74 years (i.e., eligible for mammography screening at the time of the study); 2) resident of the United States; 3) no prior diagnosis of cancer (except non-melanoma skin cancer); 4) no prior BRCA1/2 testing or genetic counseling; and 5) no bilateral mastectomy. They were mailed a study packet that included a consent cover letter, study questionnaire, tape measure with instructions, and a postage-paid return envelope. Out of list of 214 potential participants, 33 could not be contacted, 15 were ineligible and 17 declined participation. The overall acceptance rate was 89.8 % (149/149 + 17). We did not find any statistical differences in participants compared to non-participants when we compared the two groups by available information including mean age, rural vs urban residence or Utah vs other state residence. The University of Utah Institutional Review Board approved the study.
Measures and Procedures
Perceived Risk
Perceived risk was evaluated with an item assessing lifetime risk (Lipkus et al. 2000): “In your opinion, how likely is it that you will get breast cancer in your lifetime?” Response options were ‘Very Unlikely’, ‘Unikely’, ‘50–50 chance’, ‘Likely’, and ‘Very Likely’.
Cancer Worry
We measured the frequency and intensity of cancer worry with a validated 3-item scale (Jensen et al. 2010; McCaul et al. 2015). Two items measured worry intensity: “How bothered are you about getting breast cancer?” and “How worried are you about getting breast cancer?” Reponses ranged from ‘not at all’ to ‘extremely’ on a 5-point Likert scale. Worry frequency was measured by asking participants “During the past week, how often have you worried about getting breast cancer?” The items were summed to create the worry variable. Internal consistency was very good (Cronbach’s α = .82). Scores less than 7 were considered ‘low worry’ and scores greater than or equal to 7 were considered ‘high worry’.
Clinical Factors:
We assessed:
-
1)
whether or not a participant reported having talked with their provider about a family history of cancer, ‘Yes’, ‘No’,
-
2)
if the participant reported having 2 or more close blood relatives with breast cancer (based on a short family history of cancer questionnaire),
-
3)
if a participant reported having had a clinical breast exam and,
-
4)
mammography within the last 2 years based on self-reported date of last procedure.
Lifestyle
Participants were asked. “Over the past month, how many servings of fruits [vegetables] did you eat per day?” Response options were, ‘0’, ‘1’ , ‘2’ , ‘3’ , ‘4’ or ‘5 or more’. The number of fruit and vegetable servings per day from the two questions were combined and assessed as less than 5 or 5 or more in accordance with national dietary guidelines (“US Department of Agriculture. Dietary Guidelines for Americans. http://www.health.gov/dietaryguidelines/ ” 2015). For physical activity, we assessed if participants engaged in at least 150 min of self-reported moderate intensity exercise or 75 min of high intensity exercise per week using the International Physical Activity Questionnaire Short Form (IPAQ 2015).
Sociodemographics
We assessed age, marital status, income, education and rural or urban residence. Rural or urban status was ascertained using Rural-Urban Commuting Area Codes by zip code as previously described (Kinney et al. 2014).
Outcome Variables
The questionnaire included a brief summary about genetic testing with information about BRCA1/2 testing and other genetic changes that may relate to either small or moderate increases in breast cancer risk (i.e., gene-panel/multiplex testing). The primary outcome, interest in multiplex testing, was assessed by a single item asking participants “If genetic testing could tell you that you may have a slightly to moderately increased risk of developing breast cancer, how likely is it that you would want a genetic test?” We further asked participants if they were interested in this type of genetic testing if it could provide information about risk-based screening (mammograms, breast MRI, or other screening procedures); if their risk could be lowered by taking medications; and if their risk could be lowered by diet and exercise. Response options were “I would definitely not have the test”, “I would probably not have the test”, “I would probably have the test”, “I would definitely have the test”. Responses were dichotomized into “would definitely or probably not have the test” or “would definitely or probably have the test”. Items were adapted from a survey by Graves et al. 2011.
We assessed participants’ preferred method of receiving information about gene-panel testing including print or written information, web-based information, computer kiosk touch screen in a clinic, discussion with a nurse, discussion with a primary care physician, discussion with a cancer specialist such as an oncologist, discussion with a genetic counselor/cancer risk specialist. Possible responses were: “Not at All’, ‘A Little’, ‘Somewhat’, or ‘Very Much’. Responses were dichotomized by interest into “Not at All/A Little” and “Somewhat/Very Much”.
Data Analyses
Sociodemographics, clinical and behavioral factors, cancer worry, perceived risk, and preferences for receiving genetic testing information were characterized in the study population using descriptive statistics in SPSS version 22. Independent variables with non-normal distributions were dichotomized and variables were screened for collinearity. Unadjusted odds ratios (ORs) and 95 % and confidence intervals (CI) were estimated to ascertain association between each independent variable and interest in genetic testing. Logistic regression was used to delineate the independent association of potential factors with interest in genetic testing. Variables that were crudely associated with interest in testing based on a p value <0.20 were entered into the multivariate model. Variables were removed by backward elimination based on the probability of a likelihood-ratio statistic for variable removal of 0.10. To account for family clustering, we tested the final model using generalized mixed modeling in MPlus (version 7). There were 100 family clusters in the sample, with an average cluster size of 2. The design effect for genetic testing was essentially 0 and results were the same whether or not clustering was taken into account. However, the final adjusted model was evaluated taking clustering into account.
Results
Characteristics of the study population are presented in Table 1. The mean age of participants was 53 years (SD ± 9.4 yrs). A majority of women were married (80 %) and had annual incomes equal to or above $50,000 (67 %). Most participants had at least some college education (83 %). Participants indicated that they had talked with a personal healthcare provider about their family history of breast cancer (69 %) and reported having had a clinical breast exam (73 %) or a mammogram (77 %) in the last two years at the time of questionnaire completion. Sixty four percent of women reported having 2 or more first or second degree relatives with a breast cancer diagnosis. Over one-third of women reported high levels of cancer worry.
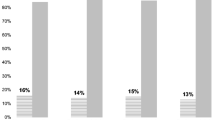
Overall the percentage of participants that reported interest in genetic testing was relatively high with 70 % of women having reported that they would either definitely or probably have a genetic test if it could tell them if they have a slightly to moderately increased risk of developing breast cancer. Interest in genetic testing increased somewhat based on behavioral modification scenarios (Fig. 1): 74 % of women indicated they would definitely or probably have genetic testing if testing could tell them whether or not they should have a mammogram, breast MRI or other screening more frequently, and 79 % of women indicated they would have a genetic test if testing could tell them whether or not their risk of breast cancer could be lowered by taking medications. Interest in genetic testing also increased in the scenario where testing could tell them whether or not their risk could be lowered by diet or exercise (77 %).
The most frequently cited preferred source of information about genetics and cancer risk was with a primary care physician (83 %; Fig. 2), followed by a genetic counselor or cancer risk specialist (78 %), and a cancer specialist such as an oncologist (77 %).
The unadjusted ORs, 95 % CIs and p values for each independent variable with overall interest in gene-panel testing are shown in Table 2. Marital status, rural/urban residence, having had a mammogram in the past two years, consuming 5 or more servings of fruits and vegetables daily, high levels of cancer worry, and increased perceived lifetime risk of developing cancer met the criteria (p < .20) for entry into the logistic model. The final adjusted logistic model included cancer worry and perceived lifetime risk of developing cancer (Table 3). Participants reporting higher levels of cancer worry were more likely to indicate interest in genetic testing than those with lower cancer worry levels (OR = 3.12, 95 % CI 1.28, 7.60, p = 0.009). Higher perceived lifetime risk was associated with interest in genetic testing (OR = 1.67, 95 % CI = 1.06 to 2.65, p = 0.031). Perceived risk and worry did not interact with each other to predict genetic testing (result not shown).
Discussion
This study is one of the first to examine interest in gene-panel testing, prior to genetic counseling, among first-degree relatives of BRCA1/2 negative breast cancer patients. We found that the percentage of women reporting interest in gene-panel testing was high for members of BRCA1/2 negative families and that the percentage of women reporting interest in testing increased if the test could inform them about customized behavior changes to reduce their risk or personalize screening. A previous study did not find increased interest in genetic testing for breast cancer risk when testing could provide recommendations for behavior change (Graves et al. 2011). Our results showed some increase and suggest that some women from BRCA negative families may be especially receptive to behavior change recommendations based on genetic test results and would prefer to receive genetic information from many sources especially their primary care provider and a genetic counselor. Family members generally are not included in their relative’s genetic counseling session. Those with high levels of worry and risk may benefit from enhanced cancer genetic information about genetic risk. Particularly if further genetic testing is not warranted for family members, addressing the limitations of genetic testing in the context of important areas of interest can help to improve cancer genetic communication for members of BRCA negative families.
In this study, interest in gene-panel testing was particularly high if testing could tell participants whether or not taking medications could reduce their risk of cancer (79 %). Currently, the US Preventive Services Task Force recommends that clinicians and patients at increased risk make shared informed decisions about taking medications to reduce their risk for breast cancer (e.g., tamoxifen or raloxifene to reduce primary breast cancer risk and recurrence) (Moyer and Force 2013; Powers and Stopfer 2014). Adherence to medication for the prevention of primary breast cancer in patients is suboptimal, and although adjuvant hormonal therapy has been shown to reduce hormone-sensitive breast cancer recurrence and mortality rates, medication adherence in this population remains low (Nelson et al. 2013). Our results suggest that members of BRCA negative families may be especially interested in information regarding medication use for cancer risk reduction.
Rural and urban residence, fruit and vegetable intake, mammography screening with the past 2 years, cancer worry, and perceived lifetime risk were associated with interest in genetic testing in bivariate analyses. Some of these factors deserve further discussion based on their potential applications. Within their small communities and with fewer health care providers to choose from, rural dwellers may perceive gene-panel testing as a greater threat to their privacy and confidentiality and may also have a greater need for accessing information to help them make informed decisions about gene-panel testing and mitigate high cancer worry (Kelly et al. 2007). The opportunity to improve access to genetic counseling is especially important for rural women because they have limited access to genetic risk specialists, are often diagnosed with late stage breast cancer compared to their urban counterparts (Nguyen-Pham et al. 2014), and experience disparities in breast cancer treatment including being less likely to receive radiation and surgery (Markossian and Hines 2012). Unmarried participants reported a greater interest in genetic testing, but this factor was not significant in multivariable analysis. The relationship between marital status and interest in cancer genetic testing is inconsistent across studies and could be a reflection of the different populations studied (Anderson et al. 2014; Graves et al. 2011; Weinrich et al. 2002). However, our current finding (unmarried women were more interested in testing) is consistent with findings from our previous study regarding interest in multiplex testing for colorectal cancer risk (Anderson et al. 2014). Younger age has been found to be associated with interest in genetic testing in other studies, but it was not significantly associated with interest in genetic testing for this population of women. Compared to our previous study that included younger men and women below age 40 who were at increased risk for familial colorectal cancer, the current study’s lower age limit was 40. It is possible that our study participants’ previous knowledge of their family BRCA1/2 mutation status led them to discuss these results with family members and share information and opinions that played a greater role in their reported interest beyond the influence of age or marital status alone. Having had a mammogram within the previous two years was also crudely associated with interest in genetic testing and may be representative of women who are already empowered to seek cancer screening based on familial risk.
Cancer worry and perceived lifetime risk were significant predictors of interest in genetic testing. Women with higher levels of cancer worry were three times as likely to report interest in gene-panel testing as those with low cancer worry levels, a finding that is consistent with other studies (Cameron and Reeve 2006; Graves et al. 2010). Cancer-specific worry has been positively correlated with intentions to have a genetic test for hereditary breast and ovarian and colorectal cancer susceptibility (Codori et al. 1999; Lerman et al. 1994). Consistent with Leventhal’s Common-Sense Model of self-regulation for health threat cognition and behavior, worry or emotional arousal can motivate protective action (i.e., engaging in strategies to reduce distress) (Cameron and Diefenbach 2001). Risk perception has also been shown to play a salient role in how cancer patients and their families cope with breast cancer and has been positively associated with interest in and utilization of genetic testing (Croyle and Lerman 1993; Graves et al. 2010; Lerman et al. 1994). Furthermore, cancer risk and illness perceptions have been shown to predict cancer worry in healthy women (Gibbons and Groarke 2015) and influence behavior change (Cameron and Muller 2009). Risk perception and worry in at-risk women from BRCA1/2 negative families may drive interest in genetic testing and behavior change. In some cases this could lead to the adoption of unnecessary tests or behaviors if a woman perceives her risk to be high and feels the need to do something to reduce this risk; overuse of cancer screening has been associated with perceived cancer risk in BRCA1/2 negative women (Milhabet et al. 2013). Additional time may need to be spent educating a patient on the limitations of gene-panel testing and clarifying who the most informative person would be to undergo testing in the family in order to maximize benefit for the patient. Based on results from this study, emotional and cognitive factors of risk and worry may help to identify family members that could most benefit from additional or targeted cancer genetic information. More studies are needed that address the psychosocial and behavioral effects of gene panel testing for members of BRCA1/2 negative families.
Genetic counseling strategies, such as the tiered-binned model, are designed to provide patients with the most pertinent information to support informed decision-making for genetic testing followed by need-based patient specific information. Bradbury et al. found that previously tested BRCA1/2 negative patients were more likely to go forward with multiplex testing after tiered/binned counseling compared to BRCA1/2 untested patients (Bradbury et al. 2015b). Patients that received testing did not have significant changes in anxiety, depression, cancer worry and uncertainty. The majority of BRCA1/2 untested patients however declined multiplex (gene-panel) testing. Breast cancer worry, greater uncertainty, and greater perceived utility were all associated with making less informed decisions about gene-panel testing. In our study, participants with no prior exposure to genetic counseling showed a high interest in genetic testing. Interest in gene-panel testing may change based on the information received in pre-test counseling and understanding the potential factors that contribute to genetic testing may further inform the clinical and personal utility of genetic communication strategies.
The vast majority of women in this study reported that they prefer to get their genetic testing information from their primary care physicians followed by a cancer risk specialist/genetic counselor. A recent study found that only a fraction of women who receive a physician referral for genetic testing also receive genetic counseling to help them make an informed decision. The ABOUT (American BRCA Outcomes and Utilization of Testing) study showed that of women who received BRCA testing ordered by their physician only 36.8 % received genetic counseling. However, women who received genetic counseling reported more knowledge about and satisfaction with BRCA testing. Since patients consistently report that they prefer to talk with their primary care providers about genetic information and testing, it is especially important that providers have a conversation about genetic counseling with their patients. Unfortunately, primary care providers often lack sufficient knowledge of hereditary cancer risk and management, which may impede recognition of appropriate times to refer patients for genetic counseling (Cohn et al. 2015).
Most of the study participants indicated that they were ‘Somewhat’ or ‘Very interested’ in getting information from a variety of sources including print materials and via the Internet whereas getting information by computer kiosk was the least preferable mode of communication. Women who participated in the REACH Project received high quality, personalized print genetic information and structured in-person or telephone genetic counseling, and may have discussed or shared this information with their relatives (i.e., the participants in this study). It is possible that many of the study participants were primed to expect high quality information from various sources. Our results agree with similar studies showing that a majority of participants prefer to receive genetic information through a physician or genetic counselor and also prefer print materials (Anderson et al. 2014; McGuire et al. 2009).
Study Limitations
Our study had several limitations. While we measured interest in gene-panel testing we did not measure reasons for this interest or whether or not women participated in follow-up genetic counseling or testing. We do not know the extent of discussions about BRCA status among family members but found that family clustering did not have a significant effect on the results (data not shown). The relatively small sample size and homogenous study population (well educated, non-Hispanic white, married) are limitations and underscore the need to replicate our findings with diverse populations. Interest in gene-panel testing may be determined by other factors such as cost, concern about genetic discrimination, and the perceived benefit of the genetic information assessed, including the likelihood of clear risk management strategies (Bradbury et al. 2015a; Cragun et al. 2015; Easton et al. 2015; Powers and Stopfer 2014). Finally, gene-panel testing is a complicated and rapidly evolving field. We did not measure participants’ genetic literacy, their cancer knowledge or how they interpret small to moderate increases in cancer risk due to genetic variants. Additional research could be especially helpful in this regard.
Practice Implications
Interest in clinic-based and direct-to-consumer gene-panel testing is increasing (Roberts and Ostergren 2013) yet physicians report lacking the time and expertise to discuss genetic test results with patients and have concerns about high VUS rates and the clinical utility of large scale genetic tests (Powell et al. 2012; Selkirk et al. 2014). Genetic counselors, report similar challenges in: 1) interpreting the results and clinical utility for some variants assessed by gene-panel tests; 2) providing appropriate informed consent; and 3) determining the most appropriate candidates for panel testing (Wolfe Schneider et al. 2014). Our study informs the need for the development of effective methods of communicating genetic information and testing strategies for members of high risk cancer families. By knowing what factors are associated with interest in testing, providers can be better prepared to offer genetic counseling referrals or other resources to the most appropriate family members. Based on our results, diverse strategies of communication with women who test negative for BRCA mutations and their family members should be utilized. Patients should be given more resources, such as information on genetic counseling and testing, and action plans, working alongside their primary care providers if appropriate, to make sure that they have access to genetic counseling so that they can better understand their cancer risk and increase their satisfaction with a testing decision. A specific opportunity for family genetic communication sharing arises after a patient receives a BRCA negative test result, yet patients with a BRCA negative test result are less likely to share genetic information with their family members who may benefit from it (Himes et al. 2016; Patenaude et al. 2006). Genetic counselors can play a significant role in developing and disseminating materials and strategies that increase genetic information sharing within BRCA negative families. The sharing of family genetic information may help to balance public perceptions about the benefits and risks of additional genetic testing.
Our study’s findings suggest that women from BRCA1/2 negative families represent a unique population with a high level of interest in gene-panel testing driven by unmitigated perceived risk and cancer worry. These women may be especially motived for customized behavior change. Clinicians should pay special attention to threat perceptions (i.e., perceived risk and worry) when designing risk communications as well as informed decision-making and behavior change interventions with the goal of effectively leveraging these potential factors with evidence-based risk communications. Interest in multiplex testing for breast cancer susceptibility may create a teachable moment that can be used by clinicians to counsel about behavior change. Health messages can include broadly applicable recommendations such as increasing fruit and vegetable intake and physical activity, healthy behaviors that are associated with decreases in incidence and mortality from cancer and other chronic disease (Kabat et al. 2015; Petersen et al. 2015).
Research Recommendations
More research is needed to:
-
1)
determine if cognitive and emotional factors are important for gene-panel testing interest and decisions and
-
2)
develop genetic communication tools for cancer families. Negative mutations testers and their family members are an understudied population that will benefit from further research.
As gene-panel testing becomes increasingly integrated into standard care, many families may need to make informed decisions about gene-panel testing and medical management. Further research should address the adoption of preventive behaviors in the context of gene-panel testing across diverse populations, and ultimately ascertain if gene-panel testing translates into improved cancer outcomes.
References
Anderson, A. E., Flores, K. G., Boonyasiriwat, W., Gammon, A., Kohlmann, W., Birmingham, W. C., & Kinney, A. Y. (2014). Interest and informational preferences regarding genomic testing for modest increases in colorectal cancer risk. Public Health Genomics, 17(1), 48–60.
Bottorff, J. L., Ratner, P. A., Balneaves, L. G., Richardson, C. G., McCullum, M., Hack, T., & Buxton, J. (2002). Women's interest in genetic testing for breast cancer risk: the influence of sociodemographics and knowledge. Cancer Epidemiology, Biomarkers & Prevention, 11(1), 89–95.
Bradbury, A. R., Patrick-Miller, L., & Domchek, S. (2015a). Multiplex genetic testing: reconsidering utility and informed consent in the era of next-generation sequencing. Genetics in Medicine, 17(2), 97–98.
Bradbury, A. R., Patrick-Miller, L. J., Egleston, B. L., DiGiovanni, L., Brower, J., Harris, D., & Domchek, S. M. (2015b). Patient feedback and early outcome data with a novel tiered-binned model for multiplex breast cancer susceptibility testing. Genetics in Medicine, 18(1), 25–33.
Cameron, L. D., & Diefenbach, M. A. (2001). Responses to information about psychosocial consequences of genetic testing for breast cancer susceptibility: influences of cancer worry and risk perceptions. Journal of Health Psychology, 6(1), 47–59.
Cameron, L. D., & Muller, C. (2009). Psychosocial aspects of genetic testing. Current Opinion in Psychiatry, 22(2), 218–223.
Cameron, L. D., & Reeve, J. (2006). Risk perceptions, worry, and attitudes about genetic testing for breast cancer susceptibility. Psychology & Health, 21(2), 211–230.
Codori, A. M., Petersen, G. M., Miglioretti, D. L., Larkin, E. K., Bushey, M. T., Young, C., & Booker, S. V. (1999). Attitudes toward colon cancer gene testing: factors predicting test uptake. Cancer Epidemiology, Biomarkers & Prevention, 8(4 Pt 2), 345–351.
Cohn, J., Blazey, W., Tegay, D., Harper, B., Koehler, S., Laurent, B., Krishnamachari, B. (2015). Physician risk assessment knowledge regarding BRCA Genetics Testing. Journal of Cancer Education, 30(3), 573–579.
Collins, F. S., & Varmus, H. (2015). A new initiative on precision medicine. The New England Journal of Medicine, 372(9), 793–795.
Cragun, D., Bonner, D., Kim, J., Akbari, M. R., Narod, S. A., Gomez-Fuego, A., & Pal, T. (2015). Factors associated with genetic counseling and BRCA testing in a population-based sample of young black women with breast cancer. Breast Cancer Research and Treatment, 151(1), 169–176.
Croyle, R. T., & Lerman, C. (1993). Interest in genetic testing for colon cancer susceptibility: cognitive and emotional correlates. Preventive Medicine, 22(2), 284–292.
Daly, M. B., Pilarski, R., Axilbund, J. E., Berry, M., Buys, S. S., Crawford, B., & Darlow, S. (2016). Genetic/familial high-risk assessment: breast and ovarian, Version 2.2015. Journal of the National Comprehensive Cancer Network, 14(2), 153–162.
Desmond, A., Kurian, A. W., Gabree, M., Mills, M. A., Anderson, M. J., Kobayashi, Y., & Ellisen, L. W. (2015). Clinical Actionability of Multigene Panel Testing for Hereditary Breast and Ovarian Cancer Risk Assessment. JAMA Oncol, 1(7), 943–951.
Easton, D. F., Pharoah, P. D., Antoniou, A. C., Tischkowitz, M., Tavtigian, S. V., Nathanson, K. L., & Foulkes, W. D. (2015). Gene-panel sequencing and the prediction of breast-cancer risk. The New England Journal of Medicine, 372(23), 2243–2257.
Evans, D. G., Warwick, J., Astley, S. M., Stavrinos, P., Sahin, S., Ingham, S., & Howell, A. (2012). Assessing individual breast cancer risk within the U.K. National Health Service Breast Screening Program: a new paradigm for cancer prevention. Cancer Prevention Research (Philadelphia, Pa.), 5(7), 943–951.
Evans, D. G., Barwell, J., Eccles, D. M., Collins, A., Izatt, L., Jacobs, C., Murray, A. (2014). The Angelina Jolie effect: how high celebrity profile can have a major impact on provision of cancer related services. Breast Cancer Research, 16(5), 442.
Gibbons, A., & Groarke, A. (2015). Can risk and illness perceptions predict breast cancer worry in healthy women? J Health Psychol. doi:10.1177/1359105315570984.
Graves, K. D., Wenzel, L., Schwartz, M. D., Luta, G., Wileyto, P., Narod, S., & Halbert, C. H. (2010). Randomized controlled trial of a psychosocial telephone counseling intervention in BRCA1 and BRCA2 mutation carriers. Cancer Epidemiology, Biomarkers & Prevention, 19(3), 648–654.
Graves, K. D., Peshkin, B. N., Luta, G., Tuong, W., & Schwartz, M. D. (2011). Interest in genetic testing for modest changes in breast cancer risk: implications for SNP testing. Public Health Genomics, 14(3), 178–189.
Henneman, L., Vermeulen, E., van El, C. G., Claassen, L., Timmermans, D. R., & Cornel, M. C. (2013). Public attitudes towards genetic testing revisited: comparing opinions between 2002 and 2010. European Journal of Human Genetics, 21(8), 793–799.
Himes, D. O., Clayton, M. F., Donaldson, G. W., Ellington, L., Buys, S. S., & Kinney, A. Y. (2016). Breast cancer risk perceptions among relatives of women with uninformative negative BRCA1/2 test results: the moderating effect of the amount of shared information. Journal of Genetic Counseling, 25(2), 258–269.
Hoberg-Vetti, H., Bjorvatn, C., Fiane, B. E., Aas, T., Woie, K., Espelid, H., & Hoogerbrugge, N. (2016). BRCA1/2 testing in newly diagnosed breast and ovarian cancer patients without prior genetic counselling: the DNA-BONus study. European Journal of Human Genetics, 24(6), 881–888.
IPAQ. (2015) International Physical Activity Questionnaire. WWW.ipaq.ki.se. Accessed March 3, 2015.
Jensen, J. D., Bernat, J. K., Davis, L. A., & Yale, R. (2010). Dispositional cancer worry: convergent, divergent, and predictive validity of existing scales. Journal of Psychosocial Oncology, 28(5), 470–489.
Kabat, G. C., Matthews, C. E., Kamensky, V., Hollenbeck, A. R., & Rohan, T. E. (2015). Adherence to cancer prevention guidelines and cancer incidence, cancer mortality, and total mortality: a prospective cohort study. The American Journal of Clinical Nutrition, 101(3), 558–569.
Kelly, K. M., Andrews, J. E., Case, D. O., Allard, S. L., & Johnson, J. D. (2007). Information seeking and intentions to have genetic testing for hereditary cancers in rural and Appalachian Kentuckians. The Journal of Rural Health, 23(2), 166–172.
Kinney, A. Y., Butler, K. M., Schwartz, M. D., Mandelblatt, J. S., Boucher, K. M., Pappas, L. M., & Campo, R. A. (2014). Expanding access to BRCA1/2 genetic counseling with telephone delivery: a cluster randomized trial. Journal of the National Cancer Institute, 106(12). doi:10.1093/jnci/dju328.
Kotsopoulos, J., Metcalfe, K., Alston, J., Nikitina, D., Ginsburg, O., Eisen, A., & Narod, S. A. (2014). Prospective study of high-risk, BRCA1/2-mutation negative women: the 'negative study. BMC Cancer, 14, 221.
Lebo, P. B., Quehenberger, F., Kamolz, L. P., & Lumenta, D. B. (2015). The Angelina effect revisited: exploring a media-related impact on public awareness. Cancer, 121(22), 3959–3964.
Lerman, C., Daly, M., Masny, A., & Balshem, A. (1994). Attitudes about genetic testing for breast-ovarian cancer susceptibility. Journal of Clinical Oncology, 12(4), 843–850.
Li, H., Feng, B., Miron, A., Chen, X., Beesley, J., Bimeh, E., & Goldgar, D. E. (2016). Breast cancer risk prediction using a polygenic risk score in the familial setting: a prospective study from the breast cancer family registry and kConFab. Genetics in Medicine. doi:10.1038/gim.2016.43.
Lincoln, S. E., Kobayashi, Y., Anderson, M. J., Yang, S., Desmond, A. J., Mills, M. A.,. Ellisen, L. W. (2015). A systematic comparison of traditional and Multigene panel testing for hereditary breast and ovarian cancer genes in more than 1000 patients. The Journal of Molecular Diagnostics, 17(5), 533–544.
Lipkus, I. M., Kuchibhatla, M., McBride, C. M., Bosworth, H. B., Pollak, K. I., Siegler, I. C., & Rimer, B. K. (2000). Relationships among breast cancer perceived absolute risk, comparative risk, and worries. Cancer Epidemiology, Biomarkers & Prevention, 9(9), 973–975.
Markossian, T. W., & Hines, R. B. (2012). Disparities in late stage diagnosis, treatment, and breast cancer-related death by race, age, and rural residence among women in Georgia. Women & Health, 52(4), 317–335.
Mavaddat, N., Pharoah, P. D., Michailidou, K., Tyrer, J., Brook, M. N., Bolla, M. K., & Garcia-Closas, M. (2015). Prediction of breast cancer risk based on profiling with common genetic variants. Journal of the National Cancer Institute, 107(5).
McBride, C. M., Birmingham, W. C., & Kinney, A. Y. (2015). Health psychology and translational genomic research: Bringing innovation to cancer-related behavioral interventions. The American Psychologist, 70(2), 91–104.
McCarthy, A. M., Bristol, M., Fredricks, T., Wilkins, L., Roelfsema, I., Liao, K., & Armstrong, K. (2013). Are physician recommendations for BRCA1/2 testing in patients with breast cancer appropriate? A population-based study. Cancer, 119(20), 3596–3603.
McCaul, K. D., & Goetz, P. W. Worry. (2015) A paper on the National Cancer Institute website http://cancercontrol.cancer.gov/Brp/constructs/worry/worry.pdf Accessed March 30, 2015.
McGuire, A. L., Diaz, C. M., Wang, T., & Hilsenbeck, S. G. (2009). Social networkers' attitudes toward direct-to-consumer personal genome testing. The American Journal of Bioethics, 9(6–7), 3–10.
Meisel, S. F., Carere, D. A., Wardle, J., Kalia, S. S., Moreno, T. A., Mountain, J. L., & Group, P. G. S (2015). Explaining, not just predicting, drives interest in personal genomics. Genome Medicine, 7(1), 74.
Milhabet, I., Duprez, C., Krzeminski, A., & Christophe, V. (2013). Cancer risk comparative perception and overscreening behaviours of non-carriers from BRCA1/2 families. European Journal of Cancer Care (Engl), 22(4), 540–548.
Moyer, V. A., & Force, U. S. P. S. T. (2013). Medications to decrease the risk for breast cancer in women: recommendations from the U.S. Preventive Services Task Force recommendation statement. Annals of Internal Medicine, 159(10), 698–708.
Nelson, H. D., Smith, M. E., Griffin, J. C., & Fu, R. (2013). Use of medications to reduce risk for primary breast cancer: a systematic review for the U.S. Preventive Services Task Force. Annals of Internal Medicine, 158(8), 604–614.
Nguyen-Pham, S., Leung, J., & McLaughlin, D. (2014). Disparities in breast cancer stage at diagnosis in urban and rural adult women: a systematic review and meta-analysis. Annals of Epidemiology, 24(3), 228–235.
Patenaude, A. F., Dorval, M., DiGianni, L. S., Schneider, K. A., Chittenden, A., & Garber, J. E. (2006). Sharing BRCA1/2 test results with first-degree relatives: factors predicting who women tell. Journal of Clinical Oncology, 24(4), 700–706.
Petersen, K. E., Johnsen, N. F., Olsen, A., Albieri, V., Olsen, L. K., Dragsted, L. O., & Egeberg, R. (2015). The combined impact of adherence to five lifestyle factors on all-cause, cancer and cardiovascular mortality: a prospective cohort study among Danish men and women. The British Journal of Nutrition, 113(5), 849–858.
Powell, K. P., Cogswell, W. A., Christianson, C. A., Dave, G., Verma, A., Eubanks, S., & Henrich, V. C. (2012). Primary care physicians' awareness, experience and opinions of direct-to-consumer genetic testing. Journal of Genetic Counseling, 21(1), 113–126.
Powers, J., & Stopfer, J. E. (2014). Risk assessment, genetic counseling, and clinical care for hereditary breast cancer. Journal of Obstetric, Gynecologic, and Neonatal Nursing, 43(3), 361–373.
Ramirez, A. G., Chalela, P., Gallion, K. J., Munoz, E., Holden, A. E., Burhansstipanov, L., Suarez, L. (2015). Attitudes Toward Breast Cancer Genetic Testing in Five Special Population Groups J Health Dispar Res Pract, 8(4), 124–135.
Roberts, J. S., & Ostergren, J. (2013). Direct-to-Consumer Genetic Testing and Personal Genomics Services: A Review of Recent Empirical Studies. Curr Genet Med Rep, 1(3), 182–200.
Selkirk, C. G., Vogel, K. J., Newlin, A. C., Weissman, S. M., Weiss, S. M., Wang, C. H., & Hulick, P. J. (2014). Cancer genetic testing panels for inherited cancer susceptibility: the clinical experience of a large adult genetics practice. Familial Cancer, 13(4), 527–536.
Sussner, K. M., Edwards, T. A., Thompson, H. S., Jandorf, L., Kwate, N. O., Forman, A., & Valdimarsdottir, H. B. (2011). Ethnic, racial and cultural identity and perceived benefits and barriers related to genetic testing for breast cancer among at-risk women of African descent in New York City. Public Health Genomics, 14(6), 356–370.
Teng, I., & Spigelman, A. (2014). Attitudes and knowledge of medical practitioners to hereditary cancer clinics and cancer genetic testing. Familial Cancer, 13(2), 311–324.
Trivers, K. F., Baldwin, L. M., Miller, J. W., Matthews, B., Andrilla, C. H., Lishner, D. M., & Goff, B. A. (2011). Reported referral for genetic counseling or BRCA 1/2 testing among United States physicians: a vignette-based study. Cancer, 117(23), 5334–5343.
US Department of Agriculture. (2015) Dietary Guidelines for Americans. http://www.health.gov/dietaryguidelines/. Retrieved July 6, 2015.
Vermeulen, E., Henneman, L., van El, C. G., & Cornel, M. C. (2014). Public attitudes towards preventive genomics and personal interest in genetic testing to prevent disease: a survey study. European Journal of Public Health, 24(5), 768–775.
Weinrich, S., Royal, C., Pettaway, C. A., Dunston, G., Faison-Smith, L., Priest, J. H., & Powell, I. (2002). Interest in genetic prostate cancer susceptibility testing among african American men. Cancer Nursing, 25(1), 28–34.
Wolfe Schneider, K., Anguiano, A., Axell, L., Barth, C., Crow, K., Gilstrap, M., & Freivogel, M. (2014). Collaboration of Colorado cancer genetic counselors to integrate next generation sequencing panels into clinical practice. Journal of Genetic Counseling, 23(4), 640–646.
Acknowledgments
We would like to thank the REACH Project participants and their relatives, who made this research possible. This work was supported by grants from the National Cancer Institute at the National Institutes of Health (1R01CA129142 to AYK and U01 CA152958) and the UNM Comprehensive Cancer Center P30 (P30CA118100). The project was also supported by the Shared Resources (P30 CA042014) at Huntsman Cancer Institute (Biostatistics and Research Design, Genetic Counseling, Research Informatics, and the Utah Population Database [UPDB]); the Utah Cancer Registry, which is funded by Contract No.HHSN261201000026C from the National Cancer Institute’s Surveillance, Epidemiology and End Results (SEER) Program with additional support from the Utah State Department of Health and the University of Utah; the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant 8UL1TR000105 (formerly UL1RR025764). This content is solely the responsibility of the authors and does not necessarily reflect the opinions or views of the funding and supporting agencies.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Authors Kristina Flores, Laurie Steffen, Christopher McLouth, Belinda Vicuna, Amanda Gammon, Lucretia Vigil, Zoneddy Dayao, Melanie Royce, and Anita Kinney have no conflict of interest.
Wendy Kohlmann received compensation within the past three years for consultation to Myriad Genetics.
Human Studies and Informed Consent
All procedures followed were in accordance with the ethical standards of University of Utah on human experimentation and with the 1964 Helsinki declaration and it later amendments or comparable ethical standards. Informed consent was obtained from all participants included in the study.
Animal Studies
No animal studies were carried out by the authors for this article.
Rights and permissions
About this article
Cite this article
Flores, K.G., Steffen, L.E., McLouth, C.J. et al. Factors Associated with Interest in Gene-Panel Testing and Risk Communication Preferences in Women from BRCA1/2 Negative Families. J Genet Counsel 26, 480–490 (2017). https://doi.org/10.1007/s10897-016-0001-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10897-016-0001-7