Abstract
A turn-on fluorescent probe (FN) for detection of hydrazine has been developed. Probe FN exhibited high selectivity and excellent sensitivity towards hydrazine with a detection limit as low as 4.6 × 10−10 M. Probe FN selectively reacts with hydrazine (N2H4) in a physiological environment, leading to an off-on fluorescence response along with the color change from colorless to yellow, allowing colorimetric detection of hydrazine by the naked eye. Furthermore, probe FN was successfully applied for visualizing hydrazine in living cells.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hydrazine is widely used as an important industrial material in chemical, pharmaceutical, and agricultural areas due to its vital roles [1–8]. And it is well-known as high-energy fuel in rocket propulsion and missile systems owning to its improved detonable properties. However, hydrazine is extremely toxic and can be easily absorbed by oral, dermal and inhalation exposure routes [9–11]. It should be noted that chronic exposure to high concentration hydrazine may damage various organs and destroy the central nervous system [4, 12, 13]. As a result, hydrazine has been classified as a potential human carcinogen substance by the U.S. Environmental Protection Agency (EPA) and World Health Organization (WHO) with allow threshold limit value (TLV) of 10 ppb [14, 15]. Therefore, it is urgently needed to develop effective analytical techniques to detect trace amount of hydrazine with satisfactory selectivity and sensitivity.
Various hydrazine detection methods, such as chemiluminescence, [16] electrochemical analysis [17, 18] and chromatography [19] have been developed for hydrazine detection. Unfortunately, they are always expensive, time-consuming and complicated, in addition, they are not suitable for real-time and on-site analysis. The fluorescent spectroscopic technique has received increasing attention due to its high sensitivity and specificity, non-invasiveness, and good compatibility for biosamples [20–25]. Till now, a number of fluorescent probes for hydrazine have been developed [26–50]. However, most of them need long response time and few of them work under neutral conditions or the unsatisfactory detection limit. So far, chemical small-molecular fluorescent probes for hydrazine are still very limited. Thus, it has been still a great challenge to develop facile and reliable small-molecule fluorescent probers for the rapid and effective determination of hydrazine both qualitatively and quantitatively.
The reported hydrazine detection probe chose malononitrile group [26–32], acetate group [33–40], 4-bromobutyrate masking unit [41–43], trifluoroacetylacetone group [44, 45], phthalimide group [46–48] or aldehyde group [49, 50] as the sensing part. In this work, we chose р-nitrobenzoic acid as the sensing part. We envision that the strong electron-withdrawing group (−NO2) will contribute to the deprotection, thus the response time or detection limit will be better. As we expected, this probe can distinguish hydrazine over other biologically relevant anions and cations in about 2 min in PBS-DMSO (10 mM, pH = 7.4, 1:4, v/v) solution at room temperature with a detection limit of 4.6 × 10−10 M. To the best of our knowledge, the detection limit is lower than most of the reported [Table S1]. It also has the shortest response time except Xu’s reported [46, 48]. Herein, we reported its simple synthesis, optical properties and application for imaging of hydrazine in living MIA PaCa-2 cells.
Experimental
Apparatus Regents and Chemical
All chemicals and reagents were used as received from commercial sources without further purification. Deionized water was used throughout all experiment. Hydrazine and other ions stock solutions (10.0 mM) were prepared in water, while stock solution of probe FN (1.0 mM) was prepared in DMSO. The stock solution was diluted to obtain the final concentration of the probe with the solution of PBS-DMSO (10 mM, pH = 7.4, 1:4, v/v) 1H and 13C NMR spectra were carried out on a Bruker DTX-400 spectrometer using TMS as internal reference. ESI mass spectra were performed on an HPLC Q-Tof HR-MS spectrometer. UV-vis spectra were measured on an Agilent 8453 spectrophotometer. Fluorescence spectra measurements were performed on a HITACHI F-7000 fluorescence spectrophotometer. The melting points were determined by an X-4 microscopic melting apparatus with a digital thermometer (Shanghai, China). The pH was measured with a Model pHs-3C meter (Shanghai, China).
Synthesis of Probe FN
р-nitrobenzoic acid (500 mg, 3 mmol), EDC (570 mg, 3 mmol) and DMAP (120 mg, 1 mmol) were combined and dissolved in DCM (25 mL). The reaction mixture was stirred for 10 min at room temperature followed by the addition of fluorescein (330 mg, 1 mmol). The reaction mixture was stirred at room temperature overnight. The solvent was removed under reduced pressure, and the crude product was purified by column chromatography on silica gel using CH2Cl2 as eluent to give FN as a white solid (79 % yield). mp 178–180 °C; 1H NMR (400 MHz, DMSO-d6, ppm): δ 8.40 (dd, J = 8.4 Hz, 8 H), 8.10 (d, J = 7.4 Hz, 1 H), 7.78–7.88 (m, 2 H), 7.57 (s, 2 H), 7.46 (d, J = 7.4 Hz, 1 H), 7.20 (d, J = 8.3 Hz, 2 H), 7.01 (d, J = 8.6 Hz, 2 H); 13C NMR (100 MHz, CDCl3, ppm): 169.03, 162.81, 152.76, 151.84, 151.64, 151.08, 135.48, 134.34, 131.44, 130.28, 129.33, 126.09, 125.42, 124.05, 123.84, 117.72, 117.14, 110.47, 81.39. HR-MS: m/z, calcd for C34H18N2O11 [M + H] + 631.0983, found: 631.0981. ( Supporting Information).
Results and Analysis
Compound FN was synthesized according the reported methods [51]. The structure of title compound FN was well characterized by 1H NMR, 13C NMR and HR-MS (Supporting information, Fig. S1-S3). The results were in good agreement with the structure shown in Scheme 1.
UV-Vis Spectral Responses of FN with Hydrazine
With this probe in hand, we first investigated its absorption spectral changes upon the addition of hydrazine and other analytes in PBS-DMSO (10 mM, pH = 7.4, 1:4, v/v) solution at room temperature. As shown in Fig.1, in the absence of hydrazine, the UV-vis spectra of FN (10 μM) exhibited only very weak absorption owing to the closed spirolactone form of fluorescein. However, upon the addition of hydrazine (10 equiv.), the absorption at 515 nm simultaneously increased resulting from the ring-opened fluorescein. The color of the solution changed from colorless to yellow after the addition of hydrazine. Whereas, no obvious response occurred upon the addition of other analytes including K+, Fe3+, Zn2+, Cu2+, Mg2+, Co2+, Ba2+, Cr3+, Cd2+, Hg2+, Mn2+, Ni2+, Pb2+, F−, Cl−, Br−, I−, CH3COO−, N3 −, NO3 −, HSO3 −, SO3 2−, HCO3 −, H2PO4 − and HPO4 2− under the same test condition. These results demonstrated that FN can be served as a visual probe for hydrazine, which would allow the colorimetric detection of hydrazine directly by the naked-eye.
Fluorescence Spectral Responses of FN with Hydrazine
Then, we investigated the fluorescence emission spectra of probe FN (Fig. 2). In the absence of hydrazine, the free probe displayed a very weak emission. However, a significant enhancement in fluorescence intensity was observed when hydrazine was added. Other biologically relevant analytes such as K+, Fe3+, Zn2+, Cu2+, Mg2+, Co2+, Ba2+, Cr3+, Cd2+, Hg2+, Mn2+, Ni2+, Pb2+, F−, Cl−, Br−, I−, CH3COO−, N3 −, NO3 −, HSO3 −, SO3 2−, HCO3 −, H2PO4 − and HPO4 2− almost could not lead to any notably changes under the same test condition.
To further demonstrate the ability of FN to detect hydrazine in the presence of other competitive cations and anions, the anti-interference experiment of FN was also conducted. As shown in Fig.3, other competitive analytes did not induce any appreciable fluorescence change at 536 nm. In the presence of these interferents, prober FN still showed the same turn-on fluorescence response to hydrazine as that in the absence of these interferents except that Hg2+ imposed little interference. The above results indicated that FN possessed high selectivity toward hydrazine over other cations and anions.
The Detection of Hydrazine
Generally, the detection limit is a very important parameter to assess a new probe. We subsequently examined the sensitivity of FN for hydrazine detection by varying concentrations of hydrazine (0–80 μM) (Fig. 4). The continuous addition of hydrazine leads to a gradual increase of emission intensity at about 536 nm. A good linear relationship between fluorescence intensity and hydrazine concentration was found. The detection limit (3σ/slope) [52] of probe FN for the determination of hydrazine was found to be 4.6 × 10−10 M, which is far below the TLV (10 ppb) recommended by EPA. These results suggested that probe FN can detect hydrazine both qualitatively and quantitatively.
Time Dependence
Reaction time is also a vital factor to evaluate the feasibility of a new probe for real time detection. Therefore, the time dependence of the response of FN to hydrazine was evaluated. As shown in Fig. 5, the fluorescence intensity of FN and hydrazine reached a plateau in about 2 min, after which the fluorescence intensity remained almost constant. However, the emission intensity of blank solution (only FN, 10 μM) almost unchanged at the same condition. The time-dependent change plot demonstrates the reaction could complete in about 2 min, which indicates that probe FN has a fast response for hydrazine.
Effect of pH
For practical applicability, the proper pH condition of this new probe for hydrazine detection was also evaluated. We investigated the fluorescence properties of probe FN and that probe FN with hydrazine under different pH values, respectively (Fig. S4). It can be seen, probe FN was pH insensitive, and its emission intensity was quite weak from pH 1 to 9. However, the fluorescent sensing toward hydrazine was obviously affected by the change of pH values. Fluorescence signal reach its maximum and keep constant around biologically relevant pH 5 to 9, indicative of its potential for application in biological systems.
Sensing Mechanism
According to the previous reported work [33, 43], we proposed that the strong fluorescence enhancement is attributed to the deprotection of р-nitrobenzoyl group and concurrent generation of fluorescein. Probe FN is colorless and non-fluorescent owning to the closed spirolactone form of fluorescein. After react with hydrazine, the ring-opened fluorescein was released and caused strong fluorescence. In order to verify our speculation, the reaction products of probe FN and hydrazine were subjected to ESI-MS spectrum. A major ion peak was founded at m/z = 331.17 (Fig. S5), corresponding to the resulting fluorescein ([M-H]−), clearly confirmed the proposed mechanism as shown in Scheme 2.
Fluorescence Imaging in Living Cells
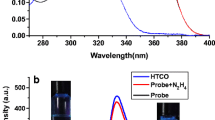
Finally, the bioimaging application of probe FN to detect hydrazine in living cells was also investigated (Fig. 6). As controls, MIA PaCa-2 cells were incubated with only FN (10 μM) for 30 min at 37 °C which showed almost no intracellular fluorescence (Fig. 6b). After treatment of N2H4 (50 μM) and then incubated for further 30 min, a significant fluorescence signal was observed and collected via fluorescence microscopy in the green channel (Fig. 6d). Furthermore, a bright field transmission image of cells treated with FN and N2H4 confirmed that these cells were viable throughout the imaging experiments (Fig. 6a and c). Therefore, these results demonstrated that probe FN was cell membrane permeable and capable of fluorescence imaging of hydrazine in biological samples.
Conclusions
In conclusion, we have rationally designed and synthesized a fluorescence off-on probe, which can exhibit a good selectivity toward hydrazine over other competitive species, with the detection limit of 4.6 × 10−10 M. The colorimetric and fluorescent response to hydrazine can be conveniently detected even by the naked eye, which provides a facile approach for visual detection of hydrazine. The living cell imaging demonstrated its value of practical application in biological systems.
Reference
Reilly CA, Aust SD (1997) Chem Res Toxicol 10:328–334
Ragnarsson U (2001) Chem Soc Rev 30:205–213
Jayasri D, Narayanan SS (2007) J Hazard Mater 144:348–354
Zelnick SD, Mattie DR, Stepaniak PC (2003) Aviat., Space. Environ. Med. 74:1285–1291
Umar A, Rahman MM, Kim SH, Hahn YB (2008) Chem, Commun:166–168
Liu JP, Li YY, Jiang J, Huang XT (2010) Dalton Trans 39:8693–8697
Zhao ZL, Zhang G, Gao Y, Yang XY, Li YH (2011) Chem Commun 47:12816–12818
Wang J, Chen L (1995) Anal Chem 67:3824–3827
Troyan JE (1953) Ind Eng Chem 45:2608–2612
Keller WC (1988) Aviat Space Environ Med 59:100–106
International Agency for Research on Cancer (1999) Re-evaluation of some organic chemicals, hydrazine, and hydrogen peroxide. IARC monographs on the evaluation of carcinogenic risk of chemicals to humans. Lyon, IARC 71:991–1013
Vieira IC, Lupetti KO, Fatibello-Filho O (2002) Anal Lett 35:2221–2231
Toth B (1975) Cancer Res 35:3693–3697
Choudhary G, Hansen H (1998) Chemosphere 37:801–843
U.S. Environmental Protection Agency (EPA) (1999) Integrated Risk Information System (IRIS) on Hydrazine/Hydrazine Sulfate, National Center for Environmental Assessment. Office of Research and Development, Washington, DC
Safavi A, Karimi MA (2002) Talanta 58:785–792
Benvidi A, Kakoolaki P, Zare HR, Vafazadeh R (2011) Electrochim Acta 56:2045–2050
Cheng J, Yang E, Ding P, Tang J, Zhang D, Zhao Y, Ye Y (2015) Sensor Actuat B-Chem 221:688–693
Sun MJ, Bai L, Liu DQ (2009) J Pharm Biomed Anal 49:529–533
Song J, Zhang D, Liu Y, Zhao Y, Ye Y (2015) New J Chem 39:6284–6288
Zhang JF, Zhou Y, Yoon J, Kim JS (2011) Chem Soc Rev 40:34163429
Li G, Tang J, Ding P, Ye Y (2016) J Fluoresc 26:155–161
Jung HS, Chen XQ, kim JS, Yoon J (2013) Chem. Soc. Rev. 42:6019–6031
Chen XQ, Pradhan T, Wang F, Kim JS, Yoon J (2012) Chem Rev 112:1910–1956
Zheng H, Zhan XQ, Bian QN, Zhang XJ (2013) Chem Commun 49:429–447
Li S, Zhang D, Wang M, Ma S, Liu J, Zhao Y, Ye Y (2016) J Fluoresc 26:769–774
Fan J, Sun W, Hu M, Cao J, Cheng G, Dong H, Song K, Liu Y, Sun S, Peng X (2012) Chem Commun 48:8117–8119
Zhao J, Xu Y, Li H, Lu A, Sun S (2013) New J Chem 37:3849–3852
Lin Y, Chow TJ (2013) RSC Adv 3:17924–17929
Goswami S, Paul S, Manna A (2013) RSC Adv 3:18872–18877
Zheng XX, Wang SQ, Wang HY, Zhang RR, Liu JT, Zhao BX (2015) Spectrochim. Acta. Part A 138:247–251
Chen B, Sun X, Li X, Agren H, Xie Y (2014) Sens. Actuators. B 199:93–100
Qu DY, Chen JL, Di B (2014) Anal Methods 6:4705–4709
Li K, Xu HR, Yu KK, Hou JT, Yu XQ (2013) Anal Methods 5:2653–2656
Zhu SS, Lin WY, Yuan L (2013) Anal Methods 5:3450–3453
Choi MG, Moon JO, Bae J, Lee JW, Chang SK (2013) Org Biomol Chem 11:2961–2965
Sun Y, Zhao D, Fan SW, Duan L (2015) Sens Actuator B 208:512–517
Choi MG, Hwang J, Moon JO, Sung J, Chang S-K (2011) Org Lett 13:5260–5263
Hu C, Sun W, Cao J, Gao P, Wang J, Fan J, Song F, Sun S, Peng X (2013) Org Lett 15:4022–4025
Liu B, Liu Q, Shah M, Wang JF, Zhang G, Pang Y (2014) Sensors Actuators B Chem 202:194–200
Goswami S, Das S, Aich K, Pakhira B, Panja S, Mukherjee SK, Sarkar S (2013) Org Lett 15:5412–5415
Goswami S, Aich K, Das S, Roy SB, Pakhira B, Sarkar S (2014) RSC Adv 4:14210–14214
Qian Y, Lin J, Han LJ, Lin L, Zhu HL (2014) Biosens Bioelectron 58:282–286
Goswami S, Das S, Aich K, Sarkar D, Mondal TK (2014) Tetrahedron Lett 55:2695–2699
Lee MH, Yoon B, Kim JS, Sessler JL (2013) Chem Sci 4:4121–4126
Cui L, Ji C, Peng Z, Zhong L, Zhou C, Yan L, Qu S, Zhang S, Huang C, Qian X, Xu Y (2014) Anal Chem 86:4611–4617
Zhao XX, Zhang JF, Liu W, Zhou S, Zhou ZQ, Xiao YH, Xi G, Miao JY, Zhao BX, Mater J (2014) Chem B 2:7344–7350
Cui L, Peng Z, Ji C, Huang J, Huang D, Ma J, Zhang S, Qian X, Xu Y (2014) Chem Commun 50:1485–1487
Chen X, Xiang Y, Li Z, Tong A (2008) Anal Chim Acta 625:41–46
Xiao LL, Tu J, Sun SG, Pei ZC, Pei YX, Pang Y, Xu YQ (2014) RSC Adv 4:41807–41811
Zhang D, Chen W, Miao ZR, Ye Y, Zhao YF, King SB, Xian M (2014) Chem Commun 50:4806–4809
IUPAC (1981) Compendium of Analytical Nomenclature, DefinitiveRules, ed. H. M. N. H. Irving, H. Freiser and T. S. West,Pergamon Press, Oxford
Acknowledgments
This work was supported by the National Science Foundation of China (Nos. 21572209) and Program for Innovative Research Team (in Science and Technology) in University of Henan Province (No. 17IRTSTHN002).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
ESM 1
(DOC 1074 kb)
Rights and permissions
About this article
Cite this article
Li, G., Liu, Y., Song, J. et al. A Fluorescein-Based Colorimetric and Fluorescent Probe for Hydrazine and its Bioimaging in Live Cells. J Fluoresc 27, 323–329 (2017). https://doi.org/10.1007/s10895-016-1961-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-016-1961-6