Abstract
A rhodamine-benzimidazole based chemosensor was designed and prepared for Fe3+ via opening of the spiro-ring to give fluorescent and colored species. L exhibited high selectivity and excellent sensitivity in both absorbance and fluorescence detection of Fe3+ in aqueous solution with comparatively wide pH range (5.8–7.4). The detection limit of this newly developed probe was shown to be up to 2.74 μM. The reversibility establishes the potential of both probes as chemosensors for Fe3+ detection. Fluorescence imaging experiments of Fe3+ in living MGC803 cells demonstrated its value of practical applications in biological systems.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Among transition metal ions, iron is the most abundant essential trace element for both plants and animals. It plays an important role in enzyme catalysis, cellular metabolism, DNA, RNA synthesis [1, 2] and as an oxygen carrier in haemoglobin and a cofactor in many enzymatic reactions involved in the mitochondrial respiratory chain [3–6]. Besides the beneficial effects, less iron in the body has been reported linked to diabetes, anemia, liver and kidney damages, and heart diseases [7]. While, deposition of iron in the central nervous system has been involved in a number of diseases, such as Parkinson’s and Alzheimer’s disease, associated with an increased quantity of iron [8]. Much effort has been focused on the development of fluorescent Fe3+ indicators, especially those that exhibit selective Fe3+-amplified emission [9–12].
Fluorescent sensors for metal ions have consistently demonstrated their potential in a variety of fields, such as biological probes, environmental sensors [13–15]. The rhodamine framework is an ideal mode to construct fluorescent chemosensors due to its excellent photophysical properties such as long absorption and emission wavelength, large absorption coefficient and high fluorescence quantum yield [16]. To data, some rhodamine based probes for Fe3+ have been reported [17–21], however, an organic cosolvent was needed to guarantee a high probe affinity for Fe3+ and there were only a few successful examples of fluorescent probes for detecting Fe3+ ions being used in natural water samples for Fe3+.
Herein, a rhodamine-benzimidazole chemosensor was prepared by a new method which is different to Han’s [22]. The title compound could sensitively and selectively detect Fe3+ in Tris-HCl buffer solution. It also displayed enhanced fluorescence intensities and clear color changes upon recognition. Moreover, fast response (in 10 s) and neutral aqueous medium for Fe3+ made it possible to be practical application. The probe could be applied in biological systems for the detection of Fe3+ through confocal laser scanning microscopy experiments.
Experimental
Apparatus Reagents and Chemicals
Fluorescence spectra measurements were performed on the F-4500 FL Spectrophotometer, and the excitation and emission wavelength band passes were both set at 5.0 nm. Absorption spectra were measured on a UV-2102 double-beam UV/VIS spectrometer, Perkin Elmer precisely. NMR spectra were recorded on a BrukerDTX-400 spectrometer in CDCl3, using TMS as internal standard. Mass spectral determination was carried on a HPLC Q-T of HR-MS.
All the materials for synthesis were purchased from commercial suppliers and used without further purification. The solutions of metal ions were prepared from their nitrate salts, except for FeCl3, FeCl2, CrCl3, AlCl3 and MnCl2. The metal ions were prepared as 10.00 mM in water solution.
Synthesis
Synthesis of Compound L
As shown in Scheme 1, compound 1 was synthesized according to the literature [23]. The concrete synthesis way of compound L was described as follows: Compound 1 (266 mg, 0.5 mmol) and Lawesson’s reagent (243 mg, 0.6 mmol) were dissolved in dry toluene, and the reaction mixture was stirred at 110 °C for 24 h under N2 atmosphere. After removing the solvent under reduced pressure, the residue was purified by column chromatography using EA/PE = 1:1 as eluent to afford L (202 mg, 78.6 %).1H-NMR(400 MHz, CDCl3): 1.14 (t, 12 H, J = 7 Hz), 3.31 (q, 8 H, J = 7 Hz), 6.15 (q, 2 H, J = 3.7 Hz), 6.31 (d, 2 H, J = 8.8 Hz), 6.49 (d, 2 H, J = 2.4 Hz),6,94 (d, 1 H, J = 8 Hz), 7.02 (t, 1 H, J = 7.4 Hz), 7.16 (t, 1 H, J = 7.4 Hz), 7.22 (d, 1 H, J = 7.6 Hz), 7.38 (q, 1 H, J = 5 Hz), 7.49 (q, 1 H, J = 5 Hz), 7.8 (d, 1 H, J = 8.4 Hz), 8.09 (s, 1 H). 13C NMR (100 MHz, CDCl3) δ (ppm): 12.6, 44.3, 97.7, 106.2, 108.1, 110.2, 120.0, 121.4, 121.9, 122.5, 124.8, 127.8, 128.3, 128.6, 130.5, 130.9, 148.5, 148.9, 153.0, 156.1, 156.4. HR-MS: C34H35N4O [M + H]+, calculated for 515.2805. Found: 515.2807. (Supporting Information, Figs. S1–S3).
Results and Analysis
The structure of compounds L was characterized by 1H NMR, 13C NMR, and HR-MS. The results were in good agreement with the structure showed in Scheme 1. Fluorescence and UV–vis studies were performed using a 10 μM solution of L in a Tris-HCl (2.5 mM) buffer solution with appropriate amounts of metal ions. Solutions were shaken for 15 min before measuring the absorption and fluorescence in order to make the metal ions chelate with the sensors sufficiently.
UV–vis Spectral Responses of L
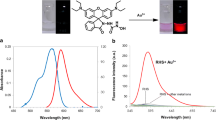
As shown in Fig. 1 , UV–vis spectrum of compound L (10 μM) exhibited only very weak bands over 450 nm. Adding of 10 equiv. Fe3+ into solution immediately resulted in a significant enhancement of absorbance at about 554 nm, and simultaneously accompanying with the color changes from colorless to red. Under the identical condition, no obvious response could be observed upon the addition of other ions including Zn2+, Cr3+, Mg2+, Ca2+, Cd2+, Al3+, Pb2+, Hg2+, Ba2+, Ni2+, Fe2+, Mn2+, K+, Li+, Ag+, Co2+ and Na+ except for Cu2+ (Fig. 1), which caused a mild effect compared to Fe3+. The results demonstrated that L was characteristic of high selectivity toward Fe3+ over other competitive metal ions.
To further investigate the interaction of Fe3+ and L, an ultraviolet titration experiment was carried out (Fig. 2). A linear increasing of absorption intensity of L could be observed accompanying with color changes from colorless to red along with the increasing concentrations of Fe3+ (Fig. 1, inset). To determine the stoichiometry of the iron–ligand complex, Job’s method for absorbance measurement was applied [24] (Fig. S4). The absorbance reached a maximum when the ratio being 0.5, indicating a 1:1 stoichiometry of the Fe3+ to L in the complex.
Fluorescence Spectral Responses of L
The selectivity of L for Fe3+ was further observed in the fluorescent spectra. As shown in Fig. 3, L exhibited a very weak fluorescence in the absence of metal ions. When 10 equiv. Fe3+ was introduced in a 10 uM solution of L in Tris-HCl buffer solution (2.5 mM, pH = 7.0), a remarkably enhancement of fluorescence spectra was observed. Competition experiments were carried out to explore the use of L as an ion-selective fluorescent probe for Fe3+. L (10 uM) was treated with 10 equiv. Fe3+ in the presence of other metal ions (10 equiv.). As shown in Fig. 4, the competing metal ions showed very low interference with the detection of Fe3+. Moreover, the competitive experiments also confirmed that the background metal ions showed very low interference with the detection of Fe3+ in water solution. The fluorescence response of L toward Fe3+ in the presence of various coexistent anions was also investigated, and it was gratifying to notice that all the tested anions had low interference (Fig. 5).
To further investigate the interaction of chemosensor L with Fe3+, a fluorescence titration experiment was conducted. As shown in Fig. S5, the fluorescence intensity of L was enhanced with the increasing concentration of Fe3+. Moreover, the time-dependence fluorescence of probe L was also evaluated in the presence of Fe3+ ions (Fig. 6). The kinetics of fluorescence enhancement at 600 nm by the probe L were recorded, and the results indicated that the recognizing event could be completed in 10 s (T = 25 °C). These results also demonstrated that compound L was a selectivity and rapidly sensor for Fe3+ over various other metal ions.
In order to investigate the influence of the different acid concentration on the spectra of L and found a suitable pH span in which L could selectively detect Fe3+ efficiently, the acid titration experiments were performed. As shown in Fig. S6, the fluorescent titration curve of free sensor L in Tris-HCl buffer solution did not show obvious characteristic color of rhodamine between pH 5.8 and 7.4, suggesting that spirolactam tautomer of L was insensitive to the pH changes in this range. However, the addition of Fe3+ led to the fluorescence enhancement over a comparatively wide pH range (5.8–7.4), which was attributed to opening of the rhodamine ring. Consequently, L might be used to detect Fe3+ in approximate physiological conditions. Generally, the detection limit of the fluorescence sensor was one of the most important and useful application. Under optimal conditions, the linear response for the fluorescence intensity response was between 0 and 1 μM (Fig. S7), and the detection limit of Fe3+ was measured to be 2.74 μM.
Mechanism
Further, it was of great interest to investigate the reversible binding nature of the sensor (shown in Fig. 7). Upon addition of 10 equiv. K3PO4 to the solution of 10 μM L with Fe3+ (10 equiv.), the fluorescence intensity at 600 nm was quenched (green line) due to the competitive binding of Fe3+ from L by K3PO4. Further addition of 10 equiv. Fe3+ could recover the strong fluorescence again (blue line). These indicated that the coordination of compound L with Fe3+ was reversible. According to the 1:1 stoichiometry of the Fe3+ to L in the complex (Fig.S4), a possible sensing mechanism was postulated (Scheme 2). It was supported by the HR-MS spectra. A directly evidence was obtained by comparing the HR-MS of L (Fig. S3) and L + Fe3+ (Fig. S8) in Tris-HCl solution (2.5 mM, pH = 7.0). An unique peak at m/z = 658.9 corresponding to [1+ Fe3++H2O + 2Cl−]+ was clearly observed when 1 equiv. of FeCl3 was added to L, whereas L without Fe3+ exhibited peaks only at m/z 515.3 which corresponded to [L + H]+. Moerover, the peaks at m/z = 533.1 and m/z = 555.1 were considered as [1+ H2O]+ and [1+ K]+.
Fluorescence intensity (at 600 nm) of L (10 μM) to Fe3+ in Tris-HCl buffer solution (2.5 mM, pH = 7.0). (1) Baseline: 10 μM L only; (2) red line: 10 μM L with 10 equiv. Fe3+; (3) green line: 10 μM L with 10 equiv. Fe3+ and then addition of 10 equiv. K3PO4;(4) blue line: 10 μM L with 10 equiv. Fe3+ and 10 equiv. K3PO4, then addition of 10 equiv.Fe3+ (λex = 520 nm, slit =5 nm)
Bioimaging Application of Compound L in MGC-803 Cells
To further assess the potential applications of the probe in living cells, fluorescent imaging in MGC-803 cells was monitored by fluorescence microscopy. As shown in Fig. 8b, very weak fluorescence of L inside the living MGC-803 cells was observed. After washing with water twice, 10 μM of Fe3+ was then supplemented to the cells. After incubated at 37 °C for 15 min, a significant increase in the fluorescence from the intracellular area was observed (Fig. 8d). A bright field transmission image of cells with Fe3+ and L confirmed that the cells were viable throughout the imaging experiments (Fig. 8a and c). These results indicated that L might be useful for detecting Fe3+ in biological samples.
Conclusion
In summary, an efficient rhodamine-based fluorescent Chemosensor L was synthesized. The probe exhibited selectivity and sensitivity in Tris-HCl buffer solution (2.5 mM, pH = 7.0) with dramatic enhanced fluorescence intensities. The significant changes in the fluorescence color could be used for naked-eye detection. L might be used to detect Fe3+ in some environmental regions in a wide pH range with a detection limit up to 2.74 μM. Moreover, it was applied for imaging in MGC803 cells to confirm that it could be used as a fluorescent sensor for monitoring Fe3+ in living cells.
References
James P, Raoul K (2005) Analyst 130:528–533
Wang L, Ye Y, Zhong S, Zhao Y (2009) Chin Chem Lett 20:58–61
Meneghini R (1997) Free Radic Biol Med 23:783–790
Wang L, Ye Y, Zhong S, Zhang D, Zhao Y (2009) Chem J Chinese U 30:493–496
Eisenstein RS (2000) Annu Rev Nutr 20:627–630
Rouault TA (2006) Nat Chem Biol 2:406–409
Brugnara C (2003) Clin Chem 49:1573–1479
Burdo JR, Connor JR (2003) Brain iron uptake and homeostatic mechanisms: an overview. Biometals 16:63–75
H. N. Kim, M. H. Lee, H. J. Kim, J. S. Kim. J. Yoon, Chem Soc Rev 37 (2008) 1465–1476.
Tumambac GE, Rosencrance CM, Wolf C (2004) Tetrahedron 60:11293–11297
Beija M, Afonso CAM, Martinho JMG (2009) Chem Soc Rev 38:2410–2418
Hu Z, Feng Y, Huang H, Ding L, Wang X, Lin C, Li M, Ma C (2011) Sensors Actuators B 156:428–433
Dylan WD, Emily LQ (2008) J C Christopher Nat Chem Bio 4:168–175
Chen X, Hong H, Han R, Zhang D, Ye Y, Zhao Y (2012) J Fluoresc 22:789–794
Shi W, Ma HM (2012) Chem Commun 48:8732–8744
Lin W, Long L, Yuan L, Cao Z, Feng J (2009) Anal Chim Acta 634:262–266
Yin W, Cui H, Yang Z, Li C, She M, Yin B, Li J, Zhao G, Shi Z (2011) Sensors Actuators B 157:675–680
Yang Z, She M, Yin B, Cui J, Zhang Y, Sun W, Li J, Shi Z (2012) J Organomet Chem 77:1143–1147
Moon K, Yang Y, Ji S, Tae J (2010) Tetrahedron Lett 51:3290–3293
Hu Z, Feng Y, Huang H, Ding L, Wang X, Lin C, Li M, Ma C (2011) Sensors Actuators B 156:428–432
Zhang D, Zou R, Wang M, Chai M, Wang X, Ye Y, Zhao Y (2013) J Fluoresc 23:13–19
Xue ZW, Chen ML, Chen JM, Han JH, Han SF (2014) RSC Adv 4:374–378
Zheng H, Shang GQ, Yang SY, Gao X, Org JGX (2008) Lett 10:2357–2360
Huang CY (1982) Methods Enzymol 87:509–525
Acknowledgments
This work was supported by the National Science Foundation of China (Nos. 21572209) and Program for New Century Excellent Talents in University (NCET-11- 0950).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
ESM 1
(DOC 927 kb)
Rights and permissions
About this article
Cite this article
Li, G., Tang, J., Ding, P. et al. A Rhodamine-Benzimidazole Based Chemosensor for Fe3+ and its Application in Living Cells. J Fluoresc 26, 155–161 (2016). https://doi.org/10.1007/s10895-015-1696-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-015-1696-9