Abstract
Genetic testing recommendations for hereditary breast and ovarian cancer involve pedigree analysis and consultation of testing guidelines. The testing landscape for hereditary cancer syndromes is shifting as multiplex panel tests become more widely integrated into clinical practice. The purpose of the current study was to assess how genetic counselors utilize pedigrees to make recommendations for genetic testing, to determine consistency of these recommendations with National Comprehensive Cancer Network (NCCN) Guidelines and to explore current use of multiplex panel testing. Sixty-nine genetic counselors were recruited through the National Society of Genetic Counselors Cancer Special Interest Group’s Discussion Forum. Participation involved pedigree analysis and completion of an online questionnaire assessing testing recommendations and use of multiplex panel testing. Pedigree analysis and test recommendations were scored for consistency with NCCN guidelines. The average score was 12.83/15 indicating strong consistency with NCCN guidelines. Participants were more likely to consider multiplex testing when pedigrees demonstrated highly penetrant dominant inheritance but were not indicative of a particular syndrome. Participant concerns about multiplex panel testing include limited guidelines for both testing eligibility and medical management. This study demonstrates high utilization of pedigree analysis and raises new questions about its use in multiplex genetic testing.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
One essential component of the genetic counseling session is construction and assessment of an individual’s pedigree. Creation of the pedigree provides an opportunity to establish rapport, learn about relationships in a family, and assess disease risk (Uhlmann et al. 2009). Use of the genetic family history as a risk assessment tool finds that a majority of individuals in an internal medicine sample have one or more genetic risk factors for disease (Frezzo et al. 2003). Additionally, the pedigree has also been described as the first genetic test (Pyeritz 2012). One of the main ways to assess an individual’s risk for a hereditary cancer syndrome is to construct and evaluate a pedigree (Bennett et al. 1995). Pedigrees can be used to determine inheritance patterns, to document family members who are at increased risk for cancer and to create a plan for genetic testing in the family (Bennett 2010). Pedigree analysis can help determine the appropriate diagnosis based on other cancers present within the family.
In families who have early onset breast cancer and/or multiple diagnoses of breast cancer, three autosomal dominant hereditary cancer syndromes are most often considered: Hereditary Breast and Ovarian Cancer (HBOC), Cowden syndrome (CS), and Li-Fraumeni syndrome (LFS) (Hemel and Domchek 2010). In addition to breast cancer, each of these syndromes has increased risks for other cancers and screening and risk reduction recommendations vary by syndrome. Briefly, HBOC is associated with germline mutations in the BRCA1 and BRCA2 genes and an estimated cancer risk to age 70 of 31–78 % for breast cancer and 11–39 % for ovarian cancer (Antoniou et al. 2003). Additional cancer risks include male breast cancer, prostate, and pancreatic cancer. (Breast Cancer Linkage Consortium 1999). CS is associated with mutations in the PTEN gene and can cause breast cancer, non-medullary thyroid cancer, renal cell carcinoma, and endometrial cancer (Tan et al. 2012). Other findings of CS include macrocephaly and benign hamartomatous overgrowth of some tissues (Pilarski 2009). LFS is associated with mutations in the TP53 gene. LFS is also known as SBLA syndrome (Sarcoma, Breast, Leukemia, and Adrenal Gland syndrome) due to the cancers that are typically associated with it. The core cancers that are associated with LFS are premenopausal breast cancer, bone and soft tissue sarcomas, brain tumors, leukemia, and adrenocortical carcinoma (Chompret et al. 2001).
There is no empirical evidence regarding the risk level that is appropriate for consideration of genetic testing (USPTF 2005), and the American Society of Clinical Oncology notes the limitations of models in estimating mutation probabilities or the lack of such models for most cancer syndromes (ASCO, 2013). Many of the models available to estimate risk of carrying a BRCA1 or BRCA2 mutation are time consuming to utilize as they require the entry of detailed family history, and at times, additional medical information (Amir et al. 2010; Culver et al. 2006). Many other hereditary breast cancer syndromes do not have comparable models to determine appropriateness of testing, leaving eligibility assessment for genetic testing dependent on clinical criteria as defined by professional organizations, such as the National Comprehensive Cancer Network (NCCN).
The NCCN is an alliance of 23 cancer centers that develop guidelines that include recommendations for genetic consultation referrals, guidance for determining genetic testing eligibility, and also provide reviews of medical management guidelines for patients at risk for HBOC, LFS, and CS in their Practice Guidelines in Oncology for Genetic/Familial High-Risk Assessment: Breast and Ovarian. They also include brief references to Hereditary Diffuse Gastric Cancer syndrome, Peutz Jeghers syndrome, and Lynch syndrome (given Lynch syndrome’s risk for ovarian cancer) in these guidelines (National Comprehensive Cancer Network 2013a).
Genetic counselors may choose to use any of these tools or testing criteria, combined with clinical judgment, to ensure that patients receive appropriate testing. Familiarity with the NCCN criteria may be particularly useful as it allows a genetic counselor to quickly assess a pedigree for eligibility, both from a clinical standpoint, and also to know whether or not the patient’s health insurance is likely to cover the cost of testing. Insurance companies often use the NCCN recommendations to guide their coverage decisions (Wang et al. 2011).
Prior to June 2013, genetic testing for the BRCA1 and BRCA2 genes were available clinically only through Myriad Genetic Laboratories (Association for Molecular Pathology, et al. v. Myriad Genetics, Inc. et al. 2013). Additional single gene testing for CS, LFS, and other hereditary cancer syndromes was available through Myriad or other clinical laboratories. Given the rarity of these gene mutations, questionable insurance coverage, and the possibility of high out-of-pocket costs, combined with the potential for testing fatigue, it is unclear how often genetic counselors pursue additional testing beyond BRCA1and BRCA2 when a family history is suggestive of other syndromes.
In February 2012, Ambry Genetic Laboratories introduced the first widely available clinical test for several genes related to an increased risk for breast cancer that went beyond BRCA1/2. Using Next Generation Sequencing (NGS), 14 additional genes beyond BRCA1/2, including PTEN and TP53, could be examined and additional genes continue to be added through Ambry and other clinical laboratories (https://doi.org/www.ambrygen.com/). Genes including BRCA1/2, TP53, and PTEN are clinically actionable genes, those which have data to guide alterations in medical management. However, these new multiplex test panels also include several moderate risk genes, for which medical management guidelines are less clear. The incorporation of testing panels will impact the process of genetic counseling in a cancer setting. New models of pre-test counseling will be needed to provide patients with appropriate education about testing for multiple genetic conditions with one test (Domchek et al. 2013).
With genetic testing evolving from single gene to several genes available in a comparable time frame and cost range, the question of the utility of testing criteria becomes important. Therefore, the purpose of the present study was to 1) assess how genetic counselors utilize pedigrees to make recommendations for genetic testing; 2) determine if these recommendations are consistent with the NCCN guideline for Genetic/Familial Risk Assessment for Breast and Ovarian Cancer (Version 4.2013, https://doi.org/www.nccn.org), and 3) assess how often and under what circumstances a genetic counselor might pursue multiplex panel testing. In the months since this survey was administered, BRCA1 and BRCA2 testing has become available at several labs other than Myriad Genetics and is now included as part of most multiplex breast cancer panels. This may further influence genetic counselor decisions about testing; however, many of the potential limitations concerning genetic counselors at the time of this survey continue to apply to current panels.
Methods
Participants
Eligible participants were full members of the National Society of Genetic Counselors (NSGC) who could access the Discussion Forum for the Familial Cancer Special Interest Group.
Recruitment
Full NSGC members were contacted using the Discussion Forum for the Familial Cancer Special Interest Group. Membership in this Special Interest Group is voluntary and requires an annual fee. There are approximately 750 members of the Special Interest Group and utilization of the discussion forum is optional. On November 1, 2012, a post to the Discussion Forum invited genetic counselors to participate in a confidential online survey examining how genetic counselors utilize pedigrees to make recommendations for genetic testing for hereditary cancer syndromes. The post included a cover letter that described participation in the online survey and a link to complete the survey. The initial invitation yielded 44 responses. On November 13, 2012, the survey invitation was posted to the Discussion Forum for the Cancer Special Interest group again, and this second request yielded an additional 25 responses. There were 69 total participants in the study. The survey was available to complete during the month of November 2012. The study was approved by the Institutional Review Board at Arcadia University in Glenside, Pennsylvania.
Questionnaire
The questionnaire was comprised of 15 pedigrees, multiple choice questions about different testing options, questions to assess use of multiplex test panels, and a section to provide demographic information. At the beginning of the questionnaire, participants were given the following instructions: “For each patient, please evaluate the pedigree and recommend testing that you feel is most appropriate. Assume that the patient’s insurance company will cover any testing that is recommended in full and that the patient is highly motivated.”
Participants were provided with an electronic informed consent form before beginning the anonymous survey. They were instructed that they could leave questions unanswered and proceed but could not return to previous questions once they had moved forward. The survey was conducted using the online survey website, Survey Monkey (1999).
Pedigree Development
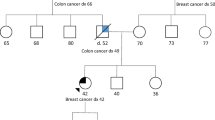
The pedigrees in the questionnaire were developed to mirror family histories of patients pursuing cancer genetic counseling. The first author created the pedigrees to include a patient with breast cancer and a family history that was indicative of HBOC, LFS, and/or CS. Some but not all of the pedigrees met NCCN testing criteria for one or more of these three syndromes. The pedigrees were reviewed by co-authors, AF and LK, who are board certified genetic counselors with expertise in hereditary cancer genetic counseling. The pedigrees were designed to have variable ethnic backgrounds, family size and structure, and family cancer diagnoses to enhance generalizeability to clinical practice. All of the pedigrees included in the study can be found in Fig. 1.
After analyzing each pedigree, participants were asked about whether or not they would recommend testing and to select which test they would recommend. For example, participants were instructed:
-
“What testing would you recommend first for this patient?
-
No Testing Recommended
-
Cowden Syndrome (PTEN)
-
Familial Adenomatous Polyposis (APC)
-
Hereditary Breast and Ovarian Cancer Syndrome (BRCA1/BRCA2)
-
Hereditary Diffuse Gastric Cancer (CDH1)
-
Li-Fraumeni (p53)
-
Lynch (MLH1, MSH2, MSH6, PMS2)
-
Peutz-Jeghers (STK11)
-
Following this question, participants were asked three additional questions about other stand-alone testing as well as multiplex panel testing, and were given the opportunity to provide open-ended comments supporting their test recommendation(s). See below for an example:
-
“If this testing came back negative, what further testing would you recommend?
-
No Further Testing Recommended
-
Cowden (PTEN)
-
Familial Adenomatous Polyposis (APC)
-
Hereditary Breast and Ovarian Cancer (BRCA1/BRCA2)
-
Hereditary Diffuse Gastric Cancer (CDH1)
-
Li-Fraumeni (p53)
-
Lynch (MLH1, MSH2, MSH6, PMS2)
-
Peutz-Jeghers (STK11)
-
-
Would you consider ordering a multi-gene breast cancer panel for this patient that contained low and moderate penetrance genes such as ATM, CHEK2, and PALB2?
-
Yes
-
No
-
Please give a brief explanation for this recommendation.”
Participants assessed 15 different pedigrees and answered the same questions after each pedigree. Following completion of the pedigree assessment and questions, the survey included three multiple choice questions to assess the clinical use of multi-gene panels. Participants were asked to record the number of times the s/he had sent testing for a multi gene panel during the previous 6 months, the frequency with which patients accepted this type of testing, and the reasons that they did not utilize these panels. For the question assessing reasons that testing was not utilized, participants selected reasons from the list and could enter a reason that was not listed. The completed survey was piloted with a small group of second year graduate students in the Arcadia Genetic counseling program to determine time needed for survey completion and for readability prior to being posted to the Discussion Forum.
Demographics
Following completion of the survey, participants provided demographic data. Data collected included if the participant was currently a practicing cancer genetic counselor, years of experience, number of new cases per week, percentage of patients referred for breast cancer, employment setting, and in which geographic region they worked. Questions were created based on the Professional Status Survey (National Society of Genetic Counselors 2012).
Demographics were collected to determine if there were any patterns regarding adherence to NCCN testing guidelines and usage of multiplex panel testing. Patterns were investigated based upon years of experience, percentage of cases referred for an indication of breast cancer, and work setting similar to items assessed in the Professional Status Survey (NSGC 2012).
Data Analyses
The participants’ genetic testing recommendations for the 15 pedigrees were reviewed for consistency with NCCN testing guidelines. The average scores and standard deviations on the testing guidelines evaluation were calculated for all participants. The results were grouped based on years of experience and on percentage of cases that were referred for an indication of breast cancer. A two tailed t-test was performed on the scores of the genetic counselors based on years of experience. Analysis of variance was performed on the scores of genetic counselors based on percentage of breast cancer referrals.
A scoring system was designed to evaluate consistency between participant testing recommendations and NCCN guidelines. For each of the 15 pedigrees, participants could recommend no testing, one stand-alone test with no additional testing, or two stand-alone tests. Each recommendation was worth 0.5 points leading to 1.0 full point possible per pedigree for a total of 15.0 points per participant. Prior to survey implementation, testing recommendations were determined by comparing the pedigrees to NCCN criteria. Points were awarded only when the participant recommended testing that was consistent with NCCN testing criteria and/or did not offer testing for pedigrees that did not meet testing criteria. For example, if a proband had breast cancer at age 50 and a family history of only one other case of breast cancer at age 75, then a participant would only receive the full 1.0 point if they did not recommend any testing.
The genetic counselors’ recommendations of multi-gene panels for the probands were calculated by percentages. The participants were asked to give reasons why they would or would not recommend testing using these panels. This open-ended question was analyzed by identifying salient themes and categorizing responses according to those themes. Percentages of responses to the use of multi-gene breast panels and their patients’ acceptance were also calculated.
Results
Sample Characteristics
Ninety five percent of the genetic counselors who completed the study were working in a cancer setting. When asked the approximate percentage of patients referred for an indication of breast cancer, nine genetic counselors (15.0 %) reported that they see less than 50 % of patients for an indication of breast cancer, 20 (33.3 %) reported that they see approximately 50–75 % of patients for an indication of breast cancer, and 30 (51.7 %) reported that they see greater than 75 % of their patients for an indication of breast cancer. Genetic counselors were asked to classify their place of employment (they could choose more than one option). Twenty five indicated that they work at a University Medical Center (42.4 %), 19 indicated that they work at a private hospital (32.2 %), and 14 indicated that they work at a public hospital (23.7 %). The remainder indicated that they worked for not-for profit organizations, diagnostic laboratories, or other unspecified locations. Of the 69 respondents, 37 had 4 or less years of experience (63.8 %) and 21 had 5 or more years of experience (36.2 %).
NCCN Testing Guidelines Evaluation
Participants reviewed 15 pedigrees and made recommendations for genetic testing based on the pedigree analysis. The testing recommendations were reviewed for consistency with NCCN testing guidelines. Each pedigree was worth 1 point, with a total score of 15 points reflecting 100 % agreement with NCCN guidelines. The average score of respondents was 12.83 with a standard deviation of 0.93. Participants were subdivided based on years of experience and percentage of breast cancer referrals (Table 1). Participants correctly interpreted that a pedigree met NCCN criteria 96.3 % of the time. Accuracy decreased when including pedigrees where participants recommended testing even when NCCN criteria were not clearly met, which the authors have defined as ‘over-testing’. Limitations and potential explanations for over-testing and a discussion of the pedigrees involved in this scenario will be reviewed.
A summary of the personal and family history for each pedigree and the corresponding NCCN criteria are summarized in Table 2. The overall average score of consistency with NCCN guidelines was 0.86. The average score for each pedigree ranged from 0.39 to 0.99 with 1.00 being the highest possible score. Pedigree numbers 10, 12, and 13 yielded the lowest average scores of 0.689, 0.393, and 0.632 out of 1.000, respectively. These pedigrees did not meet NCCN criteria for the available testing options, yet several participants recommended some type of testing. Participants who recommended more or less testing than was indicated, defined by the authors as ‘under-testing’ and ‘over-testing,’ are also summarized in Table 2. Most results that were not concordant with NCCN Guidelines were due to over-testing. For example, pedigree 12 was suggestive of CS, but did not meet NCCN criteria for testing. Many participants still recommended testing for CS in addition to HBOC. Genetic counselors recommended Lynch syndrome testing for pedigrees 4 and 7 when criteria were not met (Fig. 1). Possible reasons for these choices will be reviewed in the discussion section. The pedigrees which did not meet any testing criteria had the lowest overall average score per question.
Multiplex Breast Panels
The participants were asked if they would consider recommending one of the new multiplex breast panels for the proband in each pedigree. The percentages of genetic counselors that would offer this testing to patients based on the indication are summarized in Table 3. The percentage of participants recommending testing ranged from 9.8 to 72.1 %. These percentages varied depending on how strong the family history was and how closely the family fit with clinical presentations of known hereditary breast cancer syndromes.
Participants first completed a close ended question about whether or not they would recommend testing using multiplex breast panels. Then there was an open ended question to allow for a brief explanation for the choice to recommend or not to recommend testing with a multi-gene panel. Participant responses were analyzed to determine if there were any common themes. One thematic element was related to pedigree analysis. About half of participants (46 %) raised the importance of pedigree analysis to support ordering or not ordering a multiplex test panel. Approximately 20 % of the genetic counselors reported that they did not choose to send multiplex panels because of concern about limited clinical utility of the panels due to the lack of screening and management guidelines for individuals with mutations in moderate risk genes. Another concern raised about the panels was about the high risk of receiving a variant of uncertain significance (VUS) result. Participants indicated that they would recommend multiplex panel testing when there were multiple genes on their differential diagnosis (18.0 %) as that would be most cost effective. When genetic counselors believed that genetic testing of one to two conditions was sufficient, they would state that no other testing was indicated (18.5 %) and would not recommend use of the panels. Participants (5.1 %) indicated that the patient’s motivations played a factor in their decision to offer multi-gene breast panels.
Genetic Counselors Clinical Usage of Multiplex Breast Panels
At the time this study was conducted, multiplex breast panels had been clinically available for approximately 6 months and did not include BRCA1 or BRCA2 (Table 4). During this period, 30.0 % of participants reported that they have never offered a patient a multi-gene breast panel, while 45.0 % had ordered one approximately 1–5 times. Participants who ordered multiplex panel testing approximately 6–12 times over the past 6 months accounted for 13.3 % of the sample, with 8.3 % ordering it more than 12 times in the past 6 months. Research testing for these types of panels is still available; approximately 3.3 % of participants have only offered multi-gene panels for research studies.
Participants were asked to estimate the percentage of their patients that accepted the multiplex breast panel when it was offered clinically. The largest percentage of respondents who have offered this testing to their patients (39.0 %) stated that patient’s accepted less than 10 % of the time. Genetic counselors who responded that their patients accepted more than 90 % of the time comprised 22 % of the sample.
Participants who had not yet ordered multiplex panel testing were asked to select all explanations for why they had not ordered this testing. About 90 % of genetic counselors who had not ordered multiplex breast cancer panels had concerns about limited screening guidelines if a mutation were found in many of the genes on the panel. Many participants wrote in the open-ended question that there was also limited clinical utility of the results. The limited testing guidelines for multiplex breast cancer panels were the second most common explanation (46.2 %). Insurance coverage concerns were cited by 42.3 % of the participants. Limited professional knowledge of the panel was selected by 26.9 % of the sample.
Discussion
In this study of pedigree analysis and recommendations for hereditary cancer testing, participants demonstrated strong understanding of the NCCN testing guidelines as evidenced by the high level of consistency between the recommended testing and NCCN guidelines. The average score for pedigree assessment and testing recommendations was 12.83 points out of 15, indicating an 85.5 % consistency rate. When participants were subdivided by years of experience, participants with 4 years of experience or less did not differ significantly in their scores than participants with 5 or more years of experience (p = 0.62). Since the NCCN are updated as knowledge of hereditary breast cancer syndromes change, it can be inferred that genetic counselors are remaining up to date with the current recommendations. The participants were also subdivided based on the percentage of patients seen for breast cancer referrals. Participants’ scores increased based on the percentage of breast cancer referrals seen; although these results were not significantly different (p = 0.12). These results may indicate a trend exists, but a larger sample size would be needed to confirm this.
One interesting finding was that many genetic counselors recommended testing for families that did not meet any NCCN testing criteria. Participants in the survey had the option to recommend “no testing” for families who did not meet testing criteria, as in a clinical setting. This suggests that genetic counselors utilize testing guidelines as only a part of their overall clinical judgment when making recommendations for patients to consider genetic testing. A study comparing genetic counselor prediction of BRCA1 and BRCA2 mutation occurrence compared to the BRCAPRO computer model found similar accuracy between the two groups, although BRCAPRO had improved sensitivity (Euhus et al. 2002). Berliner and colleagues (2013) provide practice recommendations for genetic counseling for HBOC and describe that published guidelines should be consulted when determining whether or not testing may be appropriate. Published guidelines may differ from testing criteria; therefore, clinical judgment must be used in addition to guidelines to determine the appropriateness of genetic testing (Berliner et al. 2013).
Pedigree 12 is a family tree where the patient did not meet NCCN criteria for either HBOC or CS (Fig. 1). Of note, testing criteria for CS through NCCN is based only on personal history and does not take into account additional family history. However, when examining the overall family history, a genetic counselor may be alert to other features suggesting testing for the PTEN gene may be appropriate. Indeed, this pedigree shows the largest portion of “over testing” (88.52 %), supporting the idea that this family history was clinically significant to the majority of participants. Testing outcomes from this over-testing can only be hypothesized, but this may suggest a limitation to testing criteria that focus only on the proband and do not examine the entire family history.
Five of the pedigrees met testing criteria for both HBOC and another syndrome. Testing for both syndromes was appropriately recommended 90.7 % of the time (313/345 recommendations). When the second syndrome was LFS, recommendations were very accurate with the exception of an isolated case of breast cancer case under 30. Approximately 15 % (14.75 %) of participants would not offer LFS testing to this patient, despite the patient meeting NCCN criteria for LFS. This may indicate some debate about the relatively low rate of positive results in families with no additional LFS-related cancers (Gonzalez et al. 2009a, b; McCuaig et al. 2012).
Appropriate recommendations were made less frequently when the proband qualified for both HBOC and CS. For the two pedigrees that met both criteria (pedigrees 5 and 7), participants made recommendations consistent with NCCN criteria 87.7 % of the time (121/138 recommendations) with some genetic counselors opting not to pursue testing for CS. Pedigree 7 was a unique situation in that it involved a young uterine cancer case. A number of participants recommended genetic testing for Lynch syndrome as the pedigree also indicated the patient was adopted. Bethesda criteria, as outlined in the NCCN guidelines for Colorectal Cancer Screening (National Comprehensive Cancer Network 2013b), recommend molecular tumor testing for colorectal cancers diagnosed under 50, yet it does not specify testing of uterine cancers in this same way. However, the guidelines note that recent evidence supports this same test for young uterine cancers (Weissman et al. 2012). Seventeen participants, perhaps aware of the significance of a young uterine cancer as a possible indication for Lynch syndrome, recommended genetic testing for Lynch syndrome. This may have also lowered the number of respondents recommending CS testing as only two testing options were available. Two participants described their testing plans:
“I would do BRCA and IHC on the uterine tumor first, then CancerNext panel if these were normal to rule out Lynch with normal IHC and PTEN”
“In this case I would consider a panel because it is likely to include the conditions (other than BRCA) in my differential—all Lynch genes and PTEN. Since we have no idea what other cancers may have occurred in the family, it is difficult to determine which condition is most likely and may be more cost effective to test multiple genes at the same time.”
Pedigree 4 also showed a higher than expected rate of participants choosing testing for Lynch syndrome (16 participants). While the family history of ovarian cancer and late onset colon cancer may be somewhat suggestive, this family does not meet Amsterdam II criteria. Participants seemed to recognize this limitation, as suggested by one respondent stating, “Lynch syndrome testing could be considered however the family history does not meet Amsterdam or Bethesda criteria. A multi gene panel including the Lynch syndrome genes would be a better option for this patient.” Evidence suggests that Amsterdam II criteria alone will miss a significant portion of Lynch families (Hampel et al. 2005) and this finding may again suggest a greater awareness of the subtle clinical assessment required beyond strict clinical criteria for testing.
Multiplex Test Panels
Participants recommended the use of multiplex breast cancer genetic testing panels for many different indications. Genetic counselors were more likely to consider offering these panels to patients whose families were consistent with a clearly autosomal dominant inheritance pattern. A common theme that emerged for the highly penetrant pedigrees was that these panels had the potential to delineate a genetic etiology. For example, one participant stated: “The history is extremely suspicious for a genetic cause and I would CONSIDER a panel to provide this family with additional information”. Often participants noted that there were several genes on the differential and that it was more cost effective to test using panels. “I would consider this because there are a number of syndromes that increase the risk for both breast and uterine cancers, so it might be more beneficial to the patient to test for a bunch of syndromes all at once.” However, when participants did not think that more than one or two genes were indicated, they often would not recommend a panel. For example, one participant said “[I would not recommend a multi-gene panel because the] family has a clinical diagnosis of LFS; if p53 testing is negative, can consider BRCA1 and BRCA2 testing given patient’s history of early-onset bilateral breast cancer.”
Study participants raised valid concerns about multiplex test panels that apply to panels with and without the inclusion of BRCA1 and BRCA2 genes. Many of the genes on the panel are known to have moderate penetrance. Cancer predisposition syndromes arising due to moderate penetrance genes do not have well established guidelines for testing or management. Without established clinical utility from possible positive results, many genetic counselors stated that they and/or their institutions were not comfortable offering testing. One participant stated, “I do not feel there is enough data to change medical management for the [patient] or her family if she was found to have a deleterious mutation in a low or moderate penetrance gene.”
Genetic counselors are also concerned about the possibility of the identification of many variants of unknown clinical significance with the initial test while testing labs continue to collect control population data. These concerns are especially pronounced in patients from ethnic minorities. For a pedigree indicating multiple ethnic minority ancestries, one participant said “Based on the number of breast cancer cases in the family, panel testing should be presented as an option for additional testing with the caveat of higher chance of a VUS given ancestry.” Many participants stated that they are recommending patients contact them in 3–5 years, when many of these initial issues with the panels have been sorted out. Several genetic counselors indicated that they would take the patient’s wishes into consideration when recommending panels. One participant summarized many of these themes when he/she said
“I would offer BRCA1/2, but counsel that it would be low-yield in this patient. Again, if the patient was highly motivated, I would certainly consider a panel. In this family, a low/moderate penetrance gene panel may well have a higher yield than BRCA1/2. That being said, it is hard to recommend such testing clinically until we have better data regarding the pick-up rate of these tests.”
Genetic counselors also indicated that they have not sent multiplex panel testing due to limited testing guidelines and limited screening guidelines for patients who test positive for many of the genes included. Insurance coverage is also a concern for many genetic counselors. Since this survey was conducted, genetic testing for BRCA1 and BRCA2 has become available at several labs and as part of multi-gene panels. The ability to rule out HBOC, CS, LFS, and other genetic risks in one test suggests that multiplex panel testing may become more common than single gene testing. Anecdotal experience with insurance coverage also supports that this testing will be covered for appropriate patients (A. Forman, personal communication, 2013). The shift in the testing landscape as multiplex testing panels are more widely incorporated into practice may influence the way testing criteria are written and the manner in which testing criteria are used as syndrome-specific criteria may become less essential.
Study Limitations
Several limitations should be considered when drawing conclusions from this data. The small sample size may suggest that these results are not generalizable to cancer genetic counselors as a whole. However, the demographics of the participants were similar to those of the genetic counseling community in terms of work settings and years of experience (NSGC 2012). There may be differences between respondents and non-respondents that cannot be identified. For instance, individuals who have had difficulties with these panels may have been more likely to respond. One limitation of the study is the inclusion of genetic counselors who are employed by genetic testing laboratories and genetic counselors who did not specify the setting in which they worked. However, only two participants were laboratory counselors and five genetic counselors did not specify their work setting.
The pedigrees used in this study were either fictional or modified versions of pedigrees of patients seen in multiple clinical settings. In order to avoid biasing the participant towards or away from particular cancer syndromes, pathology information was not given. Pathology is a tool that is heavily utilized by genetic counselors, and many participants indicated that they would prefer to obtain that information before ordering testing. As they were not able to obtain this information, these results may not be representative of choices actually made in the clinical setting. Pedigrees were reviewed by all authors, two of whom were board-certified genetic counselors (AF and LK) with expertise in counseling patients were hereditary cancer syndromes to ensure the family history details were similar to those seen in clinic.
This data is based on participants’ self-reported accounts of what they would do if they saw this patient in a clinical setting. It cannot be verified if these responses indicate genetic counselors true actions, what they wish that they could do, or what they feel that the correct answer was supposed to be. Studies have shown that people are often unable to predict what they would do in a given situation and are often unaware of their cognitive process (Nisbett and Wilson 1977). We also do not know what the final test results would have revealed, to support counselor decisions about whether or not testing was appropriate.
Coding for thematic elements in the open-ended questions about why or why not participants would consider ordering multiplex panel testing was performed by the primary author. An additional coder would have been beneficial to determine if there was any variation in the way that the responses were coded.
Practice Implications
Despite the limitations, these data suggest that cancer genetic counselors are very skilled at interpreting pedigrees. When testing was indicated based on NCCN criteria, there was a 96.3 % rate of concordance with participants recommending testing appropriately. This finding indicates that genetic counselors analyze family histories for multiple hereditary cancer syndromes with high level of accuracy. Since many genetic counselors recommended testing of genetic syndromes when NCCN criteria were not met, they may also be overestimating risk of carrying a hereditary risk of cancer in some families. It is also possible that genetic counselors are following the recommendations of Berliner and colleagues (2013) by consulting the guidelines and then using clinical judgment to make final recommendations about which testing options are appropriate.
The field of cancer genetics has recently undergone substantive changes due to advancement in testing technologies. Multiplex testing panels still raise areas of concern for cancer genetic counselors; however, these concerns are likely to change over time. As multiplex testing panels gain use in the clinical setting, the apprehensions of cancer genetic counselors discussed in this study provide a starting point to address these issues. As this landscape further evolves, pedigree analysis will likely continue to guide genetic counselors’ testing strategies to provide patients with the most appropriate test recommendations.
Research Recommendations
Future studies can consider the role of testing guidelines as panels become more incorporated into practice. For example, will there be creation of criteria about when to use a panel versus a single gene test? Will there be guidelines about which genes should be included on panels? The most recent recommendations from NCCN (v4.2013, https://doi.org/www.nccn.org) remain vague. Future research should include a larger sample size and include pathology data to increase the generalizability of the findings. Obtaining an explanation from participants regarding the decision to recommend or not recommend testing will also help to clarify whether guidelines may be too stringent to be used without additional clinical background and familiarity with these syndromes. Further studies will be needed to determine if genetic counselors’ use of multiplex panel testing changes over time as more clearly defined guidelines for testing and follow-up management are created.
References
Ambry Genetics. (2012). BreastNext. Retrieved April 22, 2012 from https://doi.org/ambrygen.com/tests/breastnext.
American Society of Clinical Oncology. (2003). American Society of Clinical Oncology policy statement update: genetic testing for cancer susceptibility. Journal of Clinical Oncology, 21(12), 2397–2406. doi:https://doi.org/10.1200/JCO.2009.27.0660.
Amir, E., Freedman, O. C., Seruga, B., & Evans, D. G. (2010). Assessing women at high risk of breast cancer: a review of risk assessment models. Journal of the National Cancer Institute, 102(10), 680–691. doi:https://doi.org/10.1093/jnci/djq088.
Antoniou, A., Pharoah, P. D., Narod, S., Risch, H. A., Eyfjord, J. E., Hopper, J. L., et al. (2003). Average risks of breast and ovarian cancer associated with BRCA1 or BRCA2 mutations detected in case series unselected for family history: a combined analysis of 22 studies. American Journal of Human Genetics, 72(5), 1117–1130.
Association for Molecular Pathology et al. v. Myriad Genetics, Inc, et al. 12–389 US. (2013). Retrieved October 12, 2013 from https://doi.org/www.supremecourt.gov/opinions/12pdf/12–398_1b7d.pdf.
Bennett, R. L. (2010). Practical guide to the genetic family history (2nd ed.). Hoboken: Wiley-Blackwell.
Bennett, R. L., Steinhaus, K. A., Uhrich, S. B., O’Sullivan, C. K., Resta, R. G., Lochner-Doyle, D., et al. (1995). Recommendations for standardized human pedigree nomenclature. Pedigree Standardization Task Force of the National Society of Genetic Counselors. American Journal of Human Genetics, 56, 745–752.
Berliner, J. L., Fay, A. M., Shelly, A., Cummings, S. A., Burnett, B., & Tillmanns, T. (2013). NSGC practice guideline: risk assessment and genetic counseling for hereditary breast and ovarian cancer. Journal of Genetic Counseling, 22, 155–163. doi:https://doi.org/10.1007/s10897-012-9547-1.
Breast Cancer Linkage Consortium. (1999). Cancer risks in BRCA2 mutation carriers. Journal of the National Cancer Institute, 91(15), 1310–1316.
Chompret, A., Abel, A., Stoppa-Lyonnet, D., Brugieres, L., Pages, S., Feunteun, J., et al. (2001). Sensitivity and predictive value of criteria for p53 germline mutation screening. Journal of Medical Genetics, 38, 43–47.
Culver, J. O., Lowstuter, K., & Bowling, L. (2006). Assessing breast cancer risk and BRCA1/2 carrier probability. Breast Disease, 27:5–20.
Domchek, S. M., Bradbury, A., Garber, J. E., Offit, K., & Robson, M. E. (2013). Multiplex genetic testing for cancer susceptibility: out on the high wire without a net? Journal of Clinical Oncology, 31(10), 1267–70. doi:https://doi.org/10.1200/JCO.2012.46.9403.
Euhus, D. M., Smith, K. C., Robinson, L., Stucky, A., Olopade, O. I., Cummings, S., et al. (2002). Pretest prediction of BRCA1 or BRCA2 mutation by risk counselors and the computer model BRCAPRO. Journal of the National Cancer Institute, 94(11), 844–851. doi: 10.1093.
Frezzo, T. M., Rubinstein, W. S., Dunham, D., & Ormond, K. E. (2003). The genetic family history as a risk assessment tool in internal medicine. Genetics in Medicine, 5(2), 84–91. doi: 10.1097.
Gonzalez, K., Buzin, C., Noltner, K., Gu, D., Li, W., Malkin, D., et al. (2009a). High frequency of de novo mutations in Li-Fraumeni syndrome. Journal of Medical Genetics, 46(10), 689–693. doi:https://doi.org/10.1136/jmg.2008.058958.
Gonzalez, K., Noltner, K., Buzin, C., Gu, D., Wen-Fong, C., Nguyen, V., et al. (2009b). Beyond Li Fraumeni Syndrome: clinical characteristics of families with p53 germline mutations. Journal of Clinical Oncology, 27(8), 1250–1256. doi:https://doi.org/10.1200/JCO.2008.16.6959.
Hampel, H., Stephens, J. A., Pukkala, E., Sankila, R., Aaltonen, L. A., Mecklin, J. P., et al. (2005). Cancer risk in hereditary nonpolyposis colorectal cancer syndrome: later age of onset. Gastroenterology, 129(2), 415–421.
Hemel, D., & Domchek, S. M. (2010). Breast cancer predisposition syndromes. Hematology/Oncology Clinics of North America, 24, 799–814. doi:https://doi.org/10.1016/j.hoc.2010.06.004.
McCuaig, J. M., Armel, S. R., Novokmet, A., Ginsburg, O. M., Demsky, R., Narod, S. A., et al. (2012). Routine TP53 testing for breast cancer under age 30: ready for prime time? Familial Cancer, 11(4), 607–613. doi:https://doi.org/10.1007/s10689-012-9557-z.
National Comprehensive Cancer Network Guidelines. (2013a). National Comprehensive Cancer Network. Genetic/Familial High-Risk Assessment: Breast and Ovarian (version 4.2013). Accessed 10/1/2013. https://doi.org/www.nccn.org.
National Comprehensive Cancer Network Guidelines. (2013b). National Comprehensive Cancer Network. Colorectal Cancer Screening (version 2.2013). Accessed 10/16/2013. https://doi.org/www.nccn.org.
National Society of Genetic Counselor (2012). Professional Status Survey: Work Environment. Retrieved January 28, 2013 from https://doi.org/www.nsgc.org/Publications/ProfessionalStatusSurvey/tabid/142/Default.aspx.
Nisbett, R. E., & Wilson, T. E. (1977). Telling more than we can know: verbal reports on mental processes. Psychological Review, 84(3), 231–259.
Pilarski, R. (2009). Cowden syndrome: a critical review of the clinical literature. Journal of Genetic Counseling, 18(1), 13–27. doi:https://doi.org/10.1007/s10897-008-9187-7.
Pyeritz, R. (2012). The family history: the first genetic test, and still useful after all those years? Genetic Medicine, 14(1), 3–9. doi:https://doi.org/10.1038/gim.0b013e3182310bcf.
Survey Monkey. (1999) Retrieved April 22, 2012 from https://doi.org/www.surveymonkey.com.
Tan, M. H., Mester, J. L., Ngeow, J., Rybicki, L. A., Orloff, M. S., & Eng, C. (2012). Lifetime cancer risks in individuals with germline PTEN mutations. Clinical Cancer Research, 18(2), 400–407. doi:https://doi.org/10.1158/1078-0432.CCR-11-2283.
Uhlmann, W., Schuette, J., & Yashar, B. (2009). A guide to genetic counseling (2nd ed.). Hoboken: John Wiley and Sons.
United States Preventive Services Task Force (USPSTF). (2005). Genetic risk assessment and BRCA mutation testing for breast and ovarian cancer susceptibility: recommendation statement. Annals of Internal Medicine, 143(5), 355–361. doi:https://doi.org/10.7326/0003-4819-143-5-200509060-00011.
Wang, G., Beattie, M. S., Ponce, N. A., & Phillips, A. (2011). Eligibility criteria in private and public coverage policies for BRCA genetic testing and genetic counseling. Genetics in Medicine, 13(12), 1045–1050. doi:https://doi.org/10.1097/GIM.0b013e31822a8113.
Weissman, S. M., Burt, R., Church, J., Erdman, S., Hampel, H., Holter, S., et al. (2012). Identification of individuals at risk for Lynch syndrome using targeted evaluations and genetic testing: National Society of Genetic Counselors and the Collaborative Group of the Americas on Inherited Colorectal Cancer joint practice guideline. Journal of Genetic Counseling, 21(4), 484–493. doi:https://doi.org/10.1007/s10897-011-9465-7.
Acknowledgments
We are grateful to the genetic counselors who took the time to participate in this survey, Laura Conway for her support and feedback, Steven Robbins for his input in design and interpretation, and Michael Kallan and Colleen Brensinger for their assistance with the statistical analysis.
Conflict of Interest
Meghan Lundy, Andrea Forman, Kathleen Valverde and Lisa Kessler declare they have no conflict of interest.
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000. Informed consent was obtained from all participants for being included in the study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lundy, M.G., Forman, A., Valverde, K. et al. An Investigation of Genetic Counselors’ Testing Recommendations: Pedigree Analysis and the Use of Multiplex Breast Cancer Panel Testing. J Genet Counsel 23, 618–632 (2014). https://doi.org/10.1007/s10897-014-9692-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10897-014-9692-9