Abstract
Recent advancements in analytical techniques for detecting crucial chemicals and biological substances, including metal ions, have highlighted the limitations of traditional methods such as potentiometric sensors, electrochemical sensors, and spectrometry techniques, which are often costly, time-consuming, and unsuitable for real-time monitoring. Fluorescence assay methods offer high sensitivity, selectivity, and rapid analysis with simpler sample preparation. This study presents an eco-friendly, rapid, and cost-effective method for synthesizing biocompatible fluorescent carbon dots (CDs) from grapefruit peels via a one-step solvothermal process. The synthesized CDs exhibit stable photoluminescence, excellent biocompatibility, and low toxicity, making them suitable for metal ion detection and DNA sensing. Characterization using fluorescence spectrophotometry, UV-Vis spectrophotometry, FTIR spectroscopy, and TEM confirmed the CDs’ spherical morphology, optical properties, and various functional groups. The CDs demonstrated high sensitivity for detecting metal ions, with fluorescence quenching observed in the presence of Cu²⁺ (R² = 0.9998) and Al³⁺ (R² = 0.9898), and detection limits of 0.5 ppm. Stability tests showed minimal impact on fluorescence intensity with varying KCl concentration, pH, long term storage and temperature. Additionally, the CDs exhibited significant fluorescence quenching in the presence of DNA, indicating their potential for biological sensing with a detection limit of 0.4 ppm. This study underscores the potential of CDs for sensitive and selective environmental monitoring and biological applications, offering a sustainable approach to managing agricultural waste.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
In recent years, significant progress has been made in producing diverse analytical techniques aimed at detecting crucial chemicals and biological substances, including drugs, biomolecules, and metal ions, with particular emphasis on identifying harmful metal ions [1, 2]. These metal ions are commonly found in various natural and environmental water sources, such as seawater, river and lake water, drinking water, and tap water [3, 4]. These metal ions can lead to numerous neurodegenerative disorders, including Parkinson’s, Prion, and Alzheimer’s diseases [5, 6]. As a result, there is a critical need to monitor metal ion concentrations in the environment with high sensitivity and selectivity. Various analytical methods are currently available for detecting metal ions, such as potentiometric sensors, electrochemical sensors, atomic fluorescence spectrometry, atomic absorption spectrometry (AAS), and inductively coupled plasma mass spectrometry (ICP-MS) [6,7,8]. Although these methods can detect metal ions with high sensitivity their applications are restricted due to high costs, time-consuming procedures, complex sample preparation, and unsuitability for real-time monitoring. In contrast, fluorescence assay methods have been employed for detecting various metals, offering several benefits such as high sensitivity, selectivity, simple sample preparation, speed, and minimal interferences [9, 10]. Recent research indicates that fluorescent materials, such as semiconductor quantum dots and organic dyes, have been utilised for metal detection [11,12,13]. However, these methods have limitations: (1) they require multiple synthesis and purification steps. (2) The nanomaterials are made from highly toxic precursors and costly chemicals like surface modifiers. Therefore, there is a significant need to develop a new, straightforward, rapid, cost-effective, and environmentally friendly method for producing biocompatible fluorescent nanomaterials.
Fluorescent carbon nanoparticles, a novel type of nanomaterial within the nano-carbon family, have garnered significant attention in recent studies due to their unique optical and chemical characteristics [14, 15]. In contrast to conventional organic dyes and semiconductor quantum dots, fluorescent carbon nanoparticles exhibit remarkable properties, including stable photoluminescence, excellent biocompatibility, and low toxicity [16,17,18,19,20]. In 2004, carbon dots (CDs) were accidentally discovered during the purification of single-walled carbon nanotubes. CDs are quasi-spherical particles with diameters of less than 10 nm, and have an amorphous or crystalline core composed mainly of sp2 carbon, along with an oxidized carbon surface featuring carboxyl groups [21]. This material has garnered significant interest in various fields, including biological labelling, photocatalysis [22], sensing [23, 24] and biomedicine [25].
CD synthesis methods can be broadly divided into top-down and bottom-up approaches [26,27,28]. The top-down approach produces CDs from larger carbon materials using techniques like laser ablation, arc discharge, chemical oxidation, and electrochemical synthesis. In contrast, the bottom-up approach synthesizes CDs from molecular precursors through procedures such as microwave treatment, hydrothermal/solvothermal treatment, and carbonization [29]. To date, CDs have been produced from various precursors using the approaches mentioned above. Unluckily, many of these previous methods require the use of costly and harmful chemicals and solvents [30, 31]. Consequently, there is a need to explore simple, economical, and environmentally friendly alternatives for the green synthesis of CDs.
The hybrid grapefruit (Citrus paradisi) contains relatively high concentrations of flavanones, narirutin, and naringin, as well as p-coumaric acids (hydroxyl cinnamic acids), ferulic acids, gallic and vanillic acids (hydroxybenzoic acids), limonoids, carotenoids, and furocoumarins. Citrus peels, one of the most underutilized and geographically diverse biowaste leftovers, account for 50% of industrial citrus waste. Managing these wastes, which pollute the land and cause odours, remains a significant issue in the food sector. In the field of nanoscience, using Citrus paradisi peels for nanoparticle production could be an environmentally friendly strategy to develop a non-food-based market for agricultural waste, benefiting various research areas.
In the current study, we describe a completely eco-friendly method for the synthesis of luminous carbon dots. Grapefruit peels were employed as a precursor and ethanol as a solvent in a one-step, environmentally friendly method. Carbon dots were applied to detect the metal ions and this application is based on the principle that the interaction between the CDs and metals decreases the fluorescence by quenching. The synthesized CDs were also used for the detection of DNA.
2 Materials and methods
2.1 Materials
Fresh grapefruit was bought from a neighbourhood store in Charsadda, Pakistan. Ethanol and metals ion and anion solutions with high degree purity were bought from Sigma Aldrich. Sodium hydroxide and hydrochloric acid were also purchased from Sigma Aldrich. Potassium chloride (KCl) was utilized to alter the ionic strength for the ionic strength resistance experiments. A syringe filter (25 mm) having a pore size of 0.22 m was used during experiments. All glassware was cleansed with deionized water followed by drying in the oven.
2.2 Preparation of CDs
The carbon dots (CDs) were synthesized using a one-step solvothermal method. Initially, grape peels were carefully removed from the fruits and subjected to ultrasonic cleaning twice using deionized water to ensure the removal of any impurities. After cleaning, the grape peels were dried at a low temperature (60 °C) for 12 h in an oven. Once dried, the peels were ground into a fine powder using a mortar and pestle. Subsequently, 0.4 g of the grape peel powder was mixed with 20 millilitres of ethanol and the mixture was sonicated for 5 min to ensure thorough mixing. This mixture was then transferred into a 50-milliliter Teflon-lined steel autoclave. The autoclave was sealed and heated in an oven at 170 °C for 6 h. Upon completion of the heating process, the autoclave was allowed to cool to room temperature, resulting in a dark brown product. To purify the product, the suspensions were first filtered through Whatman 1-grade filter paper to remove larger particles. Further purification was achieved by filtering the filtrate with a syringe filter and then centrifuging it at 5000 rpm for 5 min. The supernatant, containing the CDs, was collected, and the solvent was evaporated under vacuum at room temperature to obtain the final product [32].
2.3 Characterization of CDs
The size, crystal structure, elemental content, and a number of other physical characteristics of CDs were determined using a wide range of analytical techniques and equipment. Fluorescence spectrophotometers (Perkin Elmer LS 45 spectrofluorimeter) were used to test the optical characteristics of the CDs, and a UV-Vis spectrophotometer was used to record the absorbance of the CDs between 200 nm and 800 nm. For the functional group identification of the synthesized CDs, a Fourier transform infrared (FTIR) spectrometer was used with a wavelength range of 500 to 4000 cm-1. Transmission electron microscopy (TEM) images were captured using TEM (JEM-2100 F) at King Fahad University of Petroleum and Minerals Dhahran Saudi Arabia. A digital pH meter was used for all pH readings.
2.4 Carbon dots Stability
To assist the stability of carbon dots under various conditions different experiments were conducted. Initially, the carbon dots were subjected to different pH levels to evaluate their stability across a range of acidic and basic environments for this hydrochloric acid (HCl) and sodium hydroxide (NaOH) was used to adjust the pH at 3, 5, 7, and 9 of the CDs solution. To examine the impact of ionic strength, potassium chloride KCl solutions with different concentrations in the range of 0.5 M to 1.0 M were introduced. Long-term stability tests were conducted by monitoring the carbon dots over an extended period of one week, one month, two months and four months. Additionally, the stability was evaluated by varying the temperature from 25 °C to 75 °C. For each experiment, 3 mL of CD solution was introduced individually, and after 10 min, Using the PL spectrum at 500 nm of emission the sample’s fluorescence intensity was evaluated. The results demonstrated that CDs’ have possessed excellent stability over a wide range of harsh conditions.
2.5 Detection of Metals ions
Different metal stock solutions were prepared and diluted to 20 ppm using a dilution formula. Metal solutions including light metals i.e. Aluminum (III) and Lithium (I) and heavy metals including Copper (II), Lead (II), Arsenic (III), Cobalt (II), Mercury (II), Iron (II), Mercury (II), Copper (II), Chromium (VI), and Gold (III). Nickel (II), Platinum (II), Silicon (II), Antimony (III), Cadmium (II), and Tungsten (VI) were prepared. Following that, 3 mL of 0.01 gL− 1 of CDs solution and different amounts of a 20 ppm metals solution were added. For 10 min at room temperature, these were allowed to react. In all metal solutions using the PL spectrum upon emission at 500 nm, the quenching performance of CDs was measured. The findings indicated that the intensity decreased with an increase in the amount of metal solution added. Based on PL quenching to calculate the quenching efficiency, the standard intensity of the PL (FLs) before the addition of CDs was compared to the reduced intensity of the PL (FLre) after the addition of CDs using the following relation:
Quenching Efficiency (%) =\(\:\frac{\text{F}\text{l}\text{s}-\text{F}\text{l}\text{r}\text{e}}{\text{F}\text{l}\text{s}}\times\:100\)
2.6 Detection of DNA
The synthesized CDs were utilized to identify DNA based on changes in the system’s relative fluorescence when DNA was introduced to the CD solution. About 20 ppm of DNA solution was made at first, and then it was diluted into different concentrations using the above dilution formula to 5, 10 and 15 ppm. Before measuring fluorescence, 1 mL of DNA solution and 3 mL of 0.01gL-1 CDs solution were completely mixed at room temperature for 10 min. At an emission wavelength of 500 nm, the relative fluorescence quenching Fo/F (where Fo and F are the relative fluorescence of the CDs solution without and with DNA, respectively) was determined.
3 Results and Discussion
3.1 Characterization
3.1.1 Surface Morphology and Optical Properties
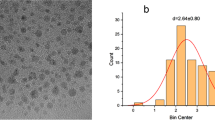
The TEM image given in Fig. 1a of the synthesized CDs showed that particles are spherical objects with an average mean particle size of 10 nm with an average diameter of 6 nm Fig. 1b. The optical properties of as-prepared CDs can be evaluated by UV-visible spectroscopy. UV absorption spectra show (Fig. 1c) a distinct absorption peak at 284 nm. In the UV-Vis spectrum, shoulders may also be observed at a wavelength of about 250 nm. The n-p* transition of C = O is responsible for the main absorption band of the synthesized CDs. The low-intensity band that appears as a shoulder around 220 nm is due to the p-p* transition of C = C in aromatic structure. The given absorption spectra have similarities to those of other published research [33,34,35]. With an excitation wavelength of 420 nm, the synthesized CDs showed their highest emission at 500 nm Fig. 1d.
3.1.2 FTIR and XRD Spectra
FTIR spectrum was recorded to characterize the surface groups of the synthesized CDs. Flavanoids and organic acids alcoholic O-H stretching showed strong absorption at 3337 cm− 1. And the formation of intra and intermolecular hydrogen bonds is indicated by its broad breadth (Fig. 2a). The bands at 2974 and 2887 cm− 1 show C-H stretching vibrations of methoxy groups [36, 37]. Aldehydic/ketonic derivative C = O stretching was attributed to the peaks at 1650 cm− 1. The aromatic rings and amide groups C = C, C-C, and C-O stretching vibrations are represented by the weak bands at 1380, 1326, and 1274 cm− 1. Strong peaks at 1045 cm− 1 and 1086 cm− 1, respectively, show the existence of C-O-C stretching of the ether and ester groups [38]. The XRD patterns of this sample in Fig. 2b demonstrate a prominent broad peak that is clearly discernible around 22.4° (with a corresponding d-spacing of 0.396 nm) in the pattern. This observation strongly suggests the presence of extensively disordered carbon atoms as well as a graphitic arrangement within the CDs. No additional peaks are evident in Fig. 2b, signifying the exclusive amorphous nature of the prepared CDs sample.
3.2 Experiment of Metal ions Detection
To investigate the potential of CDs for metal ion sensing, CDs with maximum fluorescence intensity were employed without any modification. The produced CDs were used to create a highly sensitive and selective fluorescence sensor for metallic ions [39]. When excited at 420 nm, the fluorescence intensity of the CDs was quenched in the presence of various light and heavy metal ions due to an effective charge and energy transfer process and decreased linearly as the concentration of metal ions increased between 10 µL and 130 µL, demonstrating the sensitivity of the CDs to metal ions. The quenching effect was investigated for light metal ions such as Al (III) and Li (I), and heavy metal ions such as Fe (II), Hg (II), Cu (II), Cr (VI), Pb (II), As (III), Ag (I), Au (III), Mn (II), Cd (II), Sb (III), Ni (II), Pt (II), Si (II), and W (VI). Different degrees of quenching were observed as shown in Fig. 3a and b. When metal ions come into close contact with the CD surface, they cause varying levels of disruption to the CDs’ fluorescence mechanism, depending on their interaction and electronic configuration [40, 41]. Metal ions interact with carboxylic functional groups to form coordination bonds, leading to notable quenching when electrons from the functional groups on the CD surface transfer to the metal ions [42]. Fig. 3c and 3d reveals that highest quenching in CD intensity caused by heavy metals was observed with Cu²⁺, while the highest quenching by light metals was observed with Al³⁺ ions. The relative intensity (F/F₀), where F is the maximum fluorescence intensity of the CD solution before adding metal ions and F₀ is the fluorescence intensity after adding the metal ions, showed excellent linearity with a correlation coefficient of R² = 0.9998 for Cu²⁺ and 0.9898 for Al³⁺, as shown in Fig. 3e. The data fit well with the linear Stern-Volmer equation, indicating a single reaction (static quenching) [43]. The limit of detection (LOD) for Cu²⁺ and Al³⁺ was calculated to be 0.5 ppm, using the formula 3s/k, where s is the standard deviation of the blank solution, and k is the slope of the linear equation. The quenching efficiency of Cu²⁺ and Al³⁺ ions increased from 16 to 66% and 14–65%, respectively, as the initial ion concentration increased from 10 µL to 130 µL (Fig. 3f). These findings suggest that the synthesized CDs have excellent potential for detecting and removing metallic ions in aqueous solutions. The order of quenching efficiency for heavy metal ions was: Cu²⁺ > Fe²⁺ > Cd²⁺ > Ni²⁺ > Pb²⁺ > Mn²⁺ > Sb³⁺ > Au³⁺ > Hg²⁺ > Pt²⁺ > Si²⁺ > Ag⁺ > Cr⁶⁺ > W⁶⁺ > As³⁺, and for light metal ions: Al³⁺ > Li⁺.
3.2.1 Real Sample Analysis for Cu+ 2 and Al+ 3
To assess the feasibility of the proposed method, it was employed to determine Cu+ 2 and Al+ 3 in river water samples. These samples were collected from a local river and were prepared by centrifuging at 8000 rpm, followed by filtration through Whatman 1-grade filter paper prior to analysis. A standard addition recovery experiment was conducted as part of this study. The findings, presented in Table 1, revealed recoveries ranging from 97.33 to 102% of Cu+ 2 and 93 100.81% of Al+ 3. These outcomes demonstrate that the proposed method is highly accurate and precise, indicating its strong potential for reliable Cu+ 2 and Al+ 3detection in river water samples.
3.3 Experiment of DNA Determination
Fluorescence spectroscopy was used to study the interactions of CDs and DNA by investigating the fluorescence intensities of CDs with varying DNA concentrations. The fluorescence intensity of the CDs decreased as DNA concentration increased as shown in Fig. 4a and b with a strong emission at 500 nm when excited at 420 nm. In Fig. 4c a linear relationship between DNA concentration and fluorescence quenching (F0/F) was observed in the range of 5 to 20 ppm, with a linear regression equation (R² = 0.9764) and a detection limit of 0.4 ppm. These findings revealed that the mechanism was static quenching, and that both electrostatic force and hydrophobic interactions were significantly contributors to the binding reaction [44, 45], with a quenching efficiency of 25%.
3.4 Stability of Carbon Dots
From the above study, it is obvious that in several applications CDs are very advantageous. However, to investigate the stability of the synthesized CDs in terms of pH, KCl concentration, long-term stability and temperature several experimental experiments have been carried out. In a variety of practical applications that require varying salt concentrations, pH, and high temperature the produced CDs must be stable.
3.4.1 Ionic Strength
Figure 5a demonstrates the ionic strength effect on the PL intensity. It is evident that when the concentration of potassium chloride (KCl) was increased it caused a negligible 3% decline in FL emission of CDs compared to their behaviour in deionized water. Increasing the concentration even to 1.0 M of (KCl) no precipitation is observed. This indicates that there may not be any salt interaction with the CD solution. Different (KCl) ionic strengths may assist in preventing the CDs from aggregating. This indicates that grape peel-derived CDs have resistance to salt (KCl) and can be used in the biochemistry field [46, 47].
3.4.2 Effect of pH on CDs Stability
The stability of the luminous CDs at various pH levels was evaluated to confirm a response to changes in the pH. A spectrofluorometer was used to measure the fluorescence intensity of CDs at various pH values in the range of 3 to 11. The fluorescence intensity increases steadily from pH 3 to pH 7 owing to the ionization states of either acidic or alkaline solutions [48]. Significant variations in emission intensity were detected when the pH was changed (Fig. 5b). The decrease in PL intensity of CDs in acidic solutions can be attributed to hydrogen ions. Due to the formation of hydrogen bonds with the carboxyl groups on the surface of CDs, many particles aggregate together [49]. While the alkaline state likewise showed a declining tendency. At pH levels from 9 to 11, the decrease in fluorescence emission of CDs is due to the de-protonation of chemical bonds on the CD’s surface. The carboxyl groups (COO-) on the surface of the CDs acquire a negative charge that interferes with the fluorescence activity and thus the result is PL quenching [50]. These results indicated an interesting phenomenon, the pH-dependent PL characteristic, which suggests a possible application for CDs as pH sensors.
3.4.3 Long term Stability
The CD solution exhibited a long-lasting homogenous phase at room temperature without any noticed precipitation. After being stored for four months, the fluorescence intensity recorded after one week, one month, two months, and four months showed no noticeable changes. The results, as shown in Fig. 5c, showed that the FL maximum intensity for the four CD samples was almost identical [46].
3.4.4 Effect of Temperature on Stability
The impact of heating on PL intensity is illustrated in Fig. 5b. The carbon dots (CDs) showed a mere 7% decrease in PL emission at 45 °C. However, as the temperature increased to 75 °C, the PL intensity dropped by nearly 16%. This decline is likely attributed to the precipitation of the CDs at elevated temperatures. According to the outcome, the CDs that were produced are remarkably stable at high temperatures.
The comparison of CDs synthesized through green routes from various sources and their use for the detection of various metals is shown in Table 2.
4 Conclusion
This study demonstrates the successful green synthesis of fluorescent carbon dots (CDs) from grapefruit peels using an eco-friendly one-step solvothermal method. The CDs exhibit unique optical properties, stable photoluminescence, excellent biocompatibility, and low toxicity, making them a promising alternative to conventional semiconductor quantum dots and organic dyes. Characterization confirmed their spherical morphology with an average diameter of less than 10 nm and stability across various pH levels and ionic strengths. The CDs showed high sensitivity and selectivity in detecting metal ions, with significant fluorescence quenching observed for Cu²⁺ and Al³⁺ ions and detection limits of 0.5 ppm, as well as effective DNA detection with a detection limit of 0.4 ppm. The use of grapefruit peels, a readily available biowaste, provides a sustainable approach to managing agricultural waste while offering cost effective production methods. These findings highlight the potential of CDs for environmental monitoring, particularly for harmful metal ions, and extend their utility to DNA sensing, making them promising candidates for various applications in environmental and biochemical sensing.
References
Singh AK, Bhattacharjee G, Singh R (2004) Mercury (II)-selective membrane electrode using tetrathia-diazacyclotetradeca-2, 9-diene as neutral carrier. Sens Actuators B Chem 99(1):36–41
Srivastava SK, Gupta VK, Jain S (1996) PVC-based 2, 2, 2-cryptand sensor for zinc ions. Anal Chem 68(7):1272–1275
Björklund LB, Morrison GM (1997) Determination of copper speciation in freshwater samples through SPE-spectrophotometry. Anal Chim Acta 343(3):259–266
Davenport J, Manley A (1978) The detection of heightened sea-water copper concentrations by the mussel Mytilusedulis. J Mar Biolog Assoc UK 58(4):843–850
Song Y, Qu, Konggang, Xu C, Ren J, Qu X (2010) Visual and quantitative detection of copper ions using magnetic silica nanoparticles clicked on multiwalled carbon nanotubes. Chem Commun 46(35):6572–6574
Gaetke LM, Chow CK (2003) Copper toxicity, oxidative stress, and antioxidant nutrients. Toxicology 189(1–2):147–163
Ghoreishi SM, Behpour M, Hajisadeghian E, Golestaneh M (2016) Voltammetric determination of resorcinol on the surface of a glassy carbon electrode modified with multi walled carbon nanotube. Arab J Chem 9:S1563–S1568
Gupta VK, Prasad R, Kumar A (2003) Preparation of ethambutol–copper (II) complex and fabrication of PVC based membrane potentiometric sensor for copper. Talanta 60(1):149–160
Medintz IL, Uyeda HT, Goldman ER, Mattoussi H (2005) Quantum dot bioconjugates for imaging, labelling and sensing. Nat Mater 4(6):435–446
Aragay G, Pons J, Merkoçi A (2011) Recent trends in macro-, micro-, and nanomaterial-based tools and strategies for heavy-metal detection. Chem Rev 111(5):3433–3458
Petryayeva E, Algar WR, Medintz IL (2013) Quantum dots in bioanalysis: a review of applications across various platforms for fluorescence spectroscopy and imaging. Appl Spectrosc 67(3):215–252
Zrazhevskiy P, Sena M, Gao X (2010) Designing multifunctional quantum dots for bioimaging, detection, and drug delivery. Chem Soc Rev 39(11):4326–4354
Yang H, Mao H, Wan Z, Zhu A, Guo M, Li Y, Li X, Wan J, Yang X, Shuai X (2013) Micelles assembled with carbocyanine dyes for theranostic near infrared fluorescent cancer imaging and photothermal therapy. Biomaterials 34(36):9124–9133
Shen C-L, Qing L, Chao‐Fan L, Jin‐Hao Z, Song‐Nan Q, Lin D, Shan Chong‐Xin (2019) Bright and multicolor chemiluminescent carbon nanodots for advanced information encryption. Adv Sci 6(11):1802331
Fang Y, Jiurong L, Xiujian Z, Gong Xiao (2023) Unveiling unconventional luminescence behavior of multicolor carbon dots derived from phenylenediamine. J Phys Chem Lett 14(26):5975–5984
Li H, Kang Z, Liu Y, Lee ST (2012) Carbon nanodots: synthesis, properties and applications. J Mater Chem A 22(46):24230–24253
Baker SN, Baker GA (2010) Luminescent carbon nanodots: emergent nanolights. Angew Chem Int Ed 49(38):6726–6744
Luo PG, Sahu S, Yang ST, Sonkar SK, Wang J, Wang H, LeCroy GE, Cao L, Sun YP (2013) Carbon quantum dots for optical bioimaging. J Mater Chem B 1(16):2116–2127
Huang Q, Lin X, Chen D, Tong QX (2022) Carbon Dots/α-Fe2O3-Fe3O4 nanocomposite: efficient synthesis and application as a novel electrochemical aptasensor for the ultrasensitive determination of aflatoxin B1. Food Chem 373:131415
Li J, Gong X (2022) The emerging development of multicolor carbon dots. Small 18(51):2205099
Zhu B, Sun S, Wang Y, Deng S, Qian G, Wang M, Hu A (2013) Preparation of carbon nanodots from single chain polymeric nanoparticles and theoretical investigation of the photoluminescence mechanism. J Mater Chem C 1(3):580–586
Cao L, Sahu S, Anilkumar P, Bunker CE, Xu J, Fernando KS, Wang P, Guliants EA, Tackett KN, Sun YP (2011) Carbon nanoparticles as visible-light photocatalysts for efficient CO2 conversion and beyond. J Am Chem Soc 133(13):4754–4757
Zhou L, Lin Y, Huang Z, Ren J, Qu X (2012) Carbon nanodots as fluorescence probes for rapid, sensitive, and label-free detection of hg 2 + and biothiols in complex matrices. Chem Commun 48(8):1147–1149
Li C, Lin X, Liao J, Yang M, Jiang M, Huang Y, Du Z, Chen L, Fan S, Huang Q (2024) Carbon dots-based dopamine sensors: recent advances and challenges. Chin Chem Lett. 109598
Wu YF, Wu HC, Kuan CH, Lin CJ, Wang LW, Chang CW, Wang TW (2016) Multi-functionalized carbon dots as theranosticnanoagent for gene delivery in lung cancer therapy. Sci Rep 6(1):21170
Cao M, Zhao X, Gong X (2022) Ionic liquid-assisted fast synthesis of carbon dots with strong fluorescence and their tunable multicolor emission. Small 18(11):2106683
Liu ML, Chen BB, Li CM, Huang CZ (2019) Carbon dots: synthesis, formation mechanism, fluorescence origin and sensing applications. Green Chem 21(3):449–471
Zheng XT, Ananthanarayanan A, Luo KQ, Chen P (2015) Glowing graphene quantum dots and carbon dots: properties, syntheses, and biological applications. Small 11(14):1620–1636
Wang Y, Hu A (2014) Carbon quantum dots: synthesis, properties and applications. J Mater Chem C 2(34):6921–6939
Sahu S, Behera B, Maiti TK, Mohapatra S (2012) Simple one-step synthesis of highly luminescent carbon dots from orange juice: application as excellent bio-imaging agents. Chem Commun 48(70):8835–8837
Yang Y, Cui J, Zheng M, Hu C, Tan S, Xiao Y, Yang Q, Liu Y (2012) One-step synthesis of amino-functionalized fluorescent carbon nanoparticles by hydrothermal carbonization of Chitosan. Chem Comm 48(3):380–382
Wang N, Wang Y, Guo T, Yang T, Chen M, Wang J (2016) Green preparation of carbon dots with papaya as carbon source for effective fluorescent sensing of Iron (III) and Escherichia coli. Biosens Bioelectron 85:68–75
Shariati-Rad M, Mohseninasab T, Parno F (2018) Application of response surface methodology and green carbon dots as reducing agents in speciation of iron. RSC Adv 8:2173–2180
Li J, Zheng S, Zhang S, Gong X (2024) Metal–Organic Framework-assisted rational design of Multicolor Solid‐State fluorescent Carbon nanodots and its application for LEDs. Laser Photonics Rev 18(3):2300974
Zhu S, Tang S, Zhang J, Yan B (2012) Control the size and surface chemistry of graphene for the rising fluorescent materials. ChemComm 48:4527–4539
Shereema RM, Sankar V, Raghu KG, Rao TP, Shankar SS (2015) One step green synthesis of carbon quantum dots and its application towards the bioelectroanalytical and biolabeling studies. Electrochim Acta 182:588–595
Tang X, Wang H, Yu H, Bui B, Zhang W, Wang S, Chen M, Yuan L, Hu Z, Chen W (2022) Exploration of nitrogen-doped grape peels carbon dots for baicalin detection. Mater Today 22:100576
Kumar B, Smita K, Galeas S, Sharma V, Guerrero VH, Debut A, Cumbal L (2020) Characterization and application of biosynthesized iron oxide nanoparticles using Citrus paradisi peel: a sustainable approach. 119:108116
Tan XW, Romainor ANB, Chin SF, Ng SM (2014) Carbon dots production via pyrolysis of sago waste as potential probe for metal ions sensing. J Anal Appl Pyrol 105:157–165
Tan XW, Romainor ANB, Chin SF, Ng SM (2012) Microwave-assisted synthesis of carbon nanodots through an eggshell membrane and their fluorescent application. Analyst 137:53925397
Huang Q, Li Q, Chen Y, Tong L, Lin X, Zhu J, Tong Q (2018) High quantum yield nitrogen-doped carbon dots: green synthesis and application as off-on fluorescent sensors for the determination of Fe3 + and adenosine triphosphate in biological samples. Sens Actuators B Chem 276:82–88
Abidin NHZ, Wongso V, Hui KC, Cho K, Sambudi NS, Ang WL, Saad B (2020) The effect of functionalization on rice-husks derived carbon quantum dots properties and cadmium removal. J Water Process Eng 38:101634
Kundu A, Nandi S, Das P, Nandi AK (2016) Facile and green approach to prepare fluorescent carbon dots: emergent nanomaterial for cell imaging and detection of vitamin B2. J Colloid Interface Sci 468:276–283
Kudr J, Richtera L, Xhaxhiu K, Hynek D, Heger Z, Zitka O, Adam V (2017) Carbon dots based FRET for the detection of DNA damage. Biosens Bioelectron 92:133–139
Zhang Y, Gao Z, Zhang W, Wang W, Chang J, Kai J (2018) Fluorescent carbon dots as nanoprobe for determination of lidocaine hydrochloride. Sens Actuators B Chem 262:928–937
Xue M, Zhan Z, Zou M, Zhang L, Zhao S (2016) Green synthesis of stable and biocompatible fluorescent carbon dots from peanut shells for multicolor living cell imaging. New J Chem 40:1698–1703
Balakrishnan T, Ang WL, Mahmoudi E, Mohammad AW, Sambudi NS (2022) Formation mechanism and application potential of carbon dots synthesized from palm kernel shell via microwave assisted method. Carbon Resour Convers 5:150–166
Hua C, Zhang K, Xin M, Ying T, Gao J, Jia J, Li Y (2016) High quantum yield and pH sensitive fluorescence dyes based on coumarin derivatives: fluorescence characteristics and theoretical study. RSC Adv 6:49221–49227
Jia X, Yang X, Li J, Li D, Wang E (2014) Stable Cu nanoclusters: from an aggregation-induced emission mechanism to biosensing and catalytic applications. ChemComm 50:237–239
Srisantitham S, Sukwattanasinitt M, Unarunotai S (2018) Effect of pH on fluorescence quenching of organic dyes by graphene oxide. Colloids Surf Physicochem Eng Asp 550:123–131
Zhao J, Huang M, Zhang L, Zou M, Chen D, Huang Y, Zhao S (2017) Unique approach to develop carbon dot-based nanohybrid near-infrared ratiometric fluorescent sensor for the detection of mercury ions. Anal Chem 89:8044–8049
Ramezani Z, Qorbanpour M, Rahbar N (2018) Green synthesis of carbon quantum dots using quince fruit (Cydoniaoblonga) powder as carbon precursor: application in cell imaging and As3 + determination. Colloids Surf Physicochem Eng Asp 549:58–66
Tyagi A, Tripathi KM, Singh N, Choudhary S, Gupta RK (2016) Green synthesis of carbon quantum dots from lemon peel waste: applications in sensing and photocatalysis. RSC Adv 6:72423–72432
Chatzimitakos T, Kasouni A, Sygellou L, Avgeropoulos A, Troganis A, Stalikas C (2017) Two of a kind but different: luminescent carbon quantum dots from Citrus peels for iron and tartrazine sensing and cell imaging. Talanta 175:305–312
Kailasa SK, Ha S, Baek SH, Kim S, Kwak K, Park TJ (2019) Tuning of carbon dots emission color for sensing of Fe3 + ion and bioimaging applications. Mater Sci Eng C 98:834–842
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zeeshan, M., Jan, F.A., Ali, W. et al. Green One step Synthesis of Carbon dots from Grapes peel, Characterization and Application as a Fluorescence Probe for the Detection of some Heavy, Light Metals ions and DNA Binding. Chemistry Africa (2024). https://doi.org/10.1007/s42250-024-01052-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42250-024-01052-8