Abstract
Until recently, genetic testing for hereditary breast cancer has primarily focused on pathogenic variants in the BRCA1 and BRCA2 (BRCA) genes. However, advances in DNA sequencing technologies have made simultaneous testing for multiple genes possible. We examined correlates of interest in multigene panel testing and risk communication preferences in an ethnically diverse sample of women who tested negative for BRCA mutations previously but remain at high risk based on their family history (referred to as “BRCA-uninformative”) and their at-risk female family members. Two-hundred and thirteen women with a previous breast cancer diagnosis and a BRCA-uninformative test result and their first-degree relatives completed a survey on interest in multigene panel testing, communication preferences, and sociodemographic, psychological, and clinical factors. Stepwise logistic regression was used to identify factors associated with testing interest. Chi-square analyses were used to test differences in risk communication preferences. Interest in multigene panel testing was high (84%) and did not considerably differ by cancer status or ethnicity. In multivariable analysis, factors significantly associated with interest in genetic testing were having had a mammogram in the past 2 years (odds ratio (OR) = 4.04, 95% confidence interval (CI) 1.80–9.02) and high cancer worry (OR = 3.77, 95% CI 1.34–10.60). Overall, the most commonly preferred genetic communication modes were genetic counselors, oncologists, and print materials. However, non-Hispanic women were more likely than Hispanic women to prefer web-based risk communication (p < 0.001). Hispanic and non-Hispanic women from BRCA-uninformative families have a high level of interest in gene panel testing. Cancer-related emotions and communication preferences should be considered in developing targeted genetic risk communication strategies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Until recently, genetic risk assessment for hereditary breast cancer (HBC) has focused on BRCA1 and BRCA2 (BRCA) genetic testing through conventional Sanger sequencing (Sanger et al. 1977). However, recent advances in DNA sequencing technology through next-generation sequencing (NGS) have led to plummeting costs which in turn have made clinical testing for multiple genes simultaneously (multigene panel testing) highly feasible and increasingly used (Rizzo and Buck 2012). Pathogenic variants in BRCA and other breast cancer susceptibility genes jointly account for up to 30% of breast cancers (Moran et al. 2016). Most women who were considered at high risk for hereditary breast cancer and previously had genetic sequencing limited to the BRCA genes are not found to have a mutation (i.e., BRCA negative) or have a variant in which pathogenicity has not yet been established (i.e., variant of uncertain significance), herein referred to as “BRCA-uninformative.” These women are increasingly being offered multigene panel testing with current sequencing methodologies.
Little work has been done to examine factors associated with interest in multigene panel testing and preferences for genetic risk communication strategies among BRCA-uninformative families especially with diverse populations such as Hispanic populations that have lower rates of genetic testing (Armstrong et al. 2015). Thus, it remains important to improve our understanding regarding perspectives about breast cancer risk from culturally diverse populations, to design relevant and effective risk communication strategies to help patients make informed decisions about their cancer prevention options. Also, insight into ethnic differences in interest in and preferences for multigene panel testing could help decrease disparities in genetic testing.
Multigene panel testing in BRCA-uninformative families has the potential to offer valuable information, such as identifying pathogenic variants in other genes that could prompt changes in care delivery (Frey et al. 2015; Kurian et al. 2014; Ricker et al. 2016; Yorczyk et al. 2015). While multigene panel testing has potential added benefits for individuals with suspected hereditary cancer, testing for genes of variable penetrance, some of which confer a moderate risk, can be challenging because of limited or lack of data to inform evidence-based risk management guidelines (Tung et al. 2016). It also poses challenges for cancer risk communication and assessment (Bradbury et al. 2015; Domchek et al. 2013; Norquist and Swisher 2015). Multigene panel testing also yields a higher proportion of variants of uncertain significance (VUS) with no known clinical utility (Slavin et al. 2015) which may cause patients’ psychological distress. Many clinicians report that they have limited training and expertise to help their patients interpret cancer risk from multigene panel testing (Gray et al. 2014).
Despite BRCA genetic testing being available since 1996 (Armstrong et al. 2003), underserved populations lack awareness about genetic testing and have lower utilization rates of genetic testing compared to non-Hispanic whites (Lynce et al. 2016). The prevalence of breast cancer-causing pathogenic variants among Hispanic women is similar to other major ethnic/racial population subgroups (Villarreal-Garza et al. 2015; Weitzel et al. 2013) but the majority of women underdoing BRCA testing are non-Hispanic white, with Hispanic women comprising only about 1–4% of women tested (Frank et al. 2002; Hall et al. 2009). Even after controlling for insurance coverage, Hispanic women were significantly less likely to receive BRCA testing than non-Jewish white women (Levy et al. 2011). Although Hispanics have a decreased awareness of genetic testing for cancer (Mai et al. 2014), they report a high interest in genetic testing and cancer risk assessment when informed (Gammon et al. 2011; Jagsi et al. 2015; Sussner et al. 2015), suggesting that culturally relevant information about genetic testing may not be reaching diverse populations. Improving genetic risk communication and minimizing psychological harms are especially pertinent considering the current shift toward multigene panel testing that requires communication of increasingly more complex genetic information and informed decision-making (Tung et al. 2016).
The Common Sense Model of Self-Regulation (CSMSR) (Leventhal et al. 1980) can be used to predict reactions to further genetic screening for hereditary cancer with gene panel testing (van Oostrom et al. 2007). According to the CSMSR, cognitive and emotional processes are utilized to respond to a potential health threat (Hagger and Orbell 2003) such as cancer risk and may motivate health behavior change (Cameron and Reeve 2006; van Oostrom et al. 2007). Having a family history of breast cancer can lead to high levels of worry and fear (Andersen et al. 2003), possibly most salient when being confronted with the reality of risk for hereditary cancer. In response to this, women might utilize cognitive and emotion-focused processes to estimate and understand risk, as well as to regulate their emotional reactions such as worry and fear, while deciding on potential actions to take, including whether or not to have multigene panel testing. Both cancer worry (emotional processes) as well as perceived risk for cancer (cognitive processes) may be key psychological processes in decision-making regarding multigene panel testing. The CSMSR was a guiding framework for this study
Little is known about the attitudes of members of BRCA-uninformative families toward multigene panel testing and the most effective ways to communicate information about genetic testing to promote health behaviors in general and among Hispanics in particular (McBride et al. 2015). To address this knowledge gap, we examined the association of multiple potential psychological, behavioral, demographic, and clinical factors with interest in and communication preferences regarding multigene panel in Hispanic and non-Hispanic BRCA-uninformative families. Understanding more about the factors that might influence testing decisions in high risk families can inform more effective genetic risk communication strategies to reach diverse populations.
Methods
Participants
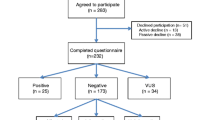
Study participants completed mailed or telephone surveys between June of 2014 and March of 2015. Women with a personal history of breast cancer were eligible if they were (1) between 18 and 74 years of age, (2) met the criteria per national guidelines for genetic testing, (3) had prior genetic counseling and testing through the Hereditary Cancer Assessment Program at the University of New Mexico Comprehensive Cancer Center, and (4) were not found to have a pathogenic variant in BRCA1 or BRCA2. Their at-risk, first-degree female relatives were also recruited if the relatives were (1) between 40 and 74 years of age (eligible for mammography screening at the time of the study), (2) had no prior diagnosis of cancer (except non-melanoma skin cancer), and (3) had no prior genetic counseling/testing. Women with a personal history of breast cancer who had received BRCA testing were the first point of contact and invited to (1) refer their first-degree female relatives to the study and (2) complete a study survey. After obtaining contact information for the first-degree relatives from these women, the relatives were then contacted and invited to participate in the study.
All potentially eligible women were mailed a study packet, which included a cover letter consent, study questionnaire, an informational brochure about the study, a postage-paid return envelope, and a $2 bill as a gift. The University of New Mexico Health Sciences Institutional Review Board approved the study.
Measures and procedures
Demographic variables included self-reported ethnicity, age, marital status, income group, and education level. Rural or urban status was ascertained using Rural-Urban Commuting Area Codes by zip code (United States Department of Agriculture 2010).
Perceived risk
Perceived risk was evaluated with an item assessing lifetime risk of breast cancer (Lipkus et al. 2000): “In your opinion, how likely is it that you will get breast cancer in your lifetime?”. Response options were “Very unlikely,” “Unlikely,” “50–50 chance,” “Likely,” and “Very Likely.” For women with a prior breast cancer diagnosis, risk for second breast cancer diagnosis was evaluated by modifying the lifetime risk questions, “… how likely is it that you will get breast cancer again…?”.
Cancer worry
The frequency and intensity of worry of breast cancer occurrence or recurrence were measured using a validated three-item scale (Jensen et al. 2010). Two items measured worry intensity: “How bothered are you about getting breast cancer [again]?” and “How worried are you about getting breast cancer [again]?” Reponses ranged from “not at all” to “extremely” on a five-point Likert scale. One item measured worry frequency, “During the past week, how often have you worried about getting breast cancer?” Response ranged from “never” to “all of the time” on a five-point scale. The items were averaged to create an average worry variable. Internal consistency was very good (Cronbach’s α = 0.88). Due to skewed data, the variable was dichotomized using the median to designate two groups. A median score of less than 2.00 being “low worry” and scores greater than or equal to 2.00 being “high worry.”
Clinical factors
The number of first- and second-degree biological relatives with a breast cancer diagnosis as well as mammogram utilization was assessed via self-report. Women were classified as having a recent mammogram within 2 years of completing the study questionnaire (yes vs. no).
Physical activity and diet
Physical activity and diet can be indicative of other positive health behaviors, and cancer survivors and their relatives may be an especially relevant and receptive population for interventions to create sustainable lifestyle behavior change (Stacey et al. 2015). Thus, lifestyle factors such as diet and physical activity were assessed in this study as they might be related to interest in multigene panel testing. Physical activity was evaluated using the International Physical Activity Questionnaire Short Form (Booth et al. 2003). According to the Guidelines for Data Processing and Analysis of the International Physical Activity Questionnaire (2005), metabolic equivalent of task (MET) scores were calculated for average weekly vigorous and moderate physical activity, as well as walking activity. Total MET scores were divided into two groups using a median split, with those engaging in the least physical activity per week in the “low” group and those engaging in the most physical activity per week in the “high” group. For diet, fruit and vegetable intake was measured with two questions: “Over the past month, how many servings of fruits [vegetables] did you eat per day?” Response options were “0,” “1,” “2,” “3,” “4,” or “5 or more.” These two questions were combined to calculate the average number of fruit and vegetable servings per day. The final diet variable was dichotomized into less than five or five or more servings per day in accordance with the World Health Organization (WHO)’s 5-a-day international dietary recommendations at the time the study was designed (World Health Organization 2003).
Interest in multigene panel testing
The primary outcome of interest was interest in multigene panel genetic testing. The survey included a brief narrative describing BRCA and multigene panel genetic testing prior to asking four separate questions assessing interest in testing. This brief narrative explained that while BRCA 1 and BRCA 2 genetic changes are associated with a high lifetime risk for breast cancer (~ 50–80%), recently identified genetic changes (in other genes) have been found to be associated with slight to moderate increases in breast cancer risk (~ 15–30%). Participants were then asked, “If genetic testing of many different genes could tell you that you may have a moderate to slightly increased risk of developing breast cancer (again), how likely it is that you would want a genetic test?” Response options were “I would definitely not have the test,” “I would probably not have the test,” “I would probably have the test,” and “I would definitely have the test.” This variable was dichotomized into high interest (“would definitely or probably not have the test”) or low interest (“would definitely not or probably not have the test”).
Additionally, women were asked about their interest in genetic testing under various circumstances: if testing might inform their own future risk-based screening (mammograms, breast MRI’s, etc.) and if testing might help inform them about reducing their risk by taking medications or through diet and exercise. Responses were along a four-point Likert-type scale ranging from “I would definitely not have the test” to “I would definitely have the test” and interest in testing for these various circumstances. The variable was dichotomized a priori into high and low interest. These items were adapted from a survey by Graves et al. (2011).
Risk communication preferences
Preferences for risk communication mode were assessed by asking participants “How much would you like to get information about genetic testing from the following methods?” The modes included print or written information, web-based information, computer kiosk touch screen in a clinic, discussion with a nurse, discussion with a primary care physician, discussion with a cancer specialist such as an oncologist, and discussion with a genetic counselor/cancer risk specialist in-person and by telephone. The possible responses for each of these modes were “Not at All,” “A Little,” “Somewhat,” or “Very Much.” Responses were dichotomized as either no/low preference (“Not at All/A Little”) or moderate/high preference (“Somewhat/Very Much”).
Data analyses
Independent variables with non-normal distributions were dichotomized and variables considered were screened for collinearity. Logistic regression was used to estimate unadjusted odds ratios (ORs) and 95% confidence intervals (CI) to ascertain associations between each independent variable and interest in genetic testing. Backward logistic regression was used to determine variables independently associated with interest in multigene panel testing. Variables that were crudely associated with interest in testing in unadjusted logistic regression analyses with a p < 0.20 were entered into the multivariable model. Variables were removed from the model by backward elimination based on the probability of a likelihood ratio statistic for variable removal not satisfying the criterion of p < 0.10. Chi-square tests were used to test for differences in interest in multigene panel testing between breast cancer patients and relatives and for differences in risk communication preferences between Hispanic and non-Hispanic women.
The effect of family clustering on interest in gene panel testing was assessed using linear mixed models. Forty-seven families participated in the study with an average cluster size of two (2.19). There was little evidence of clustering and the multigene panel testing interest intra-class correlation was essentially 0, indicating that the variability attributed to family clustering was negligible. Nonetheless, the final multilevel logistic regression model reported here accounted for family clustering.
Results
Participants characteristics
Of the 413 study-eligible women, 91 (22%) could not be contacted and 22 women (5%) refused to participate. Two-hundred and fifteen women completed a study questionnaire with an overall cooperation rate of 67%. Only contact information was collected from potentially eligible participants; therefore, it is not possible to compare women who participated to those who did not with regard to sociodemographic and other relevant characteristics. The analyses included 213 participants; two women were excluded because they did not respond to the items assessing genetic testing interest and communication preferences.
The characteristics of the study population are presented in Table 1. One hundred and forty-three women with previous breast cancer diagnoses and 70 relatives participated. Most participants (61%) identified as non-Hispanic and 38% of participants identified as Hispanic (1% missing). The majority (90%) completed the survey in English while 10% completed the Spanish version of the survey. Overall, the mean age of participants was 55 (SD = 10.9) years old. Most women were married (61%), reported at least some college education (80%), and resided in an urban area (81%). Nearly half of the participants (45%) reported a yearly household income of more than $50,000. Seventy-six percent of participants reported having a family history of breast cancer and 39% reported having two or more first- or second-degree relatives with breast cancer. Most women reported receiving a mammogram (75%) within the past 2 years. With regard to healthy lifestyle behaviors, a little less than half of the women (41%) reported having had at least five or more servings of fruits and vegetables per day and 42% of women reported high physical activity or a MET score of 1653 or greater per week.
Interest in multigene panel testing
Interest in multigene panel testing was not significantly different between cancer patients and relatives (results not shown χ 2 (1, N = 213) = 0.529, p = 0.467); therefore, all participants (breast cancer patients and relatives) were analyzed together. Comparing across the four different potential uses of gene panel testing, if genetic testing could inform participants on (1) personal risk (slight to moderate to increased risk), (2) risk-based breast cancer screening, (3) risk reduction through medication use, and (4) risk reduction through lifestyle changes (diet and exercise), participants showed high interest in multigene panel testing overall. Regardless of the perceived utility of the genetic test to potentially provide useful information about breast cancer risk and tailored screening and risk reduction recommendations, interest was overwhelmingly high (see Fig. 1). Most women (84%) reported interest in multigene panel testing if it could inform them of a small to moderate increased risk of breast cancer, which may not necessarily be linked with actionable risk management options. Most women also reported interest in testing if it could provide personalized and actionable recommendations about frequency of breast cancer screening procedures (86%), medication use to reduce their cancer risk (85%), or diet and physical activity (87%) to reduce their cancer risk.
Variables associated with interest in multigene panel testing (met the established significance criterion of p < 0.2 for entry into the multivariable logistic regression model; see Table 2) were age (OR = 0.96, 95% CI = 0.92–0.99, p = 0.02), having had a recent mammogram (OR = 4.47, 95% CI = 2.08–9.62, p < 0.001), cancer worry (OR = 4.72, 95% CI = 1.86–11.96, p = 0.001), and perceived risk for breast cancer (OR = 1.40, 95% CI = 0.99–1.96, p = 0.05).
The final multivariable logistic regression model included two variables (Table 3). The odds of being interested in multigene panel testing, controlling for cancer worry, were four times greater among women who had a recent mammogram compared to women who have not had a recent mammogram (OR = 4.04, 95% CI = 1.80–9.02, p < 0.001). The odds of being interested in multigene panel testing, controlling for mammography, were 3.7 times greater among women with high cancer worry compared to women with low levels of cancer worry (OR = 3.77, 95% CI = 1.34–10.60, p = 0.01).
Preference for cancer genetic risk communication
Preferences for receiving information about genetics and cancer risk are shown in Fig. 2. The vast majority of women (90%) reported a high preference for receiving genetic information in person with a genetic counselor/cancer risk specialist, making this the most preferred cancer risk communication mode. The other frequently preferred methods for receiving cancer genetic risk information were in person with an oncologist (89%) and print materials (81%).
Differences in preferences for risk communication between Hispanic and non-Hispanic women are presented in Table 4. Preferences for risk communication were generally similar across ethnic groups. However, non-Hispanic women were significantly more likely than Hispanic women to have a high preference for web-based cancer risk information, [χ 2 (1, N = 195) = 12.54, p < 0.001]. While 76% non-Hispanic women had a high preference for web-based information, only 51% of Hispanic women had a high preference for web-based information (see Fig. 3). Controlling for the effects of education and income level, Hispanic women were still significantly less likely to report a high preference for web-based cancer risk communication (OR = 0.46, p = 0.04, 95% CI = 0.22–0.98) compared to non-Hispanic women.
Discussion
Few studies have assessed interest in multigene panel testing and preferences for genetic risk communication among underserved, multiethnic populations, including Hispanics. We found a high level of interest in testing among an ethnically diverse sample of breast cancer survivors who previously underwent BRCA testing and a pathogenic variant was not found and their female biological relatives. High cancer-specific worry and recent mammography were independently associated with multigene panel testing interest. Unlike prior studies of interest in multigene panel testing (Elrick et al. 2016; Flores et al. 2016), our study included a relatively large proportion of Hispanics. Hispanic women did differ from non-Hispanic women in their preferences for web-based cancer risk communication. The study’s findings can help guide the development and implementation of genetic risk communication strategies to reach diverse members of BRCA-uninformative families. Further, this study can shed light on factors influencing interest in multigene panel testing in BRCA-uninformative families to promote informed decision-making about testing and medical management. Studies like this are important to help health care providers and intervention researchers address the increased complexity of communicating genetic risk information with diverse populations
The majority of participants in this study reported high interest in multigene panel testing to inform them of their personal risk (not necessarily actionable) but also actionable risk management behavior, such as receiving cancer screening, taking medication, and changing diet and exercise. In our study, we found that family members were just as likely as breast cancer patients to be interested in multigene panel testing. Perhaps this is because of relatives’ concerns about their personal cancer risks and desire for information about how they might reduce their risk for cancer (Himes et al. 2016). Because genetic risk information impacts the entire family (Peterson 2005), it can be as important to the family members as it is to the patient (Ersig et al. 2009; Vos et al. 2011).
Other studies have found that while awareness of hereditary breast cancer and genetic testing utilization are consistently low among Hispanics (Lynce et al. 2016), interest in and uptake of genetic testing are high when they are informed (Kinney et al. 2010; Ricker et al. 2006) about risk and testing. The majority of women in our study, regardless of ethnicity or educational level, demonstrated a high preference for receiving cancer risk information through a cancer genetic/risk specialist (e.g., genetic counselor) or cancer specialist (e.g., oncologist). Direct, personal communication with a cancer genetic risk specialist or oncologist may be a more acceptable mode for cancer risk communication among low-acculturated Hispanic individuals, as they see these providers as trusted, reliable sources of health information (Hamilton et al. 2015). For Hispanic women at increased risk for HBC, these providers may play a pivotal role in helping women make informed decisions about testing and relevant health behaviors and services.
Despite the wide range of electronic and online communication options available for cancer risk communication, our study found a high preference for print communication for both Hispanic and non-Hispanic women. Other research has found that participants in a biobank study preferred to receive yearly updates via convenient, inexpensive methods such as newsletters (Mester et al. 2015). Randomized trials have found that print materials can be combined with telephone or in-person counseling to help increase access genetic risk information and services and promote informed decision-making (Kinney et al. 2014a, b; Steffen et al. 2015), but these trials did not include a large proportion of Hispanics or members of other underserved minority groups.
Hispanic women were less likely to prefer web-based genetic information than non-Hispanic women after controlling for the effects of education and income. This suggests that ethnicity and culture may play a role in web-based genetic communication preferences beyond socioeconomic factors. The lower preference for online-based tools for receiving cancer-related information among Hispanics has also been observed by others. In a study comparing a telephone hotline to online messaging to deliver cancer information to Hispanics, the vast majority (98%) preferred the telephone hotline over the online messaging (Waters et al. 2009). Previous research has demonstrated the persistence of “digitally underserved” groups. For example, non-Hispanic whites have consistently been the prominent seekers of health information through web-based tools, whereas Hispanics and other ethnic minority groups, particularly those in lower socioeconomic strata, are less likely to seek health information online (Lorence et al. 2006; Peña-Purcell 2008). For Hispanics, internet access and use is associated with acculturation, fluency in English, age, health literacy, educational level, and income (Cristancho et al. 2014; Selsky et al. 2013). When Hispanics utilize technology to access online sources of health information, they place a greater importance on cultural and linguistic factors (Victorson et al. 2014). Thus, cultural and linguistic factors should be considered when designing and disseminating health-related information (Solomon et al. 2005).
Cancer worry and mammography were significant predictors of interest in multigene panel genetic testing in the final adjusted, multilevel logistic regression model. Women with a prior history of breast cancer who are BRCA-uninformative experience cancer-specific distress similar to women testing positive for BRCA pathogenic variants (Schwartz et al. 2002) and do not experience appreciable declines in cancer worry over time (van Dijk et al. 2006). Previous research has shown that cancer worry is a significant and consistent predictor of interest in testing for cancer susceptibility (Cameron and Reeve 2006; Graves et al. 2011; Graves et al. 2010; Lerman et al. 1995). Persistent worry about cancer may be particularly motivating, perhaps because women with a heightened sense of cancer worry may perceive more advantages to genetic testing to reduce their worry levels and lead them to medical recommendations to reduce their cancer risk (Cameron and Reeve 2006). According to Leventhal’s CSMSR model, attitudes and decisions toward genetic testing are formed through emotional and cognitive process. Worry about breast cancer can motivate action (interest in and uptake of testing) to help individuals cope with and protect against the threat of breast cancer (Cameron and Reeve 2006).
Women who reported having had a mammogram within the past 2 years were appreciably more interested in multigene panel testing than women who reported not having a recent mammogram. A possible explanation is that women most concerned with hereditary breast cancer may already be engaging in risk management behaviors and will likely be receptive to other risk-reducing strategies. These women may pursue genetic testing as yet another tool that could help them manage their risk. Because these women may be highly oriented toward prevention, they are more receptive toward other preventative, health-promoting avenues in general (White 2005).
Our study has several strengths. This study reveals the perspectives of Hispanic women, who have historically lacked access to cancer risk assessment services. Our survey included a high proportion of Hispanic participants (40%), similar to the New Mexico population (47% Hispanic) (“Health Indicator Report of New Mexico Population Demographics—Race/Ethnicity” 2015). Spanish-speaking Hispanic women likely have very different experiences in access to cancer risk assessment services, even when compared to English-speaking Hispanic women. In our study, Spanish-speaking Hispanic women had a higher interest in multigene panel testing compared to English-speaking Hispanic women; however, with a small number of Spanish-speakers (9.8% of participants), we could not statistically verify the moderating impact of language.
There are however some limitations to our study. Hispanic populations differ widely by region and these findings may not generalize to other Hispanic populations in other geographic regions of the USA. This study consisted of highly educated women (80% with some college or more) and these women could have greater-than-average knowledge of genetics and cancer risk. We did not assess if breast cancer patients had talked with their relatives about their cancer diagnosis or about genetic testing, but family clustering did not significantly affect the final multilevel logistic regression model. Participants were asked about their interest in multigene panel testing using a brief, simple narrative on gene panel genetic testing. The nuances of multigene panel testing were not discussed, and we did not assess genetic literacy nor perspectives on risks and benefits of gene panel testing. If participants were prompted to consider the complexity of multigene panel testing, their interest might change. In assessing ethnic differences in preferences for risk communication, we found that non-Hispanic women were more likely to be interested in web-based risk communication; however, we did not collect data on actual use of technology or online access. It is possible that there are ethnic differences in technology use and online access as well, but since this was not assessed, it remains undetermined. While health care coverage was assessed and the majority (96%) of participants reported having some level of coverage, participants’ perceptions about how health insurance coverage may impact their interest and access to multigene panel testing was not assessed.
A main aim of this study was to assess interest in multigene panel testing and to explore how sociodemographic, psychological, and clinical factors were associated with level of interest. This a key first step to better understanding reasons individuals and families access or do not access multigene panel genetic testing, especially among diverse populations. Further research is needed to understand actual testing decisions and how this information is used by patients (e.g., changes in lifestyle and medical management and family communication and cascade testing). In our study, we did not directly ask participants reasons for why they were interested or lacked interest in multigene panel testing or specific facilitators and barriers to testing. However, we assessed reasons for initial BRCA testing among those participants that had previously received BRCA testing. The top reasons for undergoing BRCA testing were (1) help explain their breast cancer diagnosis (76%), possibly to inform treatment and follow-up, (2) concern for family member’s risk (60%), and (3) a doctor or provider having recommended genetic testing (51%). Although these were reasons for undergoing BRCA testing, it is reasonable to hypothesize that reasons for seeking further multigene panel testing may be similar, for more personalized management options and out of concern for family and a desire to help manage family members’ cancer risk. Future research can yield insight into how at-risk individuals prioritize genetic testing and what factors motivate multigene panel testing in BRCA negative families.
Further research is needed to explore how cancer-related worry and preventative screening behavior play a role in decision-making regarding further testing. Future research on whether or not multigene panel testing affects cancer worry is needed to assess the psychological impact of the complexities of this type of testing. The findings from this study suggest that tailored cancer risk communication interventions may be useful in addressing disparities in access to cancer genomic risk information. Tailored cancer risk communication based on communication preferences, ethnicity, language, and the cultural context may be effective in creating culturally relevant risk communication strategies addressing the lack of knowledge among underserved populations. Language, literacy needs, and cultural perspectives should be considered in disseminating linguistically and culturally relevant cancer risk information (Kinney et al. 2010). Still more research is needed to determine the role of language/literacy in web-based tools and resources for genomic communication in monolingual and even bicultural Hispanic populations. Our study’s findings can inform public health interventions aimed at increasing utilization of cancer risk assessment among at-risk, BRCA-uninformative families and multiethnic/racial populations. Ultimately, effective cancer risk communication can lead to improved breast cancer outcomes among ethnically diverse, underserved communities.
References
Andersen MR, Smith R, Meischke H, Bowen D, Urban N (2003) Breast cancer worry and mammography use by women with and without a family history in a population-based sample. Cancer Epidemiol Biomark Prev 12(4):314–320
Armstrong J, Toscano M, Kotchko N, Friedman S, Schwartz MD, Virgo KS et al (2015) Utilization and outcomes of BRCA genetic testing and counseling in a national commercially insured population: the ABOUT Study. JAMA Oncology 1(9):1251–1260
Armstrong K, Weiner J, Weber B, Asch DA (2003) Early adoption of BRCA 1/2 testing: who and why. Genetics in Medicine 5(2):92–98. doi:10.1097/01.GIM.0000056829.76915.2A
Booth ML, Ainsworth BE, Pratt M, Ekelund U, Yngve A, Sallis JF, Oja P (2003) International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc 195(9131/03):3508–1381
Bradbury AR, Patrick-Miller L, Domchek S (2015) Multiplex genetic testing: Reconsidering utility and informed consent in the era of next-generation sequencing. Genetics in Medicine 17(2):97–98. doi:10.1038/Gim.2014.85
Cameron LD, Reeve J (2006) Risk perceptions, worry, and attitudes about genetic testing for breast cancer susceptibility. Psychol Health 21(2):211–230
Cristancho S, Peters K, Garces M (2014) Health information preferences among Hispanic/Latino immigrants in the US rural midwest. Glob Health Promot 21(1):40–49
Domchek SM, Bradbury A, Garber JE, Offit K, Robson ME (2013) Multiplex genetic testing for cancer susceptibility: out on the high wire without a net? J Clin Oncol 31(10):1267–1270
Elrick, A, Ashida, S, Ivanovich, J, Lyons, S, Biesecker, B B, Goodman, M S, & Kaphingst, K A (2016) Psychosocial and clinical factors associated with family communication of cancer genetic test results among women diagnosed with breast cancer at a young age. Journal of Genetic Counseling: 1–9
Ersig AL, Williams JK, Hadley DW, Koehly LM (2009) Communication, encouragement, and cancer screening in families with and without pathogenic variants for hereditary nonpolyposis colorectal cancer: a pilot study. Genetics in Medicine 11(10):728–734
Flores, K G, Steffen, L E, McLouth, C J, Vicuña, B E, Gammon, A, Kohlmann, W, ... & Kinney, A Y (2016) Factors associated with interest in gene-panel testing and risk communication preferences in women from BRCA1/2 negative families. Journal of Genetic Counseling: 1–11
Frank TS, Deffenbaugh AM, Reid JE, Hulick M, Ward BE, Lingenfelter B et al (2002) Clinical characteristics of individuals with germline pathogenic variants in BRCA1 and BRCA2: analysis of 10,000 individuals. J Clin Oncol 20(6):1480–1490
Frey MK, Kim SH, Bassett RY, Martineau J, Dalton E, Chern JY, Blank SV (2015) Rescreening for genetic pathogenic variants using multi-gene panel testing in patients who previously underwent non-informative genetic screening. Gynecol Oncol 139(2):211–215. doi:10.1016/j.ygyno.2015.08.006
Gammon AD, Rothwell E, Simmons R, Lowery JT, Ballinger L, Hill DA et al (2011) Awareness and preferences regarding BRCA1/2 genetic counseling and testing among Latinas and non-Latina white women at increased risk for hereditary breast and ovarian cancer. J Genet Couns 20(6):625–638. doi:10.1007/s10897-011-9376-7
Graves K, Peshkin B, Luta G, Tuong W, Schwartz M (2011) Interest in genetic testing for modest changes in breast cancer risk: implications for SNP testing. Public Health Genomics 14(3):178–189
Graves KD, Wenzel L, Schwartz MD, Luta G, Wileyto P, Narod S et al (2010) Randomized controlled trial of a psychosocial telephone counseling intervention in BRCA1 and BRCA2 pathogenic variant carriers. Cancer Epidemiol Biomark Prev 19(3):648–654. doi:10.1158/1055-9965.EPI-09-0548
Gray SW, Hicks-Courant K, Cronin A, Rollins BJ, Weeks JC (2014) Physicians’ attitudes about multiplex tumor genomic testing. J Clin Oncol JCO 2003 2052:4298
Internal Physical Activity Questionnaire (2005) Guidelines for data processing and analysis of the international physical activity questionnaire. https://sites.google.com/site/theipaq/scoring-protocol
Hagger MS, Orbell S (2003) A meta-analytic review of the common-sense model of illness representations. Psychol Health 18(2):141–184. doi:10.1080/088704403100081321
Hall MJ, Reid JE, Burbidge LA, Pruss D, Deffenbaugh AM, Frye C et al (2009) BRCA1 and BRCA2 pathogenic variants in women of different ethnicities undergoing testing for hereditary breast-ovarian cancer. Cancer 115(10):2222–2233. doi:10.1002/cncr.24200
Hamilton JG, Shuk E, Arniella G, González CJ, Gold GS, Gany F et al (2015) Genetic testing awareness and attitudes among Latinos: exploring shared perceptions and gender-based differences. Public Health Genomics 19(1):34–46
Himes, D O, Clayton, M F, Donaldson, G W, Ellington, L, Buys, S S, & Kinney, A Y (2016) Breast cancer risk perceptions among relatives of women with uninformative negative BRCA1/2 test results: the moderating effect of the amount of shared information. J Genet Couns 25:258–269
Jagsi R, Griffith KA, Kurian AW, Morrow M, Hamilton AS, Graff JJ et al (2015) Concerns about cancer risk and experiences with genetic testing in a diverse population of patients with breast cancer. J Clin Oncol 33(14):1584. doi:10.1200/Jco.2014.58.5885
Jensen JD, Bernat JK, Davis LA, Yale R (2010) Dispositional cancer worry: convergent, divergent, and predictive validity of existing scales. J Psychosoc Oncol 28(5):470–489
Kinney AY, Butler KM, Schwartz MD, Mandelblatt JS, Boucher KM, Pappas LM et al (2014b) Expanding access to BRCA1/2 genetic counseling with telephone delivery: a cluster randomized trial. Journal of the Natlonal Cancer Institute 106(12)
Kinney AY, Boonyasiriwat W, Walters ST, Pappas LM, Stroup AM, Schwartz MD et al (2014a) Telehealth personalized cancer risk communication to motivate colonoscopy in relatives of patients with colorectal cancer: the family CARE randomized controlled trial. J Clin Oncol 32(7):654–662
Kinney AY, Gammon A, Coxworth J, Simonsen SE, Arce-Laretta M (2010) Exploring attitudes, beliefs, and communication preferences of Latino community members regarding BRCA1/2 pathogenic variant testing and preventive strategies. Genetics in Medicine 12(2):105–115. doi:10.1097/Gim.0b013e3181c9af2d
Kurian AW, Hare EE, Mills MA, Kingham KE, McPherson L, Whittemore AS et al (2014) Clinical evaluation of a multiple-gene sequencing panel for hereditary cancer risk assessment. J Clin Oncol 32(19):2001–2009
Lerman C, Seay J, Balshem A, Audrain J (1995) Interest in genetic testing among first-degree relatives of breast cancer patients. Am J Med Genet 57(3):385–392
Leventhal H, Meyer D, Nerenz D (1980) The common sense representation of illness danger. Contributions to Medical Psychology 2:7–30
Levy DE, Byfield SD, Comstock CB, Garber JE, Syngal S, Crown WH, Shields AE (2011) Underutilization of BRCA1/2 testing to guide breast cancer treatment: Black and Hispanic women particularly at risk. Genetics in Medicine 13(4):349–355. doi:10.1097/Gim.0b013e3182091ba4
Lipkus IM, Rimer BK, Halabi S, Strigo TS (2000) Can tailored interventions increase mammography use among HMO women? Am J Prev Med 18(1):1–10. doi:10.1016/S0749-3797(99)00106-3
Lorence DP, Park H, Fox S (2006) Racial disparities in health information access: resilience of the digital divide. J Med Syst 30(4):241–249
Lynce F, Graves KD, Jandorf L, Ricker C, Castro E, Moreno L, Augusto B, Fejerman L, Vadaparampil ST (2016) Genomic disparities in breast cancer among Latinas. Cancer Control 23(4):359–372
Mai PL, Vadaparampil ST, Breen N, McNeel TS, Wideroff L, Graubard BI (2014) Awareness of cancer susceptibility genetic testing: the 2000, 2005, and 2010 National Health Interview Surveys. Am J Prev Med 46(5):440–448
McBride CM, Birmingham WC, Kinney AY (2015) Health psychology and translational genomic research: bringing innovation to cancer-related behavioral interventions. Am Psychol 70(2):91–104
Mester JL, Mercer M, Goldenberg A, Moore RA, Eng C, Sharp RR (2015) Communicating with biobank participants: preferences for receiving and providing updates to researchers. Cancer Epidemiol Biomark Prev 24(4):708–712
Moran, O, Nikitina, D, Royer, R, Poll, A, Metcalfe, K, Narod, S A, ... & Kotsopoulos, J (2016) Revisiting breast cancer patients who previously tested negative for BRCA mutations using a 12-gene panel. Breast Cancer Res Treat:1–8
New Mexico Population Demographics — Race/Ethnicity (2015) Mexico Department of Health, Indicator-Based Information System for Public Health. Retrieved from http://ibis.health.state.nm.us/
Norquist BM, Swisher EM (2015) More genes, more problems? Benefits and risks of multiplex genetic testing. Gynecol Oncol 139(2):209–210. doi:10.1016/j.ygyno.2015.10.013
Peña-Purcell N (2008) Hispanics' use of internet health information: an exploratory study**. J Med Lib Assoc 96(2):101
Peterson SK (2005) The role of the family in genetic testing: theoretical perspectives, current knowledge, and future directions. Health Educ Behav 32(5):627–639
Ricker C, Culver JO, Lowstuter K, Sturgeon D, Sturgeon JD, Chanock CR et al (2016) Increased yield of actionable pathogenic variants using multi-gene panels to assess hereditary cancer susceptibility in an ethnically diverse clinical cohort. Cancer Genetics 209(4):130–137
Ricker C, Lagos V, Feldman N, Hiyama S, Fuentes S, Kumar V et al (2006) If we build it… will they come? Establishing a cancer genetics services clinic for an underserved predominantly Latina cohort. J Genet Couns 15(6):505–514
Rizzo JM, Buck MJ (2012) Key principles and clinical applications of “next-generation” DNA sequencing. Cancer Prev Res 5(7):887–900
Sanger F, Nicklen S, Coulson AR (1977) DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci 74(12):5463–5467
Schwartz MD, Peshkin BN, Hughes C, Main D, Isaacs C, Lerman C (2002) Impact of BRCA1/BRCA2 pathogenic variant testing on psychologic distress in a clinic-based sample. J Clin Oncol 20(2):514–520
Selsky C, Luta G, Noone AM, Huerta EE, Mandelblatt JS (2013) Internet access and online cancer information seeking among Latino immigrants from safety net clinics. J Health Commun 18(1):58–70. doi:10.1080/10810730.2012.688248
Slavin TP, Niell-Swiller M, Solomon I, Nehoray B, Rybak C, Blazer KR, Weitzel JN (2015) Clinical application of multigene panels: challenges of next-generation counseling and cancer risk management. Front Oncol 5
Solomon FM, Eberl-Lefko AC, Michaels M, Macario E, Tesauro G, Rowland JH (2005) Development of a linguistically and culturally appropriate booklet for Latino cancer survivors: lessons learned. Health Promot Pract 6(4):405–413
Sussner KM, Edwards T, Villagra C, Rodriguez MC, Thompson HS, Jandorf L, Valdimarsdottir HB (2015) BRCA genetic counseling among at-risk Latinas in New York City: new beliefs shape new generation. Journal of Genetic Counseling 24(1):134–148. doi:10.1007/s10897-014-9746-z
Stacey FG, James EL, Chapman K, Courneya KS, Lubans DR (2015) A systematic review and meta-analysis of social cognitive theory-based physical activity and/or nutrition behavior change interventions for cancer survivors. J Cancer Surviv 9(2):305–338
Steffen LE, Boucher KM, Damron BH, Pappas LM, Walters ST, Flores KG, Edwards SL (2015) Efficacy of a telehealth intervention on colonoscopy uptake when cost is a barrier: the family CARE cluster randomized controlled trial. Cancer Epidemiol Biomark Prev 24(9):1311–1318
Tung, N, Domchek, S M, Stadler, Z, Nathanson, K L, Couch, F, Garber, J E, ... & Robson, M E (2016) Counselling framework for moderate-penetrance cancer-susceptibility mutations. Nat Rev Clin Oncol
United States Department of Agriculture 2010 Rural-urban commuting area codes. http://www.ers.usda.gov/data-products/rural-urban-commuting-area-codes.aspx
van Dijk, S, Timmermans, D R, Meijers-Heijboer, H, Tibben, A, van Asperen, C J, & Otten, W (2006) Clinical characteristics affect the impact of an uninformative DNA test result: the course of worry and distress experienced by women who apply for genetic testing for breast cancer
van Oostrom I, Meijers-Heijboer H, Duivenvoorden HJ, Bröcker-Vriends AH, van Asperen CJ, Sijmons RH et al (2007) The common sense model of self-regulation and psychological adjustment to predictive genetic testing: a prospective study. Psycho-Oncology 16(12):1121–1129
Victorson D, Banas J, Smith J, Languido L, Shen E, Gutierrez S et al (2014) Esalud: designing and implementing culturally competent ehealth research with Latino patient populations. Am J Public Health 104(12):2259–2265
Villarreal-Garza C, Weitzel JN, Llacuachaqui M, Sifuentes E, Magallanes-Hoyos MC, Gallardo L et al (2015) The prevalence of BRCA1 and BRCA2 pathogenic variants among young Mexican women with triple-negative breast cancer. Breast Cancer Res Treat 150(2):389–394. doi:10.1007/s10549-015-3312-8
Vos J, Menko F, Jansen AM, van Asperen CJ, Stiggelbout AM, Tibben A (2011) A whisper-game perspective on the family communication of DNA-test results: a retrospective study on the communication process of BRCA1/2-test results between proband and relatives. Familial Cancer 10(1):87–96
Waters EA, Sullivan HW, Finney Rutten LJ (2009) Cancer prevention information-seeking among Hispanic and non-Hispanic users of the National Cancer Institute's cancer information service: trends in telephone and livehelp use. J Health Commun 14(5):476–486. doi:10.1080/10810730903032952
Weitzel JN, Clague J, Martir-Negron A, Ogaz R, Herzog J, Ricker C et al (2013) Prevalence and type of BRCA pathogenic variants in Hispanics undergoing genetic cancer risk assessment in the Southwestern United States: a report from the Clinical Cancer Genetics Community Research Network. J Clin Oncol 31(2):210–216. doi:10.1200/Jco.2011.41.0027
White HD (2005) Adherence and outcomes: it's more than taking the pills. Lancet 366(9502):1989–1991
World Health Organization (2003) WHO fruit and vegetable promotion initiative—report of the meeting, Geneva 25–27 August 2003. Geneva, Switzerland. http://www.who.int/dietphysicalactivity/publications/f&v_promotion_initiative_report.pdf?ua=1
Yorczyk A, Robinson LS, Ross TS (2015) Use of panel tests in place of single gene tests in the cancer genetics clinic. Clin Genet 88(3):278–282. doi:10.1111/cge.12488
Acknowledgments
The study was supported by grants from the National Cancer Institute at the National Institutes of Health (1R01CA129142; A.Y.K.) and the University of New Mexico Comprehensive Cancer Center Support Grant: Development Funds (P30CA118100; C.L.W.) and the Surface Family Trust.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000. Informed consent was obtained from all patients for being included in the study.
Conflict of interest
Belinda Vicuña, Harold D. Delaney, Kristina G. Flores, Lori Ballinger, Melanie E. Royce, Zoneddy R. Dayao, Tuya Pal, and Anita Y. Kinney declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Vicuña, B., Delaney, H.D., Flores, K.G. et al. Preferences for multigene panel testing for hereditary breast cancer risk among ethnically diverse BRCA-uninformative families. J Community Genet 9, 81–92 (2018). https://doi.org/10.1007/s12687-017-0322-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12687-017-0322-8