Abstract
A simple method for the green synthesis of fluorescent carbon quantum dots (CQDs) has been developed by using roasted chickpea as carbon source in one-step without using any chemical. Interestingly, not only the carbon source of CQDs and the whole synthesis procedure are environmentally friendly, but also the synthesized CQDs have shown many advantageous properties such as high fluorescence intensity, excellent photostability, and good water solubility. CQDs which were firstly synthesized from roasted–chickpeas by a microwave-assisted pyrolysis have been characterized using UV-vis absorption spectroscopy, fluorescence spectroscopy, fourier transform infrared spectroscopy (FTIR) spectroscopy, X-ray diffraction (XRD) technique and transmission electron microscopy (TEM). In addition, the details of the structure have been revealed by the electron diffraction (SAED; selected-area electron diffraction) method based on the TEM images. The synthesized CQDs emits blue fluorescence under UV light (at 365 nm). A microwave oven (350 watts) was used to prepare CQDs in 120 s. The effect of various metal ions on the fluorescence intensity of CQDs was investigated in order to determine its utality in the detection of metal ions. It was determined that 1.38 mg/L of Fe3+ ions quenched the fluorescence intensity of the CQDs by 65%. The linear range is observed between 11.25 and 37.50 μM with limit of detection (LOD) and limit of quantification (LOQ) of 2.74 μM and 8.22 μM, respectively. CQDs which are highly selective and sensitive for Fe3+ ions was used to determine Fe3+ ions in certified reference material (CRM-SA-C). Fe3+ ions concentration was determined with precision values of more than 95% as intra-day and inter-day relative standard deviation (RSD%) is 5 at room temperature.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Carbon consists of various structures with different morphologies which has many allotropic forms including diamonds, carbon black, carbon nanotubes, fullerene, and graphene [1]. Among these forms, graphene is the most studied and forms the basis of carbon-based nanomaterials used in environmental determinations [2, 3]. CQDs are a member of fluorescent carbon-based nanomaterials as discrete, semi-spherical particles with sizes less than 10 nm, which consist of typical or disordered graphene [4]. Carbon dots generally have a sp2 conjugated core and have oxygen composed of multiple oxygen-containing species represented by the groups such as carboxyl, hydroxyl and aldehyde. Fluorescent carbon nanoparticles with about 1 nm of vertical sizes were first discovered in 2004 by Xu et al. in the electrophoretic fraction of the arc-discharge method [5]. Xu et al. reported that their discoveries would have interesting features apart from other nanomaterials. Afterwards, “these interesting carbon nanomaterials”, also referred to as carbon dots, or carbon nanodots, or carbogenic quantum dots, only 2 years later got the expected attention with the studies carried out by Sun’s group [6].
The sizes of CQDs are largely related to the preparation method independent of the starting material. During the synthesis of CQDs, the reaction temperature and time can be varied and CQDs of different sizes can be obtained. Pan et al. used graphene oxide as the starting material and implemented different thermal reduction temperature and hydrothermal deoxidation basicity to obtain two different types of CQDs having an average size of 9 nm and about 3 nm [7, 8]. CQDs are typically composed of C and H elements. However, it has been revealed in many studies that the surface structures of CQDs are usually oxidized to hydroxyl (–OH), epoxy/ether (C-O), carbonyl (C=O) and carboxylic acid (COOH) groups. The content of CQDs structures can be analyzed by FTIR and XPS analyzes. The presence of any of –OH, –C-O, –C=O and –COOH bonds indicates that the surfaces of CQDs are functionalized with hydroxyl, carbonyl and carboxylic acid groups. This functionality allows CQDs to be better soluble in water and also facilitates the surface structures of CQDs to be modified more easily. CQDs have become an alternative to semiconductor quantum dots (QD) and organic dyes over time due to their high optical-chemical stability and biocompatibility. CQDs have replaced semiconductor quantum dots (QD) due to their useful properties such as low metabolic degradation, bright fluorescence, low toxicity, water solubility and low photodegradation [9, 10].
CQDs have attracted the greatest attention with their physical properties such as the above-mentioned high biocompatibility, easy functionalization, water solubility and low toxicity as well as adjustable photoluminescence (FL) properties [3, 11]. The reason for the greatest attention that CQDs can exhibit an excitation wavelength-dependent [12] and excitation wavelength-independent [13] photoluminescence (FL) behavior. Also, because of their photoluminescence (FL) property CQDs can be used in a variety of applications such as ion determination [14,15,16], optical sensors [17], photo-catalysis [18], bio-imaging [19], drug delivery [20], white light-emitting diodes [21], and optoelectronic devices [22] and energy conversion / storage.
In addition to their many advantages in the application, the fact that they can be produced easily in an environmentally friendly way, green synthesis, without using toxic substances has recently increased the synthesis of CQDs. In the synthesis of CQDs, simple compounds such as citric acid, urea and ascorbic acid, and a variety of foods, plants, waxes and wastes as a carbon source can be used as the starting material. The synthesis of CQDs can generally be performed in a top down method or bottom up method. In the top down method, large molecules such as carbon nanotube and graphite are broken down into CQDs by physical and/or chemical processes. Various techniques have been developed for the synthesis of CQDs by the top down method. Some of these are techniques such as laser-ablation, arc-discharge, electrochemical synthesis, etc. Unlike the top down method, the formation of CQDs is carried out by growing the structures of atomic or molecular size by chemical reactions in the bottom up method. Examples of the bottom up method include hydrothermal, microwave and ultrasonic-chemical methods, as well as several ways of requiring various combustion or thermal processes.
Fe3+ ion is a clinically required metal ion which also has environmental importance. Fe3+, an important trace element in the biological processes, has an important role in the chelation with various regulatory proteins. Therefore, the presence of Fe3+ ions in the biological systems should be effectively controlled and monitored [23, 24]. Fe3+ ion can be determined in the soil samples by a variety of AAS and ICP methods [24,25,26,27,28]. These methods require expensive equipments, professional workers, adsorbents and separation reagents [29,30,31], as well as time-consuming separation processes such as extraction and re-precipitation [32]. Therefore, it is important to develop a simple, environmentally friendly, non-time consuming and cost-free method to determine the iron content in the soil samples.
Spectrofluorometric methods are simple and cost-effective analytical methods and they also have inherent advantages such as selectivity and sensitivity. The use of fluorescent molecules [33, 34] or materials [14,15,16, 35,36,37] in the sensitive and selective determination of metal ions is a very effective way. These molecules or materials contain functional groups such as hydroxyl and carbonyl, which can easily interact with the metals. In the literature, many fluorescent ligands have been used for metal determination. However, the synthesises of most of these ligands require any toxic reactive reagent and are carried out in multiple steps. In addition, water dissolution problems restrict their application fields. Therefore, the use of fluorescent nanomaterials for metal determinations provides many advantages such as not being toxic, being soluble in water and being easily synthesized.
In this study, carbon quantum dots (CQDs) were synthesized using the stale roasted chickpeas, as a carbon source, by a simple, easy and inexpensive method without any chemicals. In the fabricate of CQDs, the green synthesis was performed with a microwave-assisted method for a short period of 2 min, neither an organic solvent nor a surface passivation chemical was required. When spectroscopically assessed, they have been stated that CQDs can be used for the fluorometric determination of Fe3+ ions. There are many studies in the literature using CQDs in which Fe3+ ions are determined by fluorometric methods [38,39,40,41]. In most of these studies, applications of Fe3+ ion detection are performed in water samples [38,39,40,41]. In the literature, we have not yet encountered a study in which Fe3+ ions were determined in soil samples using CQDs. Availability of the proposed method was proven by analyzing Fe3+ ions of the certified reference material (CRM-SA-C) under the optimized conditions. The proposed method has great benefits compared to other methods based on the determination of Fe3+ ions: a) the synthesis of CQDs does not require any chemical, b) the starting material is cheap and environmentally friendly, c) the synthesis process of CQDs does not involve complex processes, d) the production time of CQDs is quite short, and e) CQDs and Fe3+ interaction is quite stable. In addition, it should be noted that CQDs have the potential to be used in bio-imaging applications.
Experimental Section
Materials
The roasted chickpeas were purchased from a local supermarket in a district of Çorum (in Turkey) as a product, the shelf-life of which was expired, and used in the experimental studies by pulverizing thoroughly using a blender. All chemicals used in the study are of analytical grade and do not require any purification. The solutions (analytical grade, 1000 mg/L) of the salts of LiNO3, NaNO3, KNO3, Mg(NO3)2, Ca(NO3)2, Sr(NO3)2, Ba(NO3)2, Be4O(C2H2O2)6, SeO2, Cr(NO3)3, Mn(NO3)2, Fe(NO3)3, Co(NO3)2, Ni(NO3)2, Cu(NO3)2, Zn(NO3)2, AgNO3, Cd(NO3)2, H(AuCl4), Hg(NO3)2, Al(NO3)3, Pb(NO3)2, Bi(NO3)3, H3BO3, Sb2O3, SnCl4, H3AsO4, Pd(NO3)2, TlNO3, Y(NO3)3, Sc2O3, NH4VO3, (NH4)2WO4, (NH4)6Mo7O24 and (NH4)2TiF6 were purchased from Merck (Darmstadt, Germany). Quinine sulfate, 98%, suitable for fluorescence, was supplied by Fluka (Buch, Swetzerland). Certified reference material (CRM-SA-C) was supplied from High Purity Standards, Inc. The digestion of CRM-SA-C was carried out by utilizing an ultra-pure concentrated solution mixture of HNO3, HCl, HF and H2O2 in a MARS 6 - Microwave Digestion System – CEM Corporation.
Synthesis of Carbon Quantum Dots (CQDs) from Chickpeas
As shown in Scheme 1, CQDs were synthesized by the microwave-assisted pyrolysis of the roasted chickpeas. The roasted chickpeas are obtained by baking and roasting the chickpeas three times. The stale roasted chickpeas purchased from the supermarket were grounded using a blender until a homogenous fine powder was obtained. 2 g of the roasted chickpeas was digested by adding 40 mL of ultra-pure water in an ultrasonic bath for 15 min. This mixture was transferred to a large volume beaker (250 mL) and then heated in microwave oven - Altus Corporation at 350 watts for 2 min. The solution cooled to room temperature was centrifuged at 3000 rpm for 15 min. The centrifugation was performed by Allegra X-30R centrifuge (Beckman Coulter). The cream solid was removed by taking the supernatant liquid portion. The liquid portion was filtered through a blue filter paper, a 0.45 μm and 0.2 μm syringe filter membrane respectively. Re-centrifugation was carried out at 12000 rpm for 15 min to remove the aggregated particles from the solution. The CQDs-containing liquid portions were combined. The resulting transparent pale yellow CQD solution was stored in the refrigerator at 4 °C for later use.
Characterization of CQDs
All fluorescence measurements were performed at room temperature using a Photon Technologies International Quanta Master-Spectrofluorimeter (model QM-4/2006) spectrofluorometer. The fluorescence spectra of CQDs diluted with ultra pure water were recorded at different excitation wavelengths (λex = 300, 310, 320, 330, 340, 350, 360, 370, 380 and 400 nm) at an interval of 10 nm. The UV − vis absorption spectrum of CQDs was obtained using an Analytik Jena Specord 210 UV − vis spectrophotometer. The UV − vis absorption measurements of CQDs were performed by diluting with water in a wavelength range of 200–800 nm at room temperature.
The presence of the surface functional groups was determined by Fourier transform infrared spectroscopy. The FTIR spectrum obtained using Perkin-Elmer 1600 spectrophotometer was recorded in a spectral range of 400–4000 cm−1 at room temperature.
Transmission electron microscope (TEM) images were recorded at 200 kV using a Talos F200S microscope (FEI, USA) in the Research Laboratory of Bayburt University. For TEM characterization, 1 mL of the stock solution of CQDs was diluted with alcohol and then treated with an ultrasonic probe for a short time. A drop of this solution was placed on a carbon-coated copper grid and allowed to dry at room temperature, and the morphological images of CQD were taken.
The crystallinity and phase purity of the synthesized CQDs were analyzed using X-Ray Diffractometer (XRD). For the XRD analysis, an aqueous solution of CQDs was poured onto a glass slide at a size of 1 × 2 cm2, and the analysis was performed with Bruker (D8 Discover XRD) XRD by applying Cu Kα irradiation (λ = 1.54 Å) at 40 kV and 30 mA.
Fluorescence Quantum Yield (FQY) of CQDs
The relative fluorescence quantum yield (FQY) of CQD was calculated according to Eq. 1 by using quinine sulfate (in 0.1 mol L−1 H2SO4) having a fluorescence quantum yield of 54% as a reference [42].
Here, φx and φstd are the fluorescence quantum yields of CQDs and quinine sulfate, respectively. mx refers to the slope when the absorbance values of CQDs are plotted against the areas of the emission spectrum. In a similar graphic, the slope for the quinine sulfate represents mstd. ɳ indicates the refractive index of the solvent. The refractive index of the aqueous solutions is 1.33. The ɳx/ɳstd value was taken as 1, because the solvents of the aqueous quinine sulfate and CQDs solutions were the same. In order to make the calculation in Eq. 1, the absorbance spectra of the CQDs and quinine sulfate which have the aqueous solutions of different concentrations were measured. Then, the integrated fluorescence intensities of these solutions for which the emission spectra were measured by an excitation at 320 nm were determined. The absorbance values of quinine sulfate and CQDs at 320 nm were plotted against the integrated fluorescence intensities and the slopes of the curves (mx and mstd) were determined. These graphics are given in the Electronic Supplementary Material (Online Resource 1).
Preparation of Reference Material for Analysis
0.5 g sandy soil certified reference material (CRM-SA-C Sandy Soil C) was digested. This process was carried out in a closed system microwave digester using concentrated solutions of 1.5 mL of HN03, 4.5 mL of HCl, 1.0 mL of HF and 2.0 mL of H202. After microwave digestion, the solution was filtered through a 0.45 μm cellulose nitrate membrane and evaporated. The final volume was then brought to 25 mL with ultra-pure water. This solution prepared was used by maintaining at 4 °C in a refrigerator during the experimental studies.
Determination of Fe3+ Ion with CQDs
CQDs were used for the selective determination of Fe3+ ions. Determination of Fe3+ ions was realized at room temperature with an excitation wavelength of 320 nm for a reaction time of 1 min. The maximum fluorescence emission intensity of the solution (blank) containing equal volume of deionized pure water (2 mL) and CQDs (2 mL) was determined as 435 nm. Thirty five kinds of metal ions such as Li+, Na+, K+, Mg2+,Ca2+, Sr2+, Ba2+, Be2+, Se2+, Cr3+, Mn2+, Fe3+, Co2+, Ni2+, Cu2+, Zn2+, Ag+, Cd2+, Au3+, Hg2+, Al3+, Pb2+, Bi3+, B+, Sb3+, Sn2+, As2+, Pd2+, Tl+, Y3+, Sc 3+, V3+ W2+, Mo2+ and Ti4+ were used in order to determine the selectivity of CQDs for Fe3+ ions. To examine the effect of this metal ions on the fluorescence emission spectrum of CQDs, 2.0 mL of 50 μM metal ion solution was added to the aqueous solution of CQDs (2 mL). 1 min later, the fluorescence emission spectrums was measured. A fluorometric titration is performed to determine the linear concentration range for Fe3+ ions determination by the synthesized CQDs. 2.0 mL of aqueous CQDs solution were added to the tubes, followed by increasing concentrations of Fe3+ ions (in the range of 1.88–45.00 μM), and final volumes of the tubes were brought to 4 mL with ultra-pure water. These solutions were kept at room temperature for 1 min and their florescence emission spectra were recorded by exciting at 320 nm. External calibration method was used for the quantitative analysis of Fe3+ ions in the sandy soil certified reference material (CRM-SA-C). Firstly, the solution of this sandy soil material was prepared for analysis. For this, 2 ml of CQDs solution was added to the certified reference material in an appropriate volume and the final volume was brought to 4 mL with ultra-pure water. For the calibration graphic, increasing amounts (in the appropriate concentration range) of standard Fe3+ ions were added to 2 mL of CQDs solutions and the final volume was brought to 4 mL with ultra pure water. Fluorescence emission spectra of these solutions were recorded after 1 min of the reaction time. The fluorescence intensities (F) of the standard Fe3+ ions solutions at 435 nm were plotted against the standard Fe3+ ions concentrations and the equation of the calibration curve was determined (in Eq. 2). At the wavelength of 435 nm, the measured fluorescence intensity for the sandy soil certified reference material solution was replaced by F in this equation, so that the concentration of Fe3+ ions was calculated ([Fe3+]). This calculation has been made by using the intra-day and inter-day analysis measurements for the sandy soil certified reference material.
Results and Discussion
Synthesis and Characterization of CQDs
Microwave-assisted pyrolysis has been accepted as an easy and effective method for the synthesis of CQDs as it is a time-saving, energy saving and cost-effective method, and provides a convenient process as compared to the other synthetic methods [43]. The microwave-assisted method provides intensive and homogenous energy to the reaction system [44]. In this study, the proposed synthesis method carried out using microwave irradiation has been a very simple and effective method. As shown in Scheme 1, the synthetic process took only 2 min and the fabricated CQDs exhibited a fluorescence quantum yield of 1.8%.
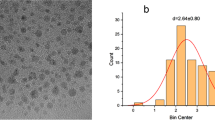
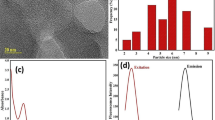
The morphological shapes and sizes of the synthesized CQDs are shown in Figs. 1 and 2 by different sample preparation techniques. The synthesized CQDs solution were diluted to the ratio of 1:1 with alcohol, treated with ultrasonic probe for 2 min and TEM images were obtained. In this TEM image shown in Fig. 1, it is seen that the CQDs’s structures are agglomerated and their sizes are distributed on a very wide scale (5–100 nm). The undiluted aqueous CQDs solution was analyzed in TEM to understand the effect of sample preparation difference on the TEM image. This TEM image is shown in Fig. 2. It is seen from the TEM image (in Fig. 2a) on a scale size of 50 mm that the structures of CQDs that do not undergo ultrasound were much more aggregated. In Fig. 2b, it is clearly seen that the shapes of CQDs with a size range of 4.5–10.3 nm are spherical. It is clearly understood from the diffusion rings of SAED (by electron diffraction technique) pattern and TEM images (Fig. 2b) that the structures of CQDs with an average diameter of 8.7 nm are amorphous, but not crystal [45].
The XRD (X-Ray Diffractometer) graphic of CQDs (Fig. 3a) shows a large diffraction peak at 2θ = 23.8°. A wide peak around 23.8° corresponding to the 002 plane of the graphite was observed in the XRD graphic of the CQDs. According to the Bragg’s Law, the interlayer spacing of the CQDs (d) was calculated as 0.374 nm. This value is a bit more than that of the interlayer spacing of the graphite (0.33 nm) [46]. This suggests that CQDs have some functional goups such as O-H and C=O on the surface of the graphitic sheets within the CQDs consistenting with the previous studies [47]. The CQDs obtained are amorphous and do not have crystalline phases in their structures. Although these synthesized CQDs consist of a large number of oxygen-containing groups, the fact that they have carbon atoms tightly packed by the surface functionalities can be shown as the cause of these amorphous structures [48]. In addition, the diffusion rings in the SAED (Selected Area Electron Diffraction) pattern of the TEM image taken at a magnification of 10 nm in Fig. 2c also support that the structure has not a crystal but an amorphous structure [45].
The synthesized CQDs were also characterized by Fourier transform infrared (FTIR) spectroscopy. FTIR analysis was performed to determine the surface functional groups of CQDs and the results are shown in Fig. 3b. The strong absorption peak (3339 cm−1) between 3300 and 3500 cm−1 belongs to OH stretching vibration [49]. It is well known that C-H stretching vibrations are between 2800 and 2990 cm−1 [50, 51]. Therefore, the absorption peaks at 2975 cm−1 and 2932 cm−1 just below 3000 cm−1 depend on aliphatic C-H stretching vibrations. The absorption peak at 1650 cm−1 belonging to C=O stretching vibrations were evident in the spectrum [52]. The presence of the absorbance bands at 1380–1400 cm−1 proves the existence of C-H bending vibration on the surface of CQDs [53]. The absorption peaks of 1087 cm−1 and 1044 cm−1 are due to C-O groups of the different functional moieties (such as C-O-H, O=C-O, C-O-C and epoxide) [54, 55].
Optical Properties of CQDs
The optical properties of the synthesized CQDs were determined by UV-vis and fluorescence spectroscopy. The resulting spectra are shown in Fig. 4. UV-vis absorption spectrophotometry was performed for analysis of the characteristic peaks proving the formation of CQDs. The absorption spectra of CQDs exhibits a typical optical absorption with a tail shape extending from the UV to the visible region. This is evident in the UV-vis spectrum of ten-fold diluted CQDs in a wavelength range of 200–800 nm in Fig. 4a. While no absorbance is observed in the visible region (400–700 nm) in the spectrum, it is observed in the ultraviolet region. The aqueous solution of the fluorescent CQDs synthesized shows an absorption peak of about 250 nm. This peak can be associated with π – π* transitions of the conjugated C=C bonds (C–C=C–C=C) [56, 57]. Furthermore, a weak shoulder at about 322 nm between 300 to 250 nm in the absorbance spectrum of the CQD is related to the n – π∗ transitions [58]. It is also clearly visible to the naked eye that the CQD excited by a UV lamp (365 nm) shows a strong blue fluorescence (inset in Fig. 4a: (right)). Another optical method that proves the formation of CQDs is the characteristic emission analysis performed by fluorescence spectroscopy. The effect of different wavelength excitation on the emission spectra of CQDs is given in Fig. 4b. In fluorescence spectroscopy, it was seen that when the aqueous solution of CQDs was excited at different wavelengths ranging from 360 to 400 nm, the emission peaks shifted to long wavelengths and fluorescence intensities were decreased. However, when CQDs solution were excited with different wavelengths between 300 and 350 nm, it was observed that the maximum wavelength at which the maximum emission occurred remained constant at about 435 nm. Thus, it was understood that CQDs showed an excitation-dependent property between 360 and 400 nm and an excitation-independent property between 300 and 350 nm. In order to better analyze the change in the emission of CQDs in the studies with metal ions, it was decided that it would be appropriate to excite CQDs in the range (300–350 nm) where the emission wavelength is not dependent on the excitation wavelength.
Thus, the wavelength of 320 nm, which has the highest fluorescence intensity in flourescence spectra of CQDs, is chosen as the excitation wavelength. The maximum emission of CQDs excited at a wavelength of 320 nm was observed at 435 nm.
Possible Mechanism of CQDs’s Fluorescence Response to Fe3+ Ions
UV-vis spectroscopy was used to understand the mechanism of interaction between CQDs and Fe3+ ion (Fig. 5). For this purpose, the absorbance spectra of the aqueous solutions of Fe3+ ion, CQDs and (Fe3+– CQDs) were measured in a wavelength range of 200–800 nm, and the maximum absorbances were found at 297 nm, 250 nm and 265 nm, respectively. Figure 5 shows that the UV-vis absorption peak of the CQDs containing Fe3+ ions at 250 nm exhibited a shift to the red by changing to 265 nm. The absorption peak seen at 265 nm by mixing CQDs with Fe3+ ions does not coincide with the absorption peak of Fe3+ ions (at 298 nm). This event of (Fe3+- CQDs) can be explained by the chelation of Fe3+ ions with the surface functional groups of CQDs such as -C=O and -OH. Strong interactions of CQDs with Fe3+ ions may promote the fluorescence quenching effect by inhibiting the radiative recombination of excitons. This case may have indicated a possible charge transfer [59].
Fluorescence Quenching Mechanism by the Effect of Fe3+ Ions
The fluorescence intensity of CQDs solution is exhibited a significant decrease with present of Fe3+ ions. CQDs to which different concentrations of Fe3+ ions were added was excited at 320 nm and its fluorescence spectra were measured (Fig. 6a). Analysis of fluorescence quenching mechanism of CQDs caused by Fe3+ ions, was performed with Stern-Volmer equation (Eq. 3) [60].
The Stern-Volmer relationship describes the efficacy of quenching the fluorophore by a quencher. F0 and F are the fluorescence intensity of CQDs without and with Fe3+ ions, respectively. KSV is Stern − Volmer quenching constant and [Q] is the concentration of the quencher. This equation represents the relationship between the relative fluorescence intensity (F0/F) and the concentration of Fe3+ ions. If a system fits to the Stern - Volmer equation when a graphic of [Q] versus F0/F is plotted, then a linear line is obtained, which intersects the y-axis with a slope of KSV. In the experimental studies, Fig. 6b shows that when a graphic of the relative fluorescence intensity of CQDs versus the concentration of Fe3+ ions is plotted, it is not linear for all concentration. The experimental data fits to the Stern-Volmer equation only in the range of 11.25–37.50 μM. A positive deviation was observed in the Stern–Volmer graphic when the concentration of Fe3+ ions exceeded 37.50 μM. A positive deviation from the linearity observed in the Stern-Volmer graphic after a certain quencher concentration indicates a system in which a dynamic and static quenchings are together [61].
At the system between CQDs and Fe3+ ions dynamic and static quenchings are effective. Static quenching requires a ground state complex. This can be explained by the temperature effect of dynamic quenching. Dynamic quenching is a kind of collisional quenching and the quenching effect increases, as the temperature increases. Online Resource 2 (Electronic Supplementary Material) shows the effect of temperature on the fluorescence efficiency. As seen in Online Resource 2, the quenching efficiency decreases, as the temperature increases. Lower fluorescence yield was obtained at a high temperature (at 333 K). In Online Resource 2, it can be said that a static quenching that supports a complex formation between CQDs and Fe3+ions is effective in the fluorescence quenching mechanism.
Determination of Fe3+ Ions
Firstly, the effects of metal ions with a concentration of 25 μM on the fluorescence spectrum of CQDs were investigated. Almost all of the metals investigated were found to cause more or less decrease in the fluorescence emission intensity of CQDs. The decrease in the fluorescence intensity of CQDs was revealed by calculating (%QE = [(FCQD-FCQD-Me)/FCQD]*100). The (W2+, Y3+, Mo2+, Li+, Al3+, Ti4+, B+ and Na+) ions, (Ca2+, V+3 and Mn2+) ions, (Ca2+, V+3 and Mn2+) ions, (Zn2+, Cr3+, Y3+, Cd2+, Ni2+, As2+, Sr2+, Cu2+ and Se2+) ions, (Bi2+, Tl+ and Ag+) ions, (Fe3+, Sn2+, Pb2+ and Pd2+) ions, (Ba2+ and Co2+) ions and (Au3+, Hg2+ and Sb3+) ions have the percentage quenching effect (%QE) with (0–10)%, (10–20)%, (20–30)%, (30–40)%, (40–50)%, (50–60)%, (60–70)%, (70–80)%, respectively. As seen, the highest quenching was caused by the metal ions Fe3+, Sn2+, Pb2+, Pd2+, Au4+, Hg2+ and Sb2+. Fluorometric titrations of CQDs were performed with increasing concentrations of all these metal ions. It was seen that only the fluorometric titration of Fe3+ ions was repeatable. It was determined that CQDs interacts with Fe3+ ions more sensitive and stable than other metal ions. The stability of the interaction between CQDs and Fe3+ ions was investigated by examining the change in the fluorescence intensity of (CQDs – Fe3+) over time (Online Resource 3 in the Electronic Supplementary Material). The fluorescence emission intensity of (CQDs – Fe3+) was measured for 1 h. When the change was examined, it was observed that the value of measured fluorescence intensity at the 1 min was approximately constant for 20 min and subsequently, the change was not greater than 5%. As a result, CQDs interacted with Fe3+ ions within 1 min and it maintained its stability for 1 h.
As shown in Fig. 6a, the regular fluorescence quenching was observed between 380 and 500 nm when the concentration of Fe3+ ions was increased during the fluorometric titration of CQDs with Fe3+ ions. An external calibration graphic was prepared for the determination of Fe3+ ions by utilizing this regular change in the fluorescence intensity at 435 nm (inset of Fig. 6b). The linear range is between 11.25 and 37.50 μM in the graphic. A deviation from the Beer’s law was observed upon a concentration of 37.50 μM for Fe3+ ion. This external calibration graphic was used to determine the amount of Fe3+ ions in the sandy soil certified reference material (CRM-SA-C) and satisfactory accuracy values were obtained. The analytical performance data of the developed method are given in Table 1.
As seen in Table 1, a correlation constant (R2) value, which is 0.9884, is almost linear (R2 = 1). The limit of detection (LOD) was calculated by dividing the three-fold of the standard deviation (N = 11) for the blank by the slope (3Ss/m). The standard deviation was calculated by measuring 11 blanks. The limit of quantification (LOQ) was calculated as 8.22 μM by taking 3 times the limit of detection (9Ss/m). In these calculations based on IUPAC, Ss refers to the standard deviation of the measurements for the blank, and m refers to the slope of the external calibration graphic.
The accuracy of the proposed method was proved by analyzing the sandy soil certified reference material (CRM-SA-C). Fe3+ ions of 21.2 μM were determined by performing necessary dilutions from the stock solution of this certified reference material (in Table 2).
A good correlation was found between the analysis and the certified values. Statistical analysis of the results obtained by using Student’s t - test shows that there is no significant difference between the obtained values and certified values. According to the results of the analysis, the calculated Student’s t – test value (0.011) was lower than the theoretical value (2.57 for N = 6) at 95% confidence level.
Conclusion
Briefly, a green synthesis of the water-soluble carbon quantum dots (CQDs) from the chickpeas, a carbon source, was carried out in order to develop an easy, simple and inexpensive method. CQDs were characterized using the results of FTIR, XRD and TEM. CQDs synthesized under the optimized reaction conditions were found to have a particle size of less than 10 nm. It was shown by the result of the SAED pattern that the structure of CQD was amorphous, but not crystalline. In addition, the XRD graphic showed that the structure of CQDs had highly disordered- carbon atoms. The optical properties of the synthesized CQDs were analyzed by UV-vis absorption and fluorescence spectroscopy. Spectroscopic studies showed that the maximum of the fluorescence band of CQDs (at 345 nm) was both excitation wavelength-dependent and excitation wavelength-independent. The linear range of CQDs for Fe3+ was 11.25–37.50 μM. The limit of detection and the limit of quantification is 2.74 μM and 8.22, respectively. CQDs were used for the sensitive and selective determination of Fe3+ ions. The accuracy of the proposed method was proved by a successful analysis of the sandy soil certified reference material (CRM-SA-C). Since the synthesized CQDs can be produced easily and interacts stably with Fe3+ ions, we think that it can be used in many chemical and biological applications.
References
Trogadas P, Fuller TF, Strasser P (2014) Carbon as catalyst and support for electrochemical energy conversion. Carbon 75:5–42. https://doi.org/10.1016/j.carbon.2014.04.005
Abi A, Kazemi G, Safavi A (2018) A carbon dot-based fluorescence method for selective quantification of sulfide in environmental samples. Sensors Actuators B Chem 277:1–7. https://doi.org/10.1016/j.snb.2018.08.150
Lu W, Qin X, Liu S, Chang G, Zhang Y, Luo Y, Asiri AM, Al-Youbi AO, Sun X (2012) Economical, green synthesis of fluorescent carbon nanoparticles and their use as probes for sensitive and selective detection of mercury (II) ions. Anal Chem 84:5351–5357. https://doi.org/10.1021/ac3007939
Zhang J, Yu SH (2016) Carbon dots: large-scale synthesis, sensing and bioimaging Cdots. Mater Today 19:382–393. https://doi.org/10.1016/j.mattod.2015.11.008
Xu XY, Ray R, Gu YL, Ploehn HJ, Gearheart L, Raker K, Scrivens WA (2004) Electrophoretic analysis and purification of fluorescent single-walled carbon nanotube fragments. J Am Chem Soc 126:12736–12737. https://doi.org/10.1021/ja040082h
Sun YP, Zhou B, Lin Y, Wang W, Fernando KAS, Pathak P, Meziani MJ, Harruff BA, Wang X, Wang HF, Luo PJG, Yang H, Kose ME, Chen BL, Veca LM, Xie SY (2006) Quantum-sized carbon dots for bright and colorful photoluminescence. J Am Chem Soc 128:7756–7757. https://doi.org/10.1021/ja062677d
Pan D, Zhang J, Li Z, Wu M (2010) Hydrothermal route for cutting graphene sheets into blue-luminescent graphene quantum dots. Adv Mater 22:734–738. https://doi.org/10.1002/adma.200902825
Pan DLG, Zhang J, Xi C, Xue Q, Huang H, Li J, Zhang Z, Yu W, Chen Z, Li Z, Wu M (2012) Cutting sp2 clusters in graphene sheets into colloidal graphene quantum dots with strong green fluorescence. J Mater Chem 22:3314–3318. https://doi.org/10.1039/C2JM16005F
Yang ST, Wang X, Wang H, Lu F, Luo PG, Cao L, Meziani MJ, Liu JH, Liu Y, Chen M, Huang Y, Sun YP (2009) Carbon dots as nontoxic and high-performance fluorescence imaging agents. J Phys Chem C 113(42):18110–18114. https://doi.org/10.1021/jp9085969
Dong Y, Pang H, Yang HB, Guo C, Shao J, Chi Y, Li CM, Yu T (2013) Carbon-based dots co-doped with nitrogen and sulfur for high quantum yield and excitation-independent emission. Angew Chem Int Ed Eng 52:7800–7804. https://doi.org/10.1002/anie.201301114
Shen P, Gao J, Cong J, Liu Z (2016) Synthesis of cellulose-based carbon dots for bioimaging. Chem Select 1:1314–1317. https://doi.org/10.1002/slct.201600216
Bhunia SK, Saha A, Maity AR, Ray SC, Jana NR (2013) Carbon nanoparticle-based fluorescent bioimaging probes. Sci Rep 3:1473. https://doi.org/10.1038/srep01473
Yang Z, Xu M, Liu Y, He F, Gao F, Su Y, Wei H, Zhang Y (2014) Nitrogen-doped, carbon-rich, highly photoluminescent carbon dots from ammonium citrate. Nanoscale 6:1890–1895. https://doi.org/10.1039/c3nr05380f
Cao B, Yuan C, Liu B, Jiang C, Guan G, Han MY (2013) Ratiometric fluorescence detection of mercuric ion based on the nanohybrid of fluorescence carbon dots and quantum dots. Anal Chim Acta 786:146–152. https://doi.org/10.1016/j.aca.2013.05.015
Niu WJ, Shan D, Zhu RH, Deng SY, Cosnier S, Zhang XJ (2016) Dumbbell-shaped carbon quantum dots/AuNCs nanohybrid as an efficient ratiometric fluorescent probe for sensing cadmium (II) ions and l-ascorbic acid. Carbon 96:1034–1042. https://doi.org/10.1016/j.carbon.2015.10.051
Li Y, Liu Y, Shang X, Chao D, Zhou L, Zhang H (2018) Highly sensitive and selective detection of Fe3+ by utilizing carbon quantum dots as fluorescent probes. Chem Phys Lett 705:1–6. https://doi.org/10.1016/j.talanta.2016.02.018
Goncalves HMR, Duarte AJ, Esteves da Silva JCG (2010) Optical fiber sensor for hg(II) based on carbon dots. Biosens Bioelectron 26:1302–1306. https://doi.org/10.1016/j.bios.2010.07.018
Lu Y, Feng Y, Wang F, Zou X (2018) Facile hydrothermal synthesis of carbon dots (CDs) doped ZnFe2O4/TiO2 hybrid materials with high photocatalytic activity. J Photochem Photobiol A Chem 353:10–18. https://doi.org/10.1016/j.apcatb.2017.02.024
Edison TNJI, Atchudan R, Sethuraman MG, Lee YR (2016) Microwave assisted green synthesis of fluorescent N-doped carbon dots: cytotoxicity and bio-imaging applications. J Photochem Photobiol B Biol 161:154–161. https://doi.org/10.1016/j.jphotobiol.2016.05.017
Zeng Q, Shao D, He X, Ren Z (2016) Carbon dots as a trackable drug delivery carrier for localized Cancer therapy in vivo. J Mater Chem B 4:5119–5126. https://doi.org/10.1039/C6TB01259K
Jang E, Jun S, Jang H, Lim J (2010) White-light-emitting diodes with quantum dot color converters for display backlights. Adv Mater 22:3076–3080. https://doi.org/10.1002/adma.201000525
Eberl K, Lipinski MO, Manz YM, Winter W, Jin-Phillipp NY, Schmidt OG (2001) Self-assembling quantum dots for optoelectronic devices on Si and GaAs. Phys E: Low-dimens Syst Nanostruct 9:164–174. https://doi.org/10.1016/S1386-9477(00)00190-9
Qu K, Wang J, Ren J, Qu X (2013) Carbon dots prepared by hydrothermal treatment of dopamine as an effective fluorescent sensing platform for the label-free detection of iron(III) ions and dopamine. Chem – A Eur J 19:7243–7249. https://doi.org/10.1002/chem.201300042
Zhou M, Zhou Z, Gong A, Zhang Y, Li Q (2015) Synthesis of highly photoluminescent carbon dots via citric acid and Tris for iron(III) ions sensors and bioimaging. Talanta 143:107–113. https://doi.org/10.1016/j.talanta.2015.04.015
Soares R, Escaleira V, Boas de Campos DV, Monteiro MIC, Santelli RE, Bernardi ACD (2013) Comparison of conventional and alternative determination methods for copper, iron, manganese and zinc in mehlich-1 extracted soil solution. Rev Bras Cienc Solo 37:707–714. https://doi.org/10.1590/S0100-06832013000300017
Akl MA (2003) Preconcentration extractive separation, speciation and spectrometric determination of iron(III) in environmental samples. Microchem J 75:199–209. https://doi.org/10.1016/S0026-265X(03)00097-3
Dominik P, Kaupenjohann M (2000) Simple spectrophotometric determination of Fe in oxalate and HCl soil extracts. Talanta 51:701–707. https://doi.org/10.1016/S0039-9140(99)00324-0
Albuquerque LGR, Fioroto AM, Paixao TRLC (2017) Evaluation of multi-mixtures of acids for the sample preparation of organic soil amendments for multi-element determination by ICP OES. Commun Soil Sci Plant Anal 48:2210–2217. https://doi.org/10.1080/00103624.2017.1408818
Khayatian G, Hassanpoor S, Nasiri F, Zolali A (2012) Preconcentration, determination and speciation of iron by solidphase extraction using dimethyl (E)-2-[(Z)-1-acetyl)-2-hydroxy-1-propenyl]-2-butenedioate. Quim Nova 35:535–540. https://doi.org/10.1590/S0100-40422012000300018
Tokalıoğlu Ş, Ergün H, Çukurovalı A (2010) Preconcentration and determination of Fe(III) from water and food samples by newly synthesized chelating reagent impregnated Amberlite XAD-16 resin. Bull Kor Chem Soc 31:1976–1980. https://doi.org/10.5012/bkcs.2010.31.7.1976
Citak D, Tüzen M, Soylak M (2009) Simultaneous coprecipitation of lead, cobalt, copper, cadmium, iron and nickel in food samples with zirconium (IV) hydroxide prior to their flame atomic absorption spectrometric determination. Food Chem Toxicol 47:2302–2307. https://doi.org/10.1016/j.fct.2009.06.021
Duran C, Özdeş D, Çelenk Kaya E, Kantekin H, Bulut VN, Tüfekçi M (2012) Optimization of a new cloud point extraction procedure for the selective determination of trace amounts of total iron in some environmental samples. Turk J Chem 36:445–456. https://doi.org/10.3906/kim-1108-10
Kumar CK, Trivedi R, Giribabu L, Niveditha S, Bhanuprakash K, Sridhar B (2015) Ferrocenyl pyrazoline based multichannel receptors for a simple and highly selective recognition of Hg2+ and Cu2+ ions. J Organomet Chem 780:20–29. https://doi.org/10.1016/j.jorganchem.2014.12.027
Veale EB, Gunnlaugsson T (2010) Fluorescent sensors for ions based on organic structures. Annu Rep Prog Chem Sect B 106:376–406. https://doi.org/10.1039/c004146g
Lina Y, Zhenga Y, Guo Y, Yang Y, Lia H, Fanga Y, Wanga C (2018) Peptide-functionalized carbon dots for sensitive and selective Ca2+ detection. Sensors Actuators B Chem 273:1654–1659. https://doi.org/10.1016/j.snb.2018.07.073
Kumari A, Kumar A, Sahuc SK, Kumara S (2018) Synthesis of green fluorescent carbon quantum dots using wastepolyolefins residue for Cu2+ ion sensing and live cell imaging. Sensors Actuators B 254:197–120. https://doi.org/10.1016/j.snb.2017.07.075
Rong M, Fengd Y, Wanga Y (2017) Chena X, one-pot solid phase pyrolysis synthesis of nitrogen-doped carbondots for Fe3+ sensing and bioimaging. Sensors Actuators B 245:868–874. https://doi.org/10.1016/j.snb.2017.02.014
Gao X, Zhou X, Ma Y, Qian T, Wang C, Chu F (2019) Facile and cost-effective preparation of carbon quantum dots for Fe3+ ion and ascorbic acid detection in living cells based on the “on-off-on” fluorescence principle. Appl Surf Sci 469:911–916. https://doi.org/10.1016/j.apsusc.2018.11.095
Chen L, Wu C, Du P, Feng X, Wu P, Cai C (2017) Electrolyzing synthesis of boron-doped graphene quantum dots for fluorescence determination of Fe3+ ions in water samples. Talanta 164:100–109. https://doi.org/10.1016/j.talanta.2016.11.019
Chen K, Qing W, Hu W, Lu M, Wang Y, Liu X (2019) On-off-on fluorescent carbon dots from waste tea: their properties, antioxidant and selective detection of CrO4 2−, Fe3+, ascorbic acid and L-cysteine in real samples. Spectrochim Acta A Mol Biomol Spectrosc 213:228–234. https://doi.org/10.1016/j.saa.2019.01.066
Yang R, Guo X, Jia L, Zhang Y, Zhao Z, Lonshakov F (2017) Green preparation of carbon dots with mangosteen pulp for the selective detection of Fe3+ ions and cell imaging. Appl Surf Sci 423:426–432. https://doi.org/10.1016/j.apsusc.2017.05.252
Gong J, An XQ, Yan XJA (2014) Novel rapid and green synthesis of highly luminescent carbon dots with good biocompatibility for cell imaging. N J Chem 38:1376–1379. https://doi.org/10.1039/c3nj01320k
Guo LL, Li L, Liu MY, Wan Q, Tian JW, Huang Q, Wen YQ, Liang SD, Zhang XY, Wei Y (2018) Bottom-up preparation of nitrogen doped carbon quantum dots with gren emission under microwave-assisted hydrothermal treatment and their biological imaging. Mater Sci Eng C 84:60–66. https://doi.org/10.1016/j.msec.2017.11.034
Yang H, He L, Long Y, Li H, Pana S, Liu H, Hua X (2018) Fluorescent carbon dots synthesized by microwave-assisted pyrolysis for chromium (VI) and ascorbic acid sensing and logic gate operation. Spectrochim Acta A Mol Biomol Spectrosc 205:12–20. https://doi.org/10.1016/j.saa.2018.07.015
Wang J, Wang CF, Chen S (2012) Amphiphilic egg-derived carbon dots: rapid plasma fabrication, pyrolysis process, and multicolor printing patterns. Angew Chem Int Ed 51:9297–9301. https://doi.org/10.1002/anie.201204381
Liu Y, Liu Y, Park SJ, Zhang Y, Kim T, Chae S, Kim HY (2015) One-step synthesis of robust nitrogen-doped carbon dots: acid-evoked fluorescence enhancement and their application in Fe3+ detection. J Mater Chem A 3:17747–17754. https://doi.org/10.1039/c5ta05189d
Essner JB, Laber CH, Ravula S, Polo-Parada L, Baker GA (2016) Pee-dots: biocompatible fluorescent carbon dots derived from the upcycling of urine. Green Chem 18(1):243–250. https://doi.org/10.1039/c5gc02032h
De B, Karak N (2013) A green and facile approach for the synthesis of water soluble fluorescent carbon dots from banana juice. RSC Adv 3:8286–8290. https://doi.org/10.1039/c3ra00088e
Zhu SJ, Meng QN, Wang L, Zhang JH, Song YB, Jin H, Zhang K, Sun HC, Wang HY, Yang B (2013) Highly photoluminescent carbon dots for multicolor patterning, sensors, and bioimaging. Angew Chem Int Ed 52:3953–3957. https://doi.org/10.1002/anie.201300519
Zhang F, Wang Y, Miao Y, He Y, Yang Y, Liu X (2016) Optimal nitrogen and phosphorus codoping carbon dots towards white light-emitting device. Appl Phys Lett 109:083103. https://doi.org/10.1063/1.4961631
Cui X, Zhu L, Wu J, Hou Y, Wang P, Wang Z, Yang M (2015) A fluorescent biosensor based on carbon dots-labeled oligodeoxyribonucleotide and graphene oxide for mercury (II) detection. Biosens Bioelectron 63:506–512. https://doi.org/10.1016/j.bios.2014.07.085
Zhou L, Qiao M, Sun LZL, Zhang Y, Liu W (2019) Green and efficient synthesis of carbon quantum dots and their luminescent properties. J Lumin 206:158–163. https://doi.org/10.1016/j.jlumin.2018.10.057
Rodrigues CV, JRCCM A, Andrade LP, Galvão PM, Costa PA, Campos AL, Pereira AJ, Ghesti GF, Felix JF, Weber IT, Neto BA, Rodrigues MO (2015) Down- and Up-Conversion Photoluminescence of Carbon-Dots from Brewing Industry Waste: Application in Live Cell-Imaging Experiments. J Braz Chem Soc 26(12):2623–2628. https://doi.org/10.5935/0103-5053.20150291
Gaddam RR, Mukherjee S, Punugupati N, Vasudevan D, Patra CR, Narayan R, Kothapalli RVSN (2017) Facile synthesis of carbon dot and residual carbon nanobeads: implications for ion sensing, medicinal and biological applications. Mater Sci Eng C 73:643–652. https://doi.org/10.1016/j.msec.2016.12.095
Shi CCY, Li M, Xing M, Wu Q (2017) Carbon quantum dots from carbonized walnut shells: structural evolution, fluorescence characteristics, and intracellular bioimaging. Mater Sci Eng C 79:473–480. https://doi.org/10.1016/j.msec.2017.05.094
Wang L, Bi Y, Hou J, Li H, Xu Y, Wang B, Ding H, Ding L (2016) Facile, green and clean one-step synthesis of carbon dots from wool: application as a sensor for glyphosate detection based on the inner filter effect. Talanta 160:268–275. https://doi.org/10.1016/j.talanta.2016.07.020
Zhang R, Chen W (2014) Nitrogen-doped carbon quantum dots: facile synthesis and application as a “turn-off” fluorescent probe for detection of Hg2+ ions. Biosens Bioelectron 55:83–90. https://doi.org/10.1016/j.bios.2013.11.074
Wei S, Zhang R, Liu Y, Ding H, Zhang YL (2016) Graphene quantum dots prepared from chemical exfoliation of multiwall carbon nanotubes: an efficient photocatalyst promoter. Catal Commun 74:104–109. https://doi.org/10.1016/j.catcom.2015.11.010
Aslandas AM, Balcı N, Arık M, Sakiroglu H, Onganer Y, Meral K (2015) Liquid nitrogen-assisted synthesis of fluorescent carbon dots from blueberry and their performance in Fe3+ detection. Appl Surf Sci 356:747–752. https://doi.org/10.1016/j.apsusc.2015.08.147
Chen RF (1990) Fluorescence of proteins and peptides. In: Guilbault GG, Dekker MM (eds) Practical fluorescence, 2nd edn. New York, p 595
Behera PK, Mukherjee T, Mishra AK (1995) Simultaneous presence of static and dynamic component in the fluorescence quenching for substituted naphthalene-CCl4 system. J Lumin 65:131–136. https://doi.org/10.1016/0022-2313(95)00067-Z
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 123 kb)
Rights and permissions
About this article
Cite this article
Başoğlu, A., Ocak, Ü. & Gümrükçüoğlu, A. Synthesis of Microwave-Assisted Fluorescence Carbon Quantum Dots Using Roasted–Chickpeas and its Applications for Sensitive and Selective Detection of Fe3+ Ions. J Fluoresc 30, 515–526 (2020). https://doi.org/10.1007/s10895-019-02428-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-019-02428-7