Abstract
Recently, natural products are the powerful carbon source to synthesize carbon dots (CDs) with interesting physical and chemical properties. In this present work, we report a facile hydrothermal synthesis method for preparing fluorescent carbon dots using a biogenic precursor of rice bran without any surface passivation agent. The synthetic methodology was easy, simple, environmental friendly and convenient. Structural and optical properties of the RB-CDs have been studied by UV-visible, Fourier transform infrared spectroscopy (FTIR), Field emission scanning electron microscopy (FESEM), Fluorescence spectra and X-ray photoelectron spectroscopy (XPS) techniques. The prepared RB-CDs exhibited green emission upon irradiation with UV light and the calculated fluorescence quantum yield (QY) was found to be 7.4%. The morphological features of the synthesized RB-CDs were characterized by High-Resolution Transmission Electron Microscopy (HR-TEM), the average size of the RB-CDs was found to be 2.96 nm. The synthesized RB-CDs were beneficially applied as a catalyst for the catalytic degradation of methylene blue (MB) dye using NaBH4 as the reducing agent in the ambient conditions. The degradation of MB dye under light illumination was 89.20% in 30 min. Further, the obtained highly fluorescent RB-CDs were efficiently utilized as a fluorescent ink for luminescent pattern printing (patterning agent) in the anti-counterfeiting applications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In recent days, semiconductor quantum dots (QDs) were mostly considered by researchers because of their outstanding luminescent and electronic properties. The prepared metal based semiconductor quantum dots commonly contains heavy metals, which is very harmful to human lives, highly toxic to environmental eco-systems and atmosphere even at very low levels [1, 2]. The highly fluorescent and innovative carbon nanoparticles having a size between 1 to 10 nm, represented as “Carbon dots” and it is mainly composed by sp2- hybridized carbon atoms and they are surface rich in functional groups [3,4,5]. The synthesized CDs having the chemical configuration such as nitrogen (N), hydrogen (H), and oxygen (O) and were found to be superior in stability, good water solubility, extraordinary photoluminescence (PL) properties, quantum yield (Φs), large stokes shifts, ease of functionalization, excellent biocompatibility, low toxicity, and low photobleaching [6]. The natural precursors have abundant Carbon content plays a vital role in the synthesis of fluorescent CDs and also it has interesting, attractive, sustainable property and eco-friendly features [7]. The diverse applications of fluorescent CDs indicate that the CDs have good biochemical, optoelectronic properties, outstanding photostability and pH sensitivity. CDs provide a wide application in many areas of research such as bio-imaging [8], drug delivery, catalysis, biologically monitoring, photovoltaic and optoelectronic devices, sensor [9,10,11,12,13,14]. Several approaches are available for the synthesis of CDs such as arc discharge [15], laser ablation [16], and chemical oxidation [17], electrochemical method. The method listed above were comes under top-down approaches. The arc discharge and laser ablation method require sophisticated, very expensive and energy-consuming equipments, even though the chemical oxidation method uses strong acids. Now a days, the bottom-up approaches includes microwave irradiation [18], hydrothermal treatment [19], ultrasonic treatment were mostly used for the synthesis of CDs. The microwave irradiation technique is the easiest way to synthesize CDs in a couple of minutes, but it is difficult to control the reaction conditions and also it is risk. Among the bottom up methods, the hydrothermal method is most widely used method due to uncomplicated experimental setup, controlled reaction conditions, easy to use, environment friendly and cost-effective. Green synthesis of CDs is more attractive in the research field, natural resources were used for the synthesis of CDs because they are less toxic, inexpensive, and easily available. Some of the reported natural carbon sources are grass, ginger, orange juice, gas soot, pomelo peel, watermelon peel, soya beans, Pineapple peel [20], pseudo-stem [21] and prickly pear cactus etc., [22].
The present article demonstrates the interesting green chemistry method. Synthesize of carbon-based nanomaterials using rice bran as it is inexpensive agro-waste materials which are largely produced, and readily available around the world. Rice bran is a byproduct of rice in the milling process and native to south India, it was a yellow colored shell. The rice bran contains high fiber, and phytochemicals such as tocopherols, tocotrienols, oryzanols, and vitamins etc. [23]. The rice bran has been chosen as a carbon source for the preparation of carbon dots due to its high phytochemicals contents.
Dyes were mostly used in textile, paints and leather industries. One of the most commonly used coloring agents in all the industries are methylene blue (MB). MB is a carcinogenic thiazine dye, it generates health issues such as allergic dermatitis, cancer, eye burns, mutations, skin irritations for animals and human [27, 28]. Photocatalysis is a well known method for the removal of organic and inorganic pollutants from industrial effluents [24]. RB-CDs were prepared by hydrothermal method and are used for the degradation of dye in presence of xenon lamp using sodium borohydride as reducing agent. The electron transfer step plays a vital role in the degradation of dye because there may be a huge redox potential difference among the electron donor and acceptor species, which can be minimized by green catalyst and also it, promotes fast electron transfer [25, 26]. Furthermore, the highly fluorescent nature of the RB-CDs were applied to the real world application through the anti-counterfeit application to identify drawings, alphabets and numbers which is printed in the paper by luminescent pattern printing technology.
In this work, we report a low-cost, simple hydrothermal method to synthesize green fluorescent carbon dots using rice bran as the raw material without any surface passivation agent. The obtained results revealed that the synthesized rice bran based carbon dots (RB-CDs) have high quantum yield and bright green fluorescence. Herein, the waste rice bran material has been efficiently used for the synthesis of CDs and their catalytic ability was examined by the photo degradation of MB dye under 400 W Xenon lamp and the obtained RB-CDs were also used as a patterning agent for the fluorescent ink applications.
Experimental Methods
Materials and Reagents
The Rice bran was acquired from the local rice field in Kancheepuram, Tamil Nadu, India. Teflon-coated stainless steel autoclave, Micro-sample tubes, Magnetic Stirrer, Remi R-8C Laboratory Centrifuge (With 8 × 15 ml Swing Out Head) machine and required glass wares was purchased from Vijaya Scientific Company. Sodium hydroxide (NaOH), Polyvinyl alcohol (PVA), Methylene Blue Dye, Sodium Borohydride was acquired from Avra synthesis. All Analytical grade reagents were used without further purification. For synthesis and solution preparation purpose the double distilled water (DD) was used.
Green Synthesis of RB-CDs
RB-CDs were synthesized by slightly modification of the reported literature [29]. About 50 g of rice bran powder was thoroughly mixed with 250 mL DD water and it was allowed to stir for 5 h in the magnetic stirrer. After 5 h, a light yellow color solution was obtained and it is used as a carbon source for the CDs synthesis. To the above mixture 2 mL of sodium hydroxide (1 M) was added. The mixture was poured into the 100 mL Teflon-coated stainless steel autoclave and it was kept in a hot oven at 200 °C for 3 h. Once the reaction is complete, the autoclave was cooled at room temperature and it was opened carefully. The obtained dark brown solution shows the formation of RB-CDs. The resultant solution was centrifuged at 3500 rpm for 45 min. A clear brown solution containing RB-CDs was collected and stored in cold storage at 4 °C for further studies.
Characterization Methods
The UV − Vis double beam spectrophotometer (Lasany, LI- 2800) was used to examine the absorption properties of the synthesized RB-CDs. The scanning range for the samples was set at 200–800 nm. “UVPROBE” software was used to record the data. FTIR spectrometer (Thermo Nicolet) was used to study the functional groups present in the CDs around the range of 4000–400 cm−1. X-ray diffraction (XRD) make by “BRUKER” (wide-angle patterns in the range of 0° - 80°) was used to test the crystalline nature of RB-CDs. The surface of rice bran was observed using FEI Quanta FEG200 (FESEM). The average diameter and morphology of prepared RB-CDs was studied using HR-TEM (JEOL JEM-2100) by placing the CDs samples over the copper grid and dried at ambient temperature and fluorescence spectra (HORIBA JOBIN YVON Fluoromax-4 spectrometer). The X-ray photo electron spectroscopy (XPS) was carried out by Kratos Axis instrument with mono-energetic Al kα x-rays.
Quantum Yield Measurement
The fluorescence quantum yield (QY) of the self-surface passivated RB-CDs were examined with a solution of quinine sulfate 0.1 M H2SO4 (QY = 0.54%) as a reference at excitation wavelength of 370 nm [30]. The quantum yields of synthesized RB-CDs are calculated using the following equation
where, Qx and Qstd are the quantum yields for RB-CDs and quinine sulfate, ƞx2 and ƞstd2 are the refractive indices of solvent used; Ix and Istd are the integrated emission area and Ax and Astd are the optical densities of the carbon dots and quinine sulfate.
Catalytic Activity of RB-CDs
The reaction was carried out by using the methylene blue, sodium borohydride, and RB-CDs. Several experiments were conducted to optimize the concentration of dye, reducing agent and catalyst. Finally, a stock solution of 3 ppm MB was prepared in distilled water. Freshly prepared 10 mL of methylene blue was added to the 2 mL of prepared ice-cold NaBH4 (0.50 M) solution and 18 mL of H2O was added to it. Then, the solution was subjected to the visible light irradiation using a 400 W Xenon lamp. In the second reaction, to the same volume of solution, 2 mL of RB-CDs was added to the reaction mixture and the degradation reaction was monitored at diverse time intervals by using the UV-vis spectrophotometer.
Preparation of Fluorescent Ink for Patterning
The fluorescent ink was prepared by adding 10 mL of 5% PVA solution to 10 mL of synthesized RB-CDs and this mixture was thoroughly stirred in a magnetic stirrer for 30 min, resulting to get highly viscous fluorescent ink. The prepared ink was poured into an empty ink pen in the refill tube and drawing the different images on the paper and it was allowed to dry in air. The dried paper having images was placed under UV light at 365 nm to get fluorescent images.
Results and Discussion
Characterization of Synthesized RB-CDs
The fluorescent RB-CDs were synthesized by hydrothermal method using rice bran solution at 200 °C for 4 h. The formation of RB-CDs was initially confirmed by the observing the color change from light yellow to dark brown. The surface characteristics of RB-CDs obtained from rice bran was studied by HR-TEM studies. Figure 1a shows that the HR-TEM images were homogeneously dispersed. It is obviously reveals that the RB-CDs are virtually spherical in shape. In (Fig. 1b) shows the lattice fringe and the measured d-spacing value is about 0.20 nm corresponding to the (002) plane of the amorphous phase [31, 32], (Fig. 1c) shows the corresponding histogram and the average diameter of RB-CDs was 2.96 nm in the narrow range from 1 to 6 nm.
The crystalline nature of the RB-CDs was identified by the XRD. The (Fig. 2) shows the peaks at 2θ = 22.34°, 38.24°, 44.51° which corresponding to the (002), (100), and (101) planes of graphitic carbon. However, RB-CDs show a broad peak at 2θ =22.34°, and a weak peak around 2θ =44.51°, indicating the amorphous phase of the RB-CDs. The d-spacing of synthesized RB-CDs was calculated by Bragg’s eq. (2).
where, n is a positive integer (1), λ is the wavelength of incident X-rays (1.54 Å) and θ is a position of the plane. The d-spacing value is calculated for the prepared RB-CDs are approximately 0.40 nm and 0.20 nm for (002), and (101) peaks, amorphous nature is due to closely filled carbon atoms by alkyl chains [33].
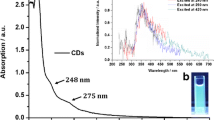
Figure 3a shows the UV-Visible absorption spectrum of synthesized RB-CDs. The synthesized RB-CDs exhibits two absorption peaks at 245 nm and 351 nm which corresponding to the π-π* transitions of C=C bonds (sp2 hybridization) of graphitic carbon core and the second peak attributed to n-π* transitions of the C=N/C=O bonds, which located on the surface or within the RB-CDs core [34,35,36]. The synthesized water dispersed RB-CDs shows green luminescence under the irradiation of 365 nm ultraviolet (UV). The clear position of the emission peak differs with the variation of excitation wavelength. There is a red shift in wavelength with a normalized intensity increases in the range from 380 nm to 430 nm, maximum emission wavelength was observed at 523 nm respectively (Fig. 3b). The quantum yield for the self-surface passivated RB-CDs was found to be 7.4%. Most commonly photoluminescence property of carbon dots is highly related to the size, shape and surface structure of the synthesized RB-CDs. High photoluminescence of RB-CDs is favorable for its applications, which is possibly appropriate in multi-color imaging, and photocatalyst extend to higher performance [37].
The functional groups of the prepared RB-CDs were identified by FTIR. Figure 4a displays the FTIR spectrum of the RB-CDs and the main characteristic absorption band appeared at 3275 cm−1, 1336 cm−1, and 1050 cm−1 parallel to stretching of O-H, C-H and C-N str vibrations. The band at 1625 cm−1 corresponding to C=C stretching of the aromatic hydrocarbons (sp2 carbon). These results confirmed the presence of carboxyl and hydroxyl groups in the water soluble RB-CDs [38]. The morphological characteristics of synthesized RB-CDs from rice bran were studied by field emission scanning electron microscope. Interestingly, the raw rice bran SEM image showed a rod-like shape as shown in (Fig. 4b) and the EDAX spectrum obviously (Fig. 4c) indicates the presence of various elemental compositions of RB-CDs such as 91.58 wt% of carbon and 8.42 wt% of oxygen in the sample. The reason for the biosynthesis of carbon nanoparticles (CNPs) is the electrostatic interactions such as hydrogen bond, bioorganic bond and capping molecules [39].
X-ray photoelectron spectroscopy (XPS) is used to identify the chemical structure and surface groups of the synthesized RB-CDs. Overall XPS full survey scan spectra in (Fig. 5a) showed two major peaks which confirmed that the presence of C, O on the surface of RB-CDs. Figure 5b shows deconvolution spectrum of C1s in the range of 282-293 eV, the three different peaks appeared at 285.12 eV, 286.65 eV, and 288.54 eV attributed to sp2 (C=C), C–H/and C=O respectively. Similarly in (Fig. 5c) shows the deconvoluted spectrum of O1s provides two dissimilar peaks at 530.6 eV and 533.1 eV corresponds to the C=O and C-OH groups, respectively. FTIR and XPS studies demonstrated that the surface of synthesized RB-CDs was functionalized with hydroxyl, carbonyl and carboxylic functional groups. The atomic percentage of Carbon, Oxygen was found to be ~75.8%, ~22.4% respectively.
Catalytic Activity of RB-CDs in the Reduction of Methylene Blue Using Sodium Borohydride (NaBH4)
The reduction of methylene blue by sodium borohydride (NaBH4) was done using prepared green catalyst (RB-CDs). The maximum absorption intensity for MB was observed at 664 nm [40, 41]. Figure 6a shows that the UV-Vis spectral responses for the reduction of methylene blue using sodium borohydride as a reducing agent in absence of catalyst (RB-CDs). Under the UV light irradiation there was no prominent change was observed in the intensity of MB even after 120 min without catalyst. The results of catalytic degradation of MB strongly suggested that the thermodynamic and kinetic feasibility of the prepared green catalyst RB-CDs by accelerating reaction rate through reducing the activation energy barrier between reactant and product. Addition of 2 mL of catalyst in the newly taken first reaction mixture (MB, NaBH4, & H2O), the degradation of MB reaction was started and this was noted as 0 min, as shown in (Fig. 6b). The intensity of methylene blue decreases linearly at the different time intervals from 0 to 30 min and reaction was completed within 30 min at the stage of no absorbance intensity was observed the and this confirms that the synthesized RB-CDs act as a proficient green catalyst to increase the rate of reduction/decomposition of the MB dye on the degradation reaction. The percentage of dye degradation in the presence and absence of the catalyst was calculated from the graph (Fig. 6c). After 120 min, degradation of Methylene blue was found to be 59.11% in the absence of green catalyst. In the presence of RB-CDs catalyst, the percentage of methylene blue dye was decomposed by 89.20% in 30 min. The color of the dye in the beginning and end of the degradation reaction was observed and corresponding photographs are shown in (Fig. 7a). The degradation of MB reaction could be clearly described through Langmuir-Hinshelwood mechanism. The MB dyes act as a electrophile and BH4− ions act as nucleophile. The simultaneous adsorption takes place on the surface of RB-CDs and the transfer of electrons from BH4− ions to methylene blue via green catalyst. RB-CDs act as the mediator for electron transfer and this implies in the minimizing the energy barrier between reactants and products [26, 42, 43]. Hence, the vast surface area and efficient electron-accepting potential of the prepared RB-CDs is the major reason of this tremendous catalytic activity [44]. Possible mechanism is shown in (Fig. 7b) and the reduction reaction of methylene blue is depicted in (Fig. 7c).
RB-CDs as Patterning Agents
In present there are number of dyes, polymers composites, and nanomaterials were used as patterning agents to identify the counterfeit printings and written hints in the different backgrounds. Herein, the synthesized RB-CDs were used as patterning agents due to their less toxic and excellent FL properties. The green fluorescent RB-CDs were used to draw different patterns on the commercial white paper further it was dried at room temperature. Figure 8a shows the image of white paper in naked eye view there were no significant patterns observed in it. On the other hand, the green fluorescent patterns were observed under the UV light irradiation (Fig. 8b). This confirmed that the synthesized RB-CDs can be efficiently utilized as a proficient patterning agent for anti-counterfeiting applications. Moreover, the RB-CDs are cost-effective and eco-friendly fluorescent ink for anti-counterfeiting applications, and could be easily removed by washed off using water [45].
Conclusion
The present work reveals the synthesis of fluorescent carbon dots (RB-CDs) from cheap, eco-friendly and easily available bio waste rice bran as carbon precursor by simple, low cost and one-pot hydrothermal process. The resultant RB-CDs exhibited bright green fluorescence and fluorescence quantum yield (QY) was found to be 7.4%. The HR-TEM studies revealed that the RB-CDs are spherical shape with 2.96 nm size. The FTIR results confirmed that the presence of -OH and -COOH groups on the surface of the RB-CDs which is evident for the high water dispensability. The synthesized RB-CDs showed significant catalytic ability on the reduction of methylene blue dye by NaBH4, the dye was decomposed by 89.20% in 30 min. The obtained RB-CDs used as a promising catalyst on the degradation of methylene blue dye and also used as patterning agent for anti-counterfeiting applications. Therefore, RB-CDs can be used as an eco- friendly nanomaterial for the future by serving as a potential candidate for environment safety, sensing and bioimaging applications.
Data Availability
All data generated or analysed during this study are included in this published article.
References
Lim SY, Shen W, Gao Z (2015) Carbon quantum dots and their applications. Chem Soc Rev 44:362–381
Chandrasekaran P, Arul V, Sethuraman MG (2020) Ecofriendly synthesis of fluorescent nitrogen-doped carbon dots from Coccinia grandis and its efficient catalytic application in the reduction of methyl Orange. J Fluoresc 30:103–112
Atchudan R, Edison TNJI, Sethuraman MG, Lee YR (2016) Efficient synthesis of highly fluorescent nitrogen-doped carbon dots for cell imaging using unripe fruit extract of Prunus mume. Appl Surf Sci 384:432–441
Zhang J, Yu S-H (2016) Carbon dots: large-scale synthesis, sensing and bioimaging. Mater Today 19:382–393
Wang Y, Hu A (2014) Carbon quantum dots: synthesis, properties and applications. J Mater Chem C 2:6921–6939
Pal T, Mohiyuddin S, Packirisamy G (2018) Facile and green synthesis of multicolor fluorescence carbon dots from curcumin: in vitro and in vivo bioimaging and other applications. ACS omega 3:831–843
Radhakrishnan K, Panneerselvam P, Marieeswaran M (2019) A green synthetic route for the surface-passivation of carbon dots as an effective multifunctional fluorescent sensor for the recognition and detection of toxic metal ions from aqueous solution. Anal Methods 11:490–506
Cao L, Wang X, Meziani MJ, Lu F, Wang H, Luo PG, Lin Y, Harruff BA, Veca LM, Murray D, Xie SY, Sun YP (2007) Carbon dots for multiphoton bioimaging. J Am Chem Soc 129:11318–11319
Baker SN, Baker GA (2010) Luminescent carbon nanodots: emergent nanolights. Angew Chemie Int Ed 49:6726–6744
Zheng XT, Ananthanarayanan A, Luo KQ, Chen P (2015) Glowing graphene quantum dots and carbon dots: properties, syntheses, and biological applications. Small 11:1620–1636
Esteves da Silva JCG, Gonçalves HMR (2011) Analytical and bioanalytical applications of carbon dots. TrAC - Trends Anal Chem 30:1327–1336
Wei J, Shen J, Zhang X, Guo S, Pan J, Hou X, Zhang H, Wang L, Feng B (2013) Simple one-step synthesis of water-soluble fluorescent carbon dots derived from paper ash. RSC Adv 3:13119–13122
Cho M-J, Park S-Y (2017) Preparation of poly (styrene)-b-poly (acrylic acid)-coupled carbon dots and their applications. ACS Appl Mater Interfaces 9:24169–24178
Gunjal DB, Gurav YM, Gore AH, Naik VM, Waghmare RD, Patil CS, Sohn D, Anbhule PV, Shejwal RV, Kolekar GB (2019) Nitrogen doped waste tea residue derived carbon dots for selective quantification of tetracycline in urine and pharmaceutical samples and yeast cell imaging application. Opt Mater (Amst) 98:109484
Jiang H, Chen F, Lagally MG, Denes FS (2010) New strategy for synthesis and functionalization of carbon nanoparticles. Langmuir 26:1991–1995
Hu S-L, Niu K-Y, Sun J, Yang J, Zhao NQ, du XW (2009) One-step synthesis of fluorescent carbon nanoparticles by laser irradiation. J Mater Chem 19:484–488
Ray SC, Saha A, Jana NR, Sarkar R (2009) Fluorescent carbon nanoparticles: synthesis, characterization, and bioimaging application. J Phys Chem C 113:18546–18551
Sachdev A, Matai I, Kumar SU, et al (2013) Supporting information a novel one-step synthesis of PEG passivated multicolour fluorescent carbon dots for potential biolabeling application. 1–9
Sachdev A, Matai I, Gopinath P (2014) Implications of surface passivation on physicochemical and bioimaging properties of carbon dots. RSC Adv 4:20915–20921
Vandarkuzhali SAA, Natarajan S, Jeyabalan S, Sivaraman G, Singaravadivel S, Muthusubramanian S, Viswanathan B (2018) Pineapple peel-derived carbon dots: applications as sensor, molecular keypad lock, and memory device. ACS omega 3:12584–12592
Vandarkuzhali SAA, Jeyalakshmi V, Sivaraman G, Singaravadivel S, Krishnamurthy KR, Viswanathan B (2017) Highly fluorescent carbon dots from pseudo-stem of banana plant: applications as nanosensor and bio-imaging agents. Sensors Actuators B Chem 252:894–900
Radhakrishnan K, Panneerselvam P (2018) RSC advances green synthesis of surface-passivated carbon dots from the prickly pear cactus as a fluorescent probe for the dual detection of arsenic (III) and hypochlorite ions from drinking water. 30455–30467
Devi RR, Arumughan C (2007) Phytochemical characterization of defatted rice bran and optimization of a process for their extraction and enrichment. Bioresour Technol 98:3037–3043
Zhang Q, Quan X, Wang H et al (2017) Constructing a visible-light-driven photocatalytic membrane by gC 3 N 4 quantum dots and TiO 2 nanotube array for enhanced water treatment. Sci Rep 7:1–7
Thomas J, Yoon M (2012) Applied catalysis B: environmental facile synthesis of pure TiO 2 ( B ) nanofibers doped with gold nanoparticles and solar photocatalytic activities. Applied Catal B Environ 111–112:502–508
Gupta N, Singh HP, Sharma RK (2011) Metal nanoparticles with high catalytic activity in degradation of methyl orange: an electron relay effect. J Mol Catal A Chem 335:248–252
Vargas AMM, Cazetta AL, Kunita MH, Silva TL, Almeida VC (2011) Adsorption of methylene blue on activated carbon produced from flamboyant pods (Delonix regia): study of adsorption isotherms and kinetic models. Chem Eng J 168:722–730
Zhu S, Meng Q, Wang L, Zhang J, Song Y, Jin H, Zhang K, Sun H, Wang H, Yang B (2013) Highly photoluminescent carbon dots for multicolor patterning, sensors, and bioimaging. Angew Chemie Int Ed 52:3953–3957
Wang W, Wang Z, Liu J, Peng Y, Yu X, Wang W, Zhang Z, Sun L (2018) One-pot facile synthesis of Graphene quantum Dots from Rice husks for Fe3+ sensing. Ind Eng Chem Res 57:9144–9150
Gajendiran J, Vijayakumar V, Senthil VP, Parthasaradhi Reddy C, Ramana Ramya J, Gokulraj S (2020) Ionic liquid assisted wet chemical synthesis CdS quantum dots and their structural, morphological, optical, electrochemical, photocatalytic, antibacterial and hemocompatibility characterization. Optik (Stuttg) 213:164638
Lu J, Yang J, Wang J et al (2009) One-Pot Synthesis of Fluorescent Carbon Graphene by the Exfoliation of Graphite in Ionic Liquids 3:2367–2375
Ming H, Ma Z, Liu Y, Pan K, Yu H, Wang F, Kang Z (2012) Large scale electrochemical synthesis of high quality carbon nanodots and their photocatalytic property. Dalt Trans 41:9526–9531
Zhao S, Lan M, Zhu X, Xue H, Ng TW, Meng X, Lee CS, Wang P, Zhang W (2015) Green synthesis of bifunctional fluorescent carbon dots from garlic for cellular imaging and free radical scavenging. ACS Appl Mater Interfaces 7:17054–17060
Sun Y-P, Zhou B, Lin Y, Wang W, Fernando KAS, Pathak P, Meziani MJ, Harruff BA, Wang X, Wang H, Luo PG, Yang H, Kose ME, Chen B, Veca LM, Xie SY (2006) Quantum-sized carbon dots for bright and colorful photoluminescence. J Am Chem Soc 128:7756–7757
Peng H, Travas-Sejdic J (2009) Simple aqueous solution route to luminescent carbogenic dots from carbohydrates. Chem Mater 21:5563–5565
S. T, D. RS (2016) Green synthesis of highly fluorescent carbon quantum dots from sugarcane bagasse pulp. Appl Surf Sci 390:435–443
Wang Z, Zeng H, Sun L (2015) Graphene quantum dots: versatile photoluminescence for energy, biomedical, and environmental applications. J Mater Chem C 3:1157–1165
Dhandapani K, Venugopal K, Kumar JV (2019) Ecofriendly and green synthesis of carbon nanoparticles from rice bran: characterization and identification using image processing technique. Int J Plast Technol 23:56–66
Arul V, Sethuraman MG (2019) Hydrothermally green synthesized nitrogen-doped carbon dots from phyllanthus emblica and their catalytic ability in the detoxification of textile effluents. ACS omega 4:3449–3457
Arul V, Edison TNJI, Lee YR, Sethuraman MG (2017) Biological and catalytic applications of green synthesized fluorescent N-doped carbon dots using Hylocereus undatus. J Photochem Photobiol B Biol 168:142–148
Edison TJI, Sethuraman MG (2012) Instant green synthesis of silver nanoparticles using Terminalia chebula fruit extract and evaluation of their catalytic activity on reduction of methylene blue. Process Biochem 47:1351–1357
Edison TNJI, Atchudan R, Kamal C, Lee YR (2016) Caulerpa racemosa: a marine green alga for eco-friendly synthesis of silver nanoparticles and its catalytic degradation of methylene blue. Bioprocess Biosyst Eng 39:1401–1408
Han Y, Huang H, Zhang H, Liu Y, Han X, Liu R, Li H, Kang Z (2014) Carbon quantum dots with photoenhanced hydrogen-bond catalytic activity in aldol condensations. ACS Catal 4:781–787
Arul V, Sethuraman MG (2018) Facile green synthesis of fluorescent N-doped carbon dots from Actinidia deliciosa and their catalytic activity and cytotoxicity applications. Opt Mater (Amst) 78:181–190
Anand SR, Bhati A, Saini D, Gunture, Chauhan N, Khare P, Sonkar SK (2019) Antibacterial nitrogen-doped carbon dots as a reversible “fluorescent nanoswitch” and fluorescent ink. ACS Omega 4:1581–1591
Acknowledgments
The authors wish to acknowledge DST-FIST (fund for improvement of S&T) for the financial assistance at the Department of Chemistry, SRM Institute of Science and Technology (grant no. SR/FST/CST-266/2015(c)).
Author information
Authors and Affiliations
Contributions
Vinoth Kumar Jothi: Conceptualization, Methodology, Writing – original draft. Kavitha Ganesan: Data curation, Formal analysis. Arulmozhi Rajaram: Visualization. Abirami Natarajan: Supervision, Investigation.
Corresponding author
Ethics declarations
Conflict of Interest
All authors declare no conflicts interest to disclose.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jothi, V.K., Ganesan, K., Natarajan, A. et al. Green Synthesis of Self-Passivated Fluorescent Carbon Dots Derived from Rice Bran for Degradation of Methylene Blue and Fluorescent Ink Applications. J Fluoresc 31, 427–436 (2021). https://doi.org/10.1007/s10895-020-02652-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-020-02652-6