Abstract
In this work, as a novel fluorescent nano-sensor, a ligand-capped CdTe QDs (CdTe-L QDs) was designed for the detection and quantification of Cu2+ ions in environmental water samples. The synthesized QDs were characterized by transmission electron microscopy (TEM), thermo-gravimetric (TG) analysis, Fourier transform infrared (FTIR), UV–Vis spectrophotometry and fluorescence spectroscopy. Optical properties of the produced nanosensor were monitored by UV–Vis and fluorescence spectrophotometry. It was observed that fluorescence intensity of the produced nano-sensor selectively quenched by adding Cu2+ ions in comparison to other metal ions tested. Using CdTe-L QDs, a rapid and facile analytical method was developed to determine Cu2+ ions in the concentration range of 5.16 ± 0.07 × 10− 8 mol L− 1–1.50 ± 0.03 × 10− 5 mol L− 1 with a detection limit of 1.55 ± 0.05 × 10− 8 mol L− 1. The nanosensor was successfully applied for the determination of Cu2+ ions in various water samples, and the results were compared with the standard method.

Graphical Abstract
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Quantum dots (QDs) are semiconductor nano-materials with remarkable optical properties, which are dramatically affected by their small size (2–12 nm), constituent materials, shape and surface chemistry [1,2,3,4]. Many investigations have been carried out regarding the importance of QDs in various fields including designing sensors [5,6,7,8,9]. Due to several advantages such as broad excitation spectra, narrow emission peak, and good photo-stability over organic dyes, QDs are excellent choices for designing fluorescent nanosensors [10,11,12].
Suitable surface modifications strategies have been utilized for designing QD-based nano-sensors. Various materials such as thioglycolic acid (TGA) [13], mercaptopropionic acid (MPA) [14] and polymeric materials [15] have been used for modification of the QDs surface [16]. In recent years, fluorescent nano-sensors have been extensively used for the detection of various types of analytes such as ions, molecules or even biomolecules [17,18,19,20,21,22,23,24].
Nowadays, due to the significance of heavy metals in human health, their detection is of great importance. Many chemical sensors have been reported for fast and inexpensive determination of these ions. Among various heavy metal ions, copper, although vital for human life, ranks second in toxicity after mercury and causes many serious diseases [25,26,27]. Hence, design and fabrication of novel sensors for detection of low levels of copper content in natural waters has specific significance. As an example, Yang et al. used phenol formaldehyde resin nanoparticles loaded with CdTe QDs for visual detection of Cu2+ ions [28]. They also used polyamine-functionalized carbon QDs as fluorescent probes for selective and sensitive detection of copper ions [29]. In addition, Jin et al. used hexadecyl trimethylammonium bromide (CTAB) modified CdSe/ZnS QDs as a fluorescent probe for the detection of Cu2+ ions [30].
In this work, based on new synthesis ligand capped CdTe QDs, a novel nano-sensor was prepared and characterized. The ligand was 4-((pyridin-2-yl) methyleneamino)-5-(1H-indol-3-yl)-4H-1,2,4-triazole-3-thiol (L), Fig. 1. Next, the applicability of the proposed L-capped CdTe QDs was studied as fluorescence probes for the monitoring of metal ions. It was found that the fluorescent nano-sensor is able to detect the copper ions selectively. When the mechanism of fluorescence quenching was investigated, parameters affecting the increasing ability for the detection of Cu2+ ions and the selectivity of the sensor towards other metal ions were optimized. In addition, the proposed nano-sensor was used to determine Cu2+ ions in real samples with satisfactory results.
Experimental
Materials
Thioglycolic acid (TGA, 98%), CdCl2. 5H2O (99.99%), tellurium (Te) powder (99%) and sodium borohydride (NaBH4, 95%) were purchased from Sigma Aldrich. All other chemicals used were of analytical grade and were obtained from Merck Co. Moreover, all solutions were prepared using double distilled water.
Instrumentation
The fluorescence studies were performed using a SCINCO’s Fluorescence Spectrometer Fluoro Mate FS-2. The UV–Vis spectrophotometer Shimadzu (model, 1650 PC) was employed for UV–Vis spectra and absorbance measurements. FT-IR spectra were recorded by Bruker Tensor 27 FT-IR spectrometer (Germany). Characterizations of size and morphology of QDs were carried out using a Zeiss TEM at an acceleration voltage of 100 kV. Thermo-gravimetric (TG) analysis was carried out using a TA Instrument 2050 thermogravimetric device under N2 atmosphere (25 mL min− 1) at a scan rate of 10 °C/min. A Varian (model: 735-OES) inductively coupled plasma optical emission spectrometer (ICP-OES) was used for the determination of copper ions.
Synthesis of CdTe-TGA QDs
Here, 0.025 g of the precursor of Te and 0.025 g of NaBH4 were dissolved in a flask containing 2.5 mL double distilled water with pure nitrogen gas for about 120 min under magnetic stirring. In the next step, 0.1 g of CdCl2 was dissolved in another flask containing 80 mL double distilled water with pure nitrogen gas under magnetic stirring. 1.4 mmol of TGA was added in precursor of Cd and the pH of solution was adjusted to 8–9. Finally, the precursor of Te was transferred into a flask containing the precursor of Cd. Then, the resulting solution was refluxed at 100 °C for 1 h. CdTe-TGA QDs were synthesized according to previous experiences [31, 32].
Synthesis of the Ligand (L)
The procedure for the preparation of 4-((pyridin-2-yl)methyleneamino)-5-(1H-indol-3-yl)-4H-1,2,4-triazole-3-thiol (L) is as follow: A mixture of indole-1,2,3-triazole-3-thiol (0.01 mol), pyridine-2-carbaldehyde (0.01 mol,) and a catalytic amount of acetic acid was refluxed for 5 h in absolute ethanol (20 ml). Then, the solvent was evaporated to 5 mL, cooled to room temperature; and the colorless crystals of the product were filtered, washed with ethanol, and dried under reduced pressure.
Synthesis of CdTe-L QDs
CdTe-L QDs were obtained based on ligand exchange strategies from CdTe-TGA QDs and an organic ligand. First, 0.03 mmol of ligand was dissolved in 20 mL of acetonitrile and diluted with double distilled water to 40 mL. Then, 20 mL of synthesized CdTe-TGA QDs were added (2 mL min− 1) with a micropipette to the system drop-wise. The resulted solution was kept for 4 days while it was sealed and stirring. Gradually, the color of the solution was changing from orange to a light yellow. The solution can be stable without any precipitation within 3 months. To explore the behavior of CdTe-L QDs, the interactions between CdTe-TGA QDs and ligand were investigated by TG analysis, FTIR, UV–Vis and fluorescence spectroscopy.
Measurements
Typical measurements were carried out as follows: the prepared of CdTe-L QDs was diluted with double distilled water (1:4), and it was suspended into Tris-buffer solution (pH 6.5, 0.01 mol L− 1). The resulting solution (2 mL) was transferred into a 1 cm × 1 cm fluorescent quartz cell and it was titrated with different metal ions. Various volumes of ion stock solutions (1 × 10− 4 mol L− 1) were gradually added to the cell using a micropipette. The fluorescence spectra were recorded in less than 1 min after adding metal ions. To explore the behavior of CdTe-L QDs and the interactions between CdTe-TGA QDs and ligand, the fluorescence spectra were taken in two excitation wavelengths, separately. For studying ligand behavior, excitation wavelength was selected 370 nm and emission spectra were recorded from 385 to 700 nm. Also, excitation wavelength was selected 390 nm and emission spectra were recorded from 405 to 700 nm for studying CdTe QDS behavior (ligand in this excitation wavelength (390 nm) does not have any emission).
Real Sample Measurements
The water samples were first filtered through Whatman filter paper. Then, 10 mL of the water samples were diluted by double distilled water to 100 mL, and the pHs of the solutions were adjusted to 6.5 using Tris-buffer (0.01 mol L− 1). A desired volume of CdTe-L QDs and double distilled water (2 ml) was transferred into the fluorescent quartz cell. Then, the solution was analyzed using the standard addition method, Cu2+ ions were added into these water samples and the resulting solutions were finally analyzed with the proposed methods, and the obtained results were compared with standard method (ICP-OES).
Results and Discussion
Optical Properties of the Nanosensor
The fluorescence spectra of the interactions between Ligand and CdTe-TGA QDs during different times at 25 °C with excitation wavelength at 370 nm (Fig. 2a) and 390 nm (Fig. 2b) are shown. An interesting observation is that the fluorescence intensity of CdTe QDs increases while the fluorescence intensity of ligand decreases by increasing the time of interaction. Changes in fluorescence intensities might be due to the modification of surface QD because of replacing L with TGA molecules. This increasing of FL intensity after addition of L to the CdTe-TGA QDs, can be considered as a good indication for successful ligand exchange process.
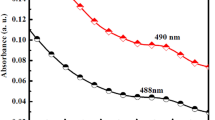
This ligand exchange can also be demonstrated with a small decrease in particle size and a small blue shift in the band-edge absorption. During the time, a blue shift occurs in UV spectra of CdTe-ligand after ligand exchanging (Fig. 3a). Moreover, comparison of UV–Vis spectra of the pure ligand, pure CdTe-TGA QDs and CdTe-L QDs are shown in Fig. 3b. Another evidence for taking part in CdTe-L QDs exchange can be found in Fig. 4. Obviously, images in Fig. 4 show the ligand, CdTe-TGA QDs, and CdTe-L QDs under (a) UV lamp (254 nm) and (b) visible light. A change in color from orange (CdTe-TGA QDS) to light yellow (CdTe-L QDs) (Fig. 4) was observed.
Characteristics of the Nanosensor
The synthesized CdTe-L QDs TEM was shown in Fig. 5. The image shows that synthetic QDs were all spherical with a suitable morphology.
FT-IR spectra were used to verify the capping of the ligand on the surface of the CdTe QDs. FTIR spectra of the CdTe-TGA QDs, ligand and CdTe-L QDs are shown in Fig. 6. The FT-IR spectra of CdTe-TGA (Fig. 6a) showed a strong band at 3446 cm− 1 assigned to stretching vibrations of hydroxyl group, and a weak but sharp band at 2550 cm− 1 assigned to the stretching vibration of the thiol group. Moreover, the stretching vibrations of carboxyl group are found at 1646 and 1562 cm− 1. The FT-IR spectrum of the ligand (Fig. 6b) exhibited a strong peak at 3030 cm− 1 corresponding to the stretching vibration of aromatic –CH groups, and a weak band at 2580 cm− 1 due to the S-H stretching vibration. The stretching vibrations of aromatic –NH groups are located 3300–3500 cm− 1. In addition, the bands at 1580 and 1600 cm− 1 correspond to aromatic ring chain vibrations of -CC groups. The FT-IR spectra of the produced nanosensor (Fig. 6c) obviously showed characteristics of both CdTe-TGA QDS and ligand spectra. Figure 6c shows that the ligand exchange has occurred; and Fig. 6c exhibits a spectrum similar to the CdTe-TGA QDS shown in Fig. 6a. It is important that there is a little shift in some absorption peaks of the FT-IR spectra of the produced nanosensor. This is due to the capping of the ligand on the surface of CdTe QDs.
The surface modification of CdTe QDs was also investigated by TG and DTG curves. As shown in Fig. 7, the TG and DTG curves showed that the maximum decomposition rate of the ligand, CdTe-L and CdTe-TGA QDs occurred at 255, 235 and 323 °C, respectively. The different thermal behavior of CdTe-L and CdTe-TGA QDs is a good indication of their dissimilar composition. It can be suggested that the thermal stability of CdTe-L QDs is decreased relative to CdTe-TGA QDs, which might be caused by the capping of ligand on the surface of QDs. In addition, the total weight loss of CdTe-L QDs at 450 °C was about 25% more than that of CdTe-TGA QDs.
Analytical Performance of the Nanosensor
Surface ligands can have important effects on fluorescence responses of QDs to metal ions. In fact, modification of QDs surfaces by suitable ligands leads to designing new fluorescent nano-sensors. As shown in Fig. 8a, the fluorescence intensity of CdTe-L QDs decreases with increasing concentration of Cu2+ ions. The quenching effect of Cu2+ ions on fluorescence, the intensity of CdTe-L QDs was analyzed by the Stern–Volmer Eq. (1):
Where F0 and F are the fluorescence intensities of CdTe-L QDs at 540 nm in the absence and presence of a quencher (Cu2+ ion is the quencher in this study), respectively, Ksv is the quenching constant of the quencher, and [Q] is the analyte (Cu2+ ion) concentration. The Stern–Volmer plot, shown in Fig. 8b, fits a good linear correlation (R2 = 0.9936) over the concentration range of 5.16 ± 0.07 × 10− 8 mol L− 1–1.50 ± 0.03 × 10− 5 mol L− 1 from the formula. The detection limit is 1.55 ± 0.05 × 10− 8 mol L− 1 (at a signal-to-noise ratio of 3). In Table 1, some previous reported works which used quantum dots for the detection of copper ions in water media have been compared. From Table 1, it can be seen that this work has a lower detection limit most of the compared probes. Furthermore, UV–Vis spectra of CdTe-L QDs, after addition of optimum amount of Cu2+ ions, are shown in Fig. 8c.
Selectivity of the Proposed Nanosensor
Generally, selectivity is an important parameter in designing new chemical sensors. The selectivity of the proposed nano-sensor was evaluated by investigating the response in the presence of other metal ions. Therefore, to evaluate the selectivity of CdTe-L QDs for Cu2+ ions, the responses from other metal ions including Mn2+, Zn2+, Ni2+, Pb2+, Al3+, Cr3+, Cu2+, Cd2+, Co2+ and Fe3+ to the fluorescence spectrum of CdTe-L QDs were studied. The fluorescence emission of CdTe-L QDs was significantly decreased without any emission band shift by adding copper ions. As shown in Fig. 9, the influence of other ions on fluorescence intensity was very weak. The produced nano-sensor was rather selective towards copper ions, which makes it very attractive for the selective detection of Cu2+ ions. Moreover, fluorescence changes of a solution of the ligand (10− 5 mol L− 1) was studied while it was titrated by adding metal cations (10− 4 mol L− 1) separately. The emission of the ligand with excitation wavelength at 370 nm did not show any change by adding cations. Thus, the proposed nano-sensor only responds to copper ions rather than other cations.
Optimization of the Experimental Conditions
In order to obtain the maximum sensitivity for the quenching system, the effects of pH and the amount of produced nano-sensor solutions on the fluorescence quenching efficiency were investigated. The effect of pH on the fluorescence intensity of this system was studied to obtain an optimum pH for effective sensing Cu2+ ions. The fluorescence intensity was studied against pH in the range of 4.5 to 9.5 in order to obtain the optimum conditions to determine copper ions. The optimum fluorescence intensity was obtained in the pH range between 6.5 and 7.5 (Fig. 10a). At the acidic pH, increase in the concentration of H+ is the result of the protonation of the heteroatoms group on the capped ligand; and at higher pH, the concentration of copper ion decreases due to precipitation of Cu(OH)2 [39]. The effect of the amount of produced nano-sensor solutions on the quenching efficiency was also investigated in the range of 200–1000 μL of stock solution. As shown in Fig. 10b, the optimal amount of CdTe-L QDs (500 μL) gives the highest sensitivity (i.e., the slope of calibration function) and the widest linear range of the calibration function. The quenching efficiency increased by increasing the produced nano-sensor volume up to 500 μL. At a higher concentration, self-quenching or aggregation of CdTe-L QDs may occur.
Detection of Copper Ions in Environmental Water Samples
In order to evaluate the capability of the designed nano-sensor in real sample analysis, a standard addition method has been utilized to detect Cu2+ ions in various water samples (under optimal conditions). After analyzing the samples, the results were compared with the standard method (ICP-OES). The obtained results, summarized in Table 2, demonstrated that the proposed fluorescence nano-sensors are sensitive and dependable enough for practical detection of copper ions in water samples. Moreover, as shown in Fig. 11a, the fluorescence intensity of CdTe-L QDs is decreased with an increase in the concentration of Cu2+ ions (in one water sample). The Stern–Volmer plot, shown in Fig. 11b, fits a good linear correlation (R2 = 0.9973).
Conclusions
In this work, a novel fluorescent nano-sensor was introduced and successfully fabricated based on CdTe-L QDs for selective and sensitive detection of Cu2+ ions in aqueous solutions. CdTe-L QDs were characterized by fluorescence spectroscopy, UV–Vis spectrophotometry, FT-IR and TG analyses. FT-IR and TG analyses confirmed the presence of ligand around CdTe QDs. The size of CdTe-L QDs was estimated around 2 nm from TEM images. Moreover, CdTe-L QDs were directly applied to selective detection of Cu2+ ions in comparison to other cations. The fluorescence intensity of CdTe-L QDs was quenched when adding Cu2+ ions into the solution of nano-sensor. Under optimum conditions, the calibration plot was linear in the range between 5.16 ± 0.07 × 10− 8 mol L− 1–1.50 ± 0.03 × 10− 5 mol L− 1. The detection limit of produced nano-sensor was 1.55 ± 0.05 × 10− 8 mol L− 1. Furthermore, the proposed nano-sensor was applied to determine Cu2+ ions in various water samples with satisfactory results.
References
Cui L, He XP, Chen GR (2015) Recent progress in quantum dot based sensors. RSC Adv 5:26644–26653
Bonilla JC, Bozkurt F, Ansari S, Sozer N, Kokini JL (2016) Applications of quantum dots in food science and biology. Trends Food Sci Technol 53:75–89
Frigerio C, Ribeiro DSM, Rodrigues SSM, Abreu VLRG, Barbosa JAC, Prior JAV, Marques KL, Santos JLM (2012) Application of quantum dots as analytical tools in automated chemical analysis. Anal Chim Acta 735:9–22
Karakoti AS, Shukla R, Shanker R, Singh S (2015) Surface functionalization of quantum dots for biological applications. Adv Colloid Interface Sci 215:28–45
Santos-Figueroa LE, Moragues ME, Climent E, Agostini A, Martinez-Manez R, Sancenon F (2013) Chromogenic and fluorogenic chemosensors and reagents for anions. A comprehensive review of the years 2010–2011. Chem Soc Rev 42:3489–613
Chen X, Pradhan T, Wang F, Kim JS, Yoon J (2012) Fluorescent chemosensors based on spiroring-opening of xanthenes and related derivatives. Chem Rev 112:1910–56
Kim HN, Ren WX, Kim JS, Yoon J (2012) Fluorescent and colorimetric sensors for detection of lead, cadmium, and mercury ions. Chem Soc Rev 41:210–44
Zhou Y, Zhang JF, Yoon J (2014) Fluorescence and colorimetric chemosensors for fluoride-ion detection. Chem Rev 114: 5511–71
Gai L, Mack J, Lu H, Nyokong T, Li Z, Kobayashi N, Shen Z (2015) Organosilicon compounds as fluorescent chemosensors for fluoride anion recognition. Coord Chem Rev 285:24–51
Qian ZS, Shan XY, Chai LJ, Chen JR, Feng H (2015) A fluorescent nanosensor based on graphene quantum dots–aptamer probe and graphene oxide platform for detection of lead (II) ion. Biosens Bioelectron 68:225–231
Li M, Zhou X, Guo S, Wu N (2013) Detection of lead (II) with a “turn-on” fluorescent biosensor based on energy transfer from CdSe/ZnS quantum dots to graphene oxide. Biosens Bioelectron 15:69–74
Sadeghi S, Jahani M, Belador F (2016) The development of a new optical sensor based on the Mn doped ZnS quantum dots modified with the molecularly imprinted polymers for sensitive recognition of florfenicol. Spectrochim Acta Mol Biomol Spectrosc 159:83–89
Ananth DA, Rameshkumar A, Jeyadevi R, Jagadeeswari S, Nagarajan N, Renganathan R, Sivasudha T (2015) Antibacterial potential of rutin conjugated with thioglycolic acid capped cadmium telluride quantum dots (TGA-CdTe QDs). Spectrochim Acta Mol Biomol Spectrosc 138:684–692
Narayanan SS, Sinha SS, Verma PK, Pal SK (2008) Ultrafast energy transfer from 3-mercaptopropionic acid-capped CdSe/ZnS QDs to dye-labelled DNA. Chem Phys Lett 463:160–165
Dogana U, Kasap E, Cetin D, Suludere Z, Boyaci IH, Turkyılmaz C, Ertas N, Tamera U (2016) Rapid detection of bacteria based on homogenous immunoassay using chitosan modified quantum dots. Sens Actuators B Chem 233:369–378
Stanisavljevic M, Krizkova S, Vaculovicova M, Kizek R, Adam V (2015) Quantum dots-fluorescence resonance energy transfer-based nanosensors and their application. Biosens Bioelectron 74:562–574
Amjadi M, Shokri R, Hallaj T (2016) A new turn-off fluorescence probe based on graphene quantum dots for detection of Au(III) ion. Spectrochim Acta Mol Biomol Spectrosc 153:619–624
Barati A, Shamsipur M, Abdollahia H (2016) Metal-ion-mediated fluorescent carbon dots for indirect detection of sulfide ions. Sens Actuators B Chem 230:289–297
Chen J, Zhu Y, Zhang Y (2016) Glutathione-capped Mn-doped ZnS quantum dots as a room-temperature phosphorescence sensor for the detection of Pb2+ ions.Spectrochim Acta Mol Biomol Spectrosc 164:98–102
Blanco-Canosa JB, Wud M, Susumub K, Petryayeva E, Jennings TL, Dawson PE, Algar WR, Medintz IL (2014) Recent progress in the bioconjugation of quantum dots. Coord Chem Rev 263:101–137
Xua L, Mao W, Huang J, Li S, Huang K, Li M, Xia J, Chen Q (2016) Economical, green route to highly fluorescence intensity carbon materials based on ligninsulfonate/graphene quantum dots composites: application as excellent fluorescent sensing platform for detection of Fe3+ions. Sens Actuators B Chem 230:54–60
Koneswaran M, Narayanaswamy R (2009) Mercaptoacetic acid capped CdS quantumdots as fluorescence single shot probe for mercury (II). Sens Actuators B 139:91–96
Chen Y, Rosenzweig Z (2002) Luminescent CdS quantum dots as selective ion probes. Anal Chem 74:5132–5138
Wang L, Liu S, Hao C, Zhang X, Wang C, He Y (2016) A dual-fluorescence biosensor assembled by quantum dots and phenazinium dyes: a comparative study for DNA detection. Sens Actuator B Chem 229:145–154
Peric J, Trgo M, Medvidovic NV (2004) Removal of zinc, copper and lead bynatural zeolite-a comparison of adsorption isotherms. Water Res 38:1893–1899
Rahman MM, Alamry KA, Saleh TS, Asiri AM (2016) Sensitive and selective Cu2+ sensor based on 4-(3-(thiophen-2-yl)-9Hcarbazol-9-yl) benzaldehyde (TPCBZ) conjugated copper complex. J Organomet Chem 817:43–49
Ferenci P, Litwin T, Seniow J, Czlonkowska A (2015) Encephalopathy in Wilson disease: copper toxicity or liver failure. J Clin Exp Hepatol 5:88–95
Yang P, Zhao Y, Lu Y, Xu QZ, Xu XW, Dong L, Yu SH (2011) Phenol formaldehyde resin nanoparticles loaded with CdTe quantum dots: a fluorescence resonance energy transfer probe for optical visual detection of copper (II) ions. ACS Nano 5:2147–2154
Yang P, Zhao Y, Lu Y, Xu QZ, Xu XW, Dong L, Yu SH (2012) Polyamine-functionalized carbon quantum dots as fluorescent probes for selective and sensitive detection of copper ions. Anal Chem 84:6220–6224
Jin LH, Han CS (2014) Ultrasensitive and selective fluorimetric detection of copper ions using thiosulfate-involved quantum dots. Anal Chem 86:7209–7213
Bardajee GR, Hooshyar Z (2016) Probing the interaction of a new synthesized CdTe quantum dots with human serum albumin and bovine serum albumin by spectroscopic methods. Mater Sci Eng C 62:806–815
Bardajee GR, Hooshyar Z, Mizani F (2014) Improving optical properties of CdTe quantum dots by a new multidentae biopolymer based on salep. Mater Sci Semicond Process 19:89–94
Boonme C, Noipa T, Tuntulani T, Ngeontae W (2013) Cysteamine capped CdS quantum dots as a fluorescence sensor for the determination of copper ion exploiting fluorescence enhancement and long-wave spectral shifts. Spectrochim Acta Mol Biomol Spectrosc 169:161–168
Yang P, Zhu B, Zhao J, Yu H, Yan L, Wei Q, Dua B (2013) A novel reusable glutathione-modified magnetic fluorescent nanosensor for highly sensitive determination and removal of Cu2+. Inorg Chim Acta 408:120–125
Kim MS, Jo TG, Hye MA, Cheal K (2017) A colorimetric and fluorescent chemosensor for the selective detection of Cu2 + and Zn2 + ions. J Fluoresc 27:357–367
Das P, Ganguly S, Bose M, Mondal S, Das AK, Banerjee S, Das NC (2017) A simplistic approach to green future with eco-friendly luminescent carbon dots and their application to fluorescent nano-sensor ‘turn-off’ probe for selective sensing of copper ions. Mater Sci Eng C Mater Biol Appl 75:1456–1464
Tian J, Liu Q, Asiri AM, Al-Youbi AO, Sun X (2013) Ultrathin graphitic carbon nitride nanosheet: a highly efficient fluorosensor for rapid, ultrasensitive detection of Cu2+. Anal Chem 85:5595–5599
Zong C, Ai K, Zhang G, Li H, Lu L (2011) Dual-emission fluorescent silica nanoparticle-based probe for ultrasensitive detection of Cu2+. Anal Chem 83:3126–3132
Faridbod F, Jamali A, Ganjali MR, Hosseini M, Norouzi P (2015) A novel cobalt-sensitive fluorescent chemosensor based on ligand capped CdS quantum dots. J Fluoresc 25:613–619
Acknowledgements
The authors are grateful to the Research Council of Imam Khomeini International University (IKIU), University of Tehran and Payame Noor University for their financial support of this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Elmizadeh, H., Soleimani, M., Faridbod, F. et al. Ligand-Capped CdTe Quantum Dots as a Fluorescent Nanosensor for Detection of Copper Ions in Environmental Water Sample. J Fluoresc 27, 2323–2333 (2017). https://doi.org/10.1007/s10895-017-2174-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-017-2174-3