Abstract
In this work, a ligand capped CdS QDs was synthesized, characterized and its fluorescence behavior was studied. The surface of the CdS QDs was modified using N-(3-methyl-2-(thiophene-2-carboxamido) phenyl) thiophene-2-carboxamide. The immobilized ligand on the surface of the CdS QDs can interact by cationic species due to the existence of donating atoms in its structures. Thus, effect of some metal cations on the fluorescent intensity of the ligand capped CdS QDs were studied. It was found that fluorescence intensity of the modified CdS QDs quenched selectively by addition of Co(II) ion in comparison with other cations tested. The ligand capped CdS QDs can be used as a fluorescent bulk chemosensor for detection of Co(II) ions. The fluorescent quenching is linear in the range of 1.0 × 10−5 to 1.5 × 10−4 mol L−1 of Co(II) ions. The limit of detection was obtained 8.3 × 10−7 mol L−1. The nanosensor exhibits high selectivity toward Co(II) ions in comparison with common metal ions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Quantum dots (QDs) are semiconductor nanostructured materials. Their sizes range from 2 to 12 nm in diameter. Due to their small size, they show unique optical and electrical properties which are different from the bulk semiconductor materials. Their optical and electrical characteristics are closely related to the size and shape of their individual crystal.
Luminescent colloidal QDs can be used as fluorescent probes in analytical applications. Organic dyes have been quickly replaced with QDs because of their high extinction coefficient, high quantum yield, wide excitation spectrum, narrow emission spectrum, and their long-term photostability. It has been estimated that quantum dots are 20 times brighter and 100 times more stable than traditional fluorescent reagents [1–3]. The wavelength of their emissions depends on the material which QDs is made from and their size. Since fluorescence properties of quantum dots are directly related to their surface, by controlling the surface chemistry of QDs, it is possible to makes suitable fluorescent nanosensors.
Ion sensing based on analyte-induced changes in photoluminescence of QDs is a very attractive field of research. Capping strategies have been applied for designing QDs based nanosensors. Various capping agents, such as thioalkyl acid ligands (e.g., thioglycolic acid, TGA; dihydrolipoic acid, DHLA) were used for modification of the QDs surface [3]. In this way, the ligand is responsible for participating in sensing the metal ions. The modification of the surface can also increase the stability of the QDs and sometimes the modification can increase the solubility of QDs in the aqueous environment [3, 4].
Interaction of ions with ligand-capped QDs causes the controlling of their luminescence emission. As an example, fluorescent emission of CdS QD increased in presence of Zn2+ ion if it was functionalized by L-cysteine, and quenched in presence of Cu2+ ion if QDs were capped with thioglycerol [5].
In this work, a new ligand capped CdS QDs was synthesized and characterized. The used ligand for surface modification was N-(3-methyl-2-(thiophene-2-carboxamido)phenyl)thiophene-2-carboxamidee (MTPT), Fig. 1. Then, its fluorescent behavior in presence of metal cations were studied.
Experimental Section
Reagents and Materials
The ligand, N-(3-methyl-2-(thiophene-2-carboxamido)phenyl)thiophene-2-carboxamidee (MTPT) were synthesized and purified as previously described method [6]. Cd(NO3)2,4H2O, Na2S.9H2O, tris(hydroxymethyl)aminomethane, and other chemicals were purchased from Merck Co. (Germany). All aqueous solutions were prepared with double distilled water. The stock solutions of ions were prepared by dissolving suitable amount of nitrate salt in 5 mL doubly distilled water, and was further diluted whenever necessary.
Instruments
The fluorescence studies were performed using a Perkin-Elmer LS50 spectrofluorimeter. UV–Vis spectra and absorbance measurements were recorded on a PerkinElmer Lambda 2 UV–vis spectrophotometer. A Metrohm pH-meter with a double junction glass electrode was used for pH adjustment. FT-IR spectra were recorded by Bruker EQUINOX 55 Fourier Transform spectrometer. Hitachi FESEM S4160, and Philips CM 10 HT, 300 kv TEM instruments were used for QDs characterizations.
Synthesize of Ligand Base-Capped CdS QDs
MTPT capped CdS QDs were synthesized by the colloidal synthesis procedures which previously described [7, 8]. Colloidal QDs are synthesized from precursor compounds dissolved in solutions, like traditional chemical processes. The synthesis of colloidal quantum dots is based on a three-component system composed of precursors, organic surfactants, and solvents. The temperature during the process is one of the critical factors in determining optimal conditions for the nanocrystal growth. The temperature should be high enough to allow rearrangement and annealing of atoms during the synthesis process and being low enough to promote crystal growth. Another critical factor which should be controlled during nanocrystal growth is the monomer concentration. Here, 1.0 mmol of MTPT dissolved in 10 mL of acetonitrile and diluted with tris-buffer (0.5 mol L−1, pH = 10) to 100 mL and transfer into a three necked round bottom 250 mL flask. The solution was purged with pure nitrogen gas for about 60 min in 45 °C under magnetic stirring. 0.5 mmol of Cd(NO3)2 was dissolved in 100 mL of distilled water and then added to the flask and purged and stirred again. Finally, 0.05 mmol L−1 solution of S2− (from dissolving 0.5 mmol Na2S in 10-mL water) was added drop wise slowly into the flask. The seeds solution (prepared by mixing 2 mL of 10−4 mol L−1 Cd2+ solution and the same amount of S2− solution) was injected into this mixture solution under strong magnetic stirring at 45 °C. The bright yellow-green colloid was obtained after 2 h. To remove most of the un-reacted precursors, the colloidal solution was washed by ethanol several times. Then, the colloid solution was stored at room temperature in dark. The solution can be stable without any precipitation during several months. Then the resulting MTPT-capped CdS QDs were characterized.
Fluorescent Studies
3.0 mg L−1 of MTPT-capped CdS QDs solution was suspended into tris-chloride buffer solution (pH 7.5, 0.01 mol L−1). 3 mL of the MTPT-capped CdS QDs solution was then transferred into a 1 cm × 1 cm fluorescent quartz cell. The fluorescence spectrum was taken. Excitation wave length was selected 370 nm and maximum of emission wave length was at 470 nm. Both excitation and emission slit were 10 nm. In titration of MTPT-capped CdS QDs with metal cations, different volumes of ion stock solutions (1 × 10−3 mol L−1) were added to the cell. The mixture was briefly shaken for a few seconds before making the fluorescence measurements then spectrum was taken. The relative fluorescence intensity was measured at the same excitation wavelength.
UV–vis Studies
The absorption spectrum of the 3.0 mg L−1 of MTPT-capped CdS QDs solution suspended into Tris-chloride buffer solution (pH 7.5, 0.01 mol L−1) was taken in 1 cm × 1 cm quartz cell.
Results and Discussion
Characteristics of the MTPT-Capped CdS QDs
MTPT-capped CdS QDs was characterized by FESEM and TEM (Fig. 2). The image shows that the shapes of the MTPT-capped particles are dispersed spherical and it seems the sizes of the particles are under 10 nm in diameter. Also the homogeneity in the size of the particles can be seen.
The UV–Vis and fluorescence spectra are powerful tools to confirm the QDs properties. The optical properties of MTPT-capped CdS QDs were characterized by UV–Vis absorption spectrometry and fluorescence spectroscopy. The absorption peak of the MTPT-capped CdS nanoparticles and its fluorescence were shown in Fig. 3. The fluorescence emission maximum of the functionalized CdS QD is obtained at 470 nm when excited by the radiation of 370 nm. It should be noted that MTPT ligand has no intrinsic fluorescence emission.
The average particles size of QDs can be calculated using the relation between particles size and absorption edge [9–12]. The absorption edge (λe) is converted into the corresponding particle size by using Henglein’s empirical curve:
Where (λe) is the absorption edge and (2R) is the diameter of the particles. From Fig. 4, the absorption edge (λe) is obtained 265 nm. Since the particle size was estimated around 1.29 nm from Eq. (1).
Diameter of CdS QDs can be also calculated from the wave length of the first excitonic absorption peak of the corresponding sample by an empirical equation [13]:
where D (nm) is the size of a given nanocrystal sample, and λ(nm) is the wavelength of the first excitonic absorption peak of the corresponding sample, here, 265 nm and the D was calculated around 1.31 nm.
Fluorescent Nanosensor
The use of luminescent QDs in the selective detection of cations has been almost grown [14]. Surface ligands can play an important role in ion selective sensing. They can have significant effects on fluorescence responses of QDs to metal ions. In fact, the ligands are responsible for sensing the metal ions. Hence, modification of QDs surfaces by suitable ligands possessing heteroatoms in their structures, leads to designing new fluorescent nanosensors.
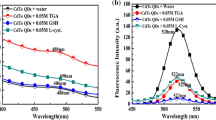
Transition metal ions can reduce the fluorescence intensity of QDs by interaction of the ions on the surface of nanoparticles [14, 15]. In this work, we found that the fluorescence intensity of MTPT-capped CdS QDs can be quenched by addition of Co(II) (Fig. 4), but other metal ions do not have significant effect on fluorescence of MTPT-capped CdS QDs. Thus, we suggest that the fluorescence quenching could be attributed to binding of Co(II) ions to the surface-capping molecules on CdS QDs through charge-dipole interaction.
The new fluorescent nanosensor was synthesized based on MTPT-capped CdS QDs for determination of Co(II) ions. The effect of Co(II) ions on the fluorescence emission of the new synthesized CdS QDs is Shown in Fig. 4. The fluorescence intensity of MTPT-capped CdS QDs was significantly decreased by addition of Co(II). Fig. 4, picture inserted, shows the effect of addition of cobalt ions on the new synthesized quantum dots under UV lamp (254 nm). It was found that Co(II) quenches the fluorescence of QDs in a concentration-dependent manner that was best described by the Stern–Volmer equation [16–19], which is given by the following equation:
Where F0 and F are the fluorescence intensity in the absence and presence of the quencher (Co(II)), KSV is the Stern-Volmer quenching constant, and [Q] is the concentration of the quencher. Fig. 5 shows the fluorescence intensity vs. concentration of the quencher (a), stern-volmer plots for different concentration range of Co(II) concentrations (b). Also, to have a better determination coefficient (R2) for the measurements, modified stern-volmer curve (Fig. 5c) also plotted. Under the optimum condition, the linearity of the Stern–Volmer plot is in the range of 1.0 × 10−5 to 1.5 × 10−4 mol L−1. The detection limit of this sensor is 8.3 × 10−6 mol L−1.
The Effect of the Concentration of the MTPT-Capped CdS QDs and pH on Fluorescence Intensity
The effect of the concentration of the MTPT-capped CdS QDs on fluorescence intensity was studied. The optimal concentration of the MTPT-capped CdS QDs QDs (3.0 mg. L−1) gives the highest sensitivity (i.e., the slope of calibration function) and the widest linear range of the calibration function. Fluorescence intensity decreases with further increase of the concentration of MTPT-capped CdS QDs due to possible self quenching effect of fluorescence of QDs.
pH is one of the major parameters that have effect on the measurements. Therefore the effect of pH on the fluorescence intensity of this system was studied to obtain an optimum pH to develop a sensitive fluorescence sensor for Co(II) ion. The fluorescence intensity was studied against pH in the range of 3.0 and 9.0 in order to obtain the optimum conditions for the determination of cobalt ion. The optimum fluorescence intensity was obtained in the pH range between 6.5-8.5. Therefore a Tris buffer of pH of 7.5 was used for the determination studies. The reason for the low fluorescence intensity in acidic medium is the result of dissociation of the ligand from the surface of nanoparticles due to the protonation of the hetroatoms group on the capped ligand. At higher pH the concentration of cobalt ion decreases due to precipitation of Cd(OH)2.
Effect of Other Interfering Ions
The fluorescence emission of MTPT-capped CdS QDs was significantly decreased without any emission band shift with the addition of Co(II). As it can be seen in Fig. 6, the influence of other ions was very weak, i.e., MTPT-capped CdS QDs were rather selective towards cobalt ion which makes it very attractive for the selective recognition of Co(II). Fig. 6 displays the typical fluorescence spectra as functions of the concentration of ions. It is observed that the emission intensity reduces successively with the increasing of Co(II) ion concentration.
Conclusions
A novel fluorescent nano-chemosensor based on ligand capped CdS QDs for selective and sensitive detection of Co(II) in aqueous solution was successfully constructed. These ion-sensitive QDs showed good selectivity to copper ions in comparison with other cations. The proposed nano-chemosensor was based on a strong and selective quenching of QDs fluorescence emission. Under the optimum conditions, the calibration plot was linear in the range of 1.0 × 10−6 to 1.5 × 10−4 mol L−1 and the detection limit of this sensor is calculated 8.3 × 10−7 mol L−1.
References
Schaller RD, Klimove VI (2004) High efficiency carrier multiplication in PbSe nanocrystals: implications for solar energy conversion. Phys Rev Lett 92:186601–186601
Bowers MJ, McBride JR, Rosenthal SJ (2005) Bright white light emission from ultrasmall cadmium selenide nanocrystals. J Am Chem Soc 127:15378–15379
Frasco MF, Chaniotakis N (2009) Semiconductor quantum dots in chemical sensors and biosensors. Sensors 9:7266–7286
Jamieson T, Bakhshi R, Petrova D, Pocock R, Imani M, Seifalian AM (2007) Biological applications of quantum dots. Biomaterials 28:4717–4732
Chen YF, Rosenzweig Z (2002) Luminescent CdS quantum dots as selective Ion probes. Anal Chem 74:5132–5138
Meghdadi S, Amirnasr M, Habibi MH, Amiri A, Ghodsi V, Rohani A, Harrington RW, Clegg W (2008) Synthesis, structure, and electrochemistry of pyridinecarboxamide cobalt(III) complexes; the effect of bridge substituents on the redox properties. Polyhedron 27:2771–2278
Wang Q, Pan D, Ji X (2005) A new two-phase route to high-quality CdS nanocrystals. Chem Eur J 11:3843–3848
Murray CB, Sun SH, Gaschler W, Doyle H, Betley TA, Kagan CR (2001) Colloidal synthesis of nanocrystals and nanocrystal Superlattices. IBM J Res Dev 45:47–56
He R, Qian XF, Yin J, Xi HA, Bian LJ, Zhu ZK (2003) Formation of monodispersed PVP-capped ZnS and CdS nanocrystals under microwave irradiation. Coll Surf A 220:151–157
Huy BT, Seo MH, Lim JM, Shin DS, Lee YI (2011) A systematic study on preparing the CdS quantum dots. J Korean Phys Soc 59:3293–3299
Zhang Y, Chen Y, Niu H, Gao M (2006) Formation of CdS nanoparticle necklaces with functionalized dendronized polymers. Small 2:1314–1319
Shankara Narayanan S, Kumar Pal S (2006) Aggregated CdS quantum dots: host of biomolecular ligands. J Phys Chem B 110:24403–24409
Yu WW, Qu L, Guo W, Peng X (2003) Experimental determination of the extinction coefficient of CdTe, CdSe, and CdS nanocrystals. Chem Mater 15:2854–2860
Xie HY, Liang HG, Zhang ZL, Liu Y, He ZK, Pang DW (2004) Uminescent CdSe-ZnS quantum dots as selective Cu2+ probe. Spectrochim Acta A 60:2527–2530
Liang JG, Ai XP, He ZK, Pang DW (2004) Functionalized CdSe quantum dots as selective silver ion chemodosimeter. Analyst 129:619–622
Hosseini M, Gupta VK, Ganjali MR, Rafiei-Sarmazdeh Z, Faridbod F, Goldooz H, Badiei AR, Norouzi P (2012) A novel dichromate-sensitive fluorescent nano-chemosensor using new functionalized SBA-15. Anal Chim Acta 715:80–85
Ganjali MR, Gupta VK, Hosseini M, Rafiei-Sarmazdeh Z, Faridbod F, Goldooz H, Badiei AR, Norouzi P (2012) A novel permanganate-sensitive fluorescent nano-chemosensor assembled with a new 8-hydroxyquinoline-functionalized SBA-15. Talanta 88:684–688
Hosseini M, Ganjali MR, Veismohammadi B, Faridbod F, Norouzi P, Abkenar SD (2010) Novel selective optode membrane for terbium ion based on fluorescence quenching of the 2-(5-(dimethylamino) naphthalen-1-ylsulfonyl)-N-henylhydrazinecarbothioamid. Sensors Actuators B 147:23–30
Hosseini M, Ganjali MR, Veismohammadi B, Faridbod F, Abkenar SD, Norouzi P (2010) Determination of terbium in phosphate rock by Tb3+-selective fluorimetric optode based on dansyl derivative as a neutral fluorogenic ionophore. Anal Chim Acta 664:172–177
Acknowledgments
The authors thank the research council of University of Tehran for financial support of this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Faridbod, F., Jamali, A., Ganjali, M.R. et al. A Novel Cobalt-Sensitive Fluorescent Chemosensor Based on Ligand Capped CdS Quantum Dots. J Fluoresc 25, 613–619 (2015). https://doi.org/10.1007/s10895-015-1544-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-015-1544-y