Abstract
Nano phosphors have been prepared with 1 mol % and 5 mol % doping of Eu in vanadate by sol-gel method. X-ray pattern indicated that nano phosphors did not lead to phase change and has tetragonal crystal structure. Excitation spectra revealed that the band at 308 nm arises due to the charge transfer transitions among Eu3+ and VO4 3- ions. The peak position of fluorescence shifted from 518 to 638 nm on varying the excitation wavelength from 257 nm to 318 nm. It is also noticed that the fluorescence intensity increases with the rise of calcination temperatures of the samples.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Extensive studies of yttrium orthovanadate (YVO4) have been carried out since past three decades in both bulk as well as nano-crystalline form because of its high sensitivity in lanthanide ions [1–7]. The red light emitting rare earth vanadates doped with europium ion (Eu3+) show intense absorption bands in the near UV and blue wavelength region based on charge transfer (CT) absorption between O2- and Eu3+ groups and 4f-4f transitions. Yttrium orthovanadate (YVO4) is one of the most promising inorganic luminescent materials which possess zirconia type tetragonal structure. The lanthanide ion substitute for yttrium in YVO4 lattice (site symmetry D2d). The introduction of the rare earth ion in the crystal results into the splitting of the degenerate levels and shift of the gravity centre of the level. This last effect is related to nephelauxetic effect [8], which is a measure of metal ligand covalency. For nano-crystals doped with the rare earth ions, a valuable tool in the investigation of the morphology changes produced by various thermal treatments is the optical spectroscopy. In case of europium ion, the luminescence spectrum is relatively easy to analyse, since strongest transitions originate on energy state (5D0) not split by crystal field. Consequently, the europium ion has been extensively used to probe the local environment of dopant sites [9–11]. There are few studies regarding the effect of the thermal treatments on the YVO4: Eu nano-crystals. Evolution of particle size as a function of calcination temperature for YVO4: Eu was presented [12]. Generally the sizes of the nano-crystals increase with the calcination temperature, though the coherence domain does not increase linearly with the calcination temperature [15]. As a consequence of the increase of the particle size, the Eu3+ emission lines become narrower and asymmetry ratio decreases. This behaviour denotes an increase of local symmetry at Eu3+ sites (improvement of crystallinity). The improved crystallinity and removal of various impurities adsorbed at the crystallite surface lead generally, to better emission efficiency.
In this paper Y(0.001-x)EuxVO4 (x = 0.0001, 0.0005) nano phosphors were synthesized by sol-gel method. Samples were calcined at different temperatures. Meanwhile, the temperature dependence of luminescence intensities and energy transfer mechanism of Eu3+ ions in YVO4 host are discussed in details. In addition to this the fluorescence behaviour of YVO4: Eu phosphors have been studied as a function of excitation wavelength. During the excitation of YVO4: Eu phosphor by UV light, red emission takes place due to the intra-configurational f-f transitions of Europium ions. On the other hand when the same phosphor is excited by far UV light ranging from 257 nm to 298 nm, the emission of different transition at different excitation takes place by dominating the characterstic red emission (5D0-7Fj, j = 1,2,3 and 4) of YVO4:Eu phosphor.
Materials and Methods
Material Used
Ammonium metavanadate (NH4VO3), yttrium acetate [Y(CH3CO2)] and europium acetate[Eu(CH3CO2)3] materials with the purity 99.99 % were supplied from Aldrich chemical company. All the procedure was carried out in dry atmosphere of nitrogen. Besides this ethylene glycol (C6H6O2), citric Acid (C6H8O7) and deionised water were also used.
Powder Preparation
Two solutions A and B were prepared for YVO4-Eu synthesis. Solution A is comprised of 0.01 mole of ammonium metavanadate, 20 ml of ammonium hydroxide and 100 ml of deionised water. Solution B is prepared by mixing yttrium acetate (0.01-x) mole, europium acetate (x = 0.0001 and 0.0005 mol) and 100 ml of deionised water. The prepared solutions were mixed together, result in the change of colour from colourless to pure white. The solution is stirred magnetically for half an hour. After this, added 0.02 mole citric acid and 1.1 ml of ethylene glycol. The solution turned into the dark pale yellow. Further stirring for 20 minutes resulted light, transparent pale yellow solution. All this procedure was carried out through the slow flow of nitrogen [16]. Finally solution was kept at 120 °C for one day. Dried precursor was obtained, which was given thermal treatments for 3 h.
Characterizations
The synthesized samples were characterized by a X-ray diffractometer (XRD, Philips). Fluorescence spectra of the phosphor were studied with the Fluor meter at different excitation wavelengths.
Results and Discussion
X-Ray Diffraction
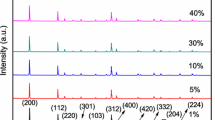
To check the phase purity of the samples XRD measurements were performed. The XRD pattern of the synthesized phosphors at 800 °C of 1 mol % and 5 mol % is presented in the Fig. 1. It is found that both the samples have tetragonal structure of YVO4. The samples also show crystallinity of YVO4-Eu corresponding to JCPDS-17-0341 showing the prominent peaks (200), (112) and (312). Among the materials with different amount of Eu ions there was no significant change in the different profiles indicating no change in the crystal structure. The absence of the significant changes in the XRD pattern with increasing europium concentration may have strict relationship with the ionic radii between the Y3+ ions of matrix (0.900 Å) and Eu3+ ion (0.947 Å) which acts as a dopant. Gasparatto et al., (2009) reported that there is a direct relationship between lattice parameters and occupation fraction of rare earth ions in a crystalline structure. In fact, for the samples with higher thermodynamic perturbation (i e 800 °C) the changes in the atomic coordinates and unit cell were almost negligible. The volume cell was 130.759 Å3 for both the samples. One of the favourable conditions for doping of the europium ion in the host lattice is that the difference in the ionic radii between both Y3+ and Eu3+ ions is less than 5 %. This behaviour is strong indication that Eu3+ ion is easily accommodated into the crystalline structure of host matrix. No reflection assigned to secondary phases was observed in diffractograms.
The lattice parameters were calculated using the following relationship between Bragg diffraction spacing and tetragonal cell parameter, which were given in the Table 1.
where dhkl is the Bragg diffraction spacing for (hkl) plane and h,k,l are miller indices, a and c are cell parameters.
Fluorescence study
The excitation spectrum obtained by monitoring the 5D0-7 F2 transition of Eu3+ at 617 nm for the sample YVO4: Eu (x = 0.0001, 0.0005) is as shown in the Fig. 2. The broad excitation band of the YVO4: Eu nano-phosphors at approximately 308 nm arose from a charge transfer transition between Eu3+ and VO4 3- anions. With the addition of europium to the YVO4 host lattice, the compound exhibits the charge transfer transitions causing the transfer of electrons from 2p orbital of oxygen to the 4f orbital of europium [17–20].
The emission spectrum was composed of sharp lines ranging from 400 to 750 nm that were associated with the 5DJ-7FJ transitions in europium ion. 5D0-7 F2 transition is the strongest red light emission assigned to be at the wavelength of 618 nm. The deep red emission line at 698 nm was due to 5D0-7 F4 transition.
The emission and excitation spectra in Figs. 2, 3 and 4 indicate that highest excitation and emission intensities were obtained at 5 mol% due to increase in the optically active centres at higher concentrations.
Under the 308 nm light excitation, both the samples show the strong red emission, which contains exclusively characterstic emission transition lines of Eu3+ ion listed in Table 2, including 5D0-7 F1 and 5D0-7 F2 transitions. We can also find the relative intensity of 5D0-7 F2 is higher than that of 5D0-7 F1, since Eu3+ ion occupies non-centro-symmetric site in the tetragonal YVO4 crystalline phase.
UV laser irradiation results in the excitation of different optical centres at different excitation wavelength, which is onus for the emission in nano-phosphors. This resulted in the shift of emission peak with different excitation wavelengths.
The Raman shift is calculated by using the relation
where λexc and λem are the excitation and emission wavelengths and h is the Planks constant. So the observation of emission and change in the emission peak with the excitation wavelength could be interpreted in context of myriad optical centres present in the YVO4-Eu nano-phosphors.
Different excitation wavelengths relate to the dominance of emission peaks at different wavelengths, whose intensity might be decreasing with the rise in the excitation wavelengths. Figures 5 and 6 show the fluorescence spectra at 1and 5 mol % doping concentration of europium in the samples at different excitation wavengths.The intensity of 5D0-
7 F1 peak is maximum at the 296 nm. On the other hand at 308 nm the 5D0-7 F2 peak is in dominance. But it is found that no emission peak can be observed upon 397 nm excitation corresponding to the emission intensity of 617 nm. Different emission at different excitation has been tabulated in the Table 3. Besides this we have also calculated stokes shift in YVO4-Eu as in the Table 3 [21].
Figure 7 presents the fluorescence spectra of YVO4:Eu3+ phosphor at different calcined temperatures under the excitation wavelength of 308 nm. The spectra consist of band ranging from 530 to 690 nm, which is attributed to the electronic transitions of europium ions [12–14]. The most intense peak at 619 nm is due to the 5D0-7 F2 electric dipole transition with energy 2.015 eV and is sensitive to the chemical bond in the vicinity of Eu3+ ions.
Peak at 594 nm is due to 5D0-7 F1 magnetic dipole transition with energy 2.086 eV and it hardly changes with the crystal field strength around the Eu3+. The intensity of the peak at 619 nm is stronger than the peak at 594 nm confirming that there is no inversion symmetry site for Eu3+ ion in the tetragonal structure. The other low intensity peak is at 538 nm due to 5D1-7 F1 transition having energy 2.30 eV. Figure 8 shows the variation of integral luminescence intensity with temperature. It reflects that integral intensity increases with the rise of calcination temperature of the sample.It is due evaporation of impurities (OH-1, NO3- etc.) which are adsorbed at the particle surface during synthesis.
Conclusion
In summary, we have demonstrated synthesis of nano phosphors by sol-gel method. Samples were calcined up to 1,000 °C temperatures. XRD study concluded that the samples have single phase. Fluorescence study strongly reflects that the transitions depend on the excitation wavelength. It is also noticed that fluorescence intensity increases with the rise of the calcination temperatures.
References
Xu HY, Wang H, Jin TN, Yan H (2005) Nanotechnology 16:65–99
Zhang H, Fu X, Niu S, Sun G, Xin Q (2004) J Solid State Chem 177:2649–2654
Kandarakis I, Cavouras D, Kanellopoulos E, Nomicos CD, Panyitakis GS (1998) Radiat Meas 29:481–486
Sun L, Zhang Y, Zhang J, Yan C, Liao C (2002) Solid State Commun 124:35–38
Mauders EA, Deshazer LG (1971) J Opt Soc 61:684–685
Golab S, Solaz P, Dzik GD, Lukasiewicz T, Romanowski WR (2002) J Alloys Compd 341:165–169
Erdei S, Ainger FW, Ravichandran D, White WB, Cross LE (1997) Mater Lett 30:389–393
Jorgensen CK (1971) North-Holland, Amsterdam
Blasse G (1973) Chem Phys Lett 20:573–574
Reisfeld R, Zigansky E, Gaft M (2004) Mol Phys 102:1319–1330
Babu S, Babu P, Jayasankar CK, Troster TH, Sievers W, Wortmann G (2006) J Phys: Condens Matter 18:1927–1936
Thakur S, AK Gathania (2014) Int J Lumin 4(I):53-55
Dillip GR, Mallikarjuna K, Dhoble SJ, Deva Prasad Raju B (2014) J Phys Chem Solids 75:8–14
Song L, Shao X, Du P, Cao H, Hui Q, Xing T, Xiong J (2013) Mater Res Bull 48:4896–4900
Georgescu S, Cotoi E, Voiculescu AM, Toma O, Grec MN, Borca E, Hodorogea S (2009) Adv Mater Opt Electron 3:1383–1387
Shin Y, Feng- Ming Huang C, Tsai YY, Teoh LG (2009) J Lumin 129:1181–1185
Blasse G, Bril A (1969) J Chem Phys 50(7):2974
Henrie DE, Fellows RL, Choppin GR (1976) Coord Chem Rev 18:199
Ekambaram S, Patil KC, Maaza M (2005) J Alloys Compd 393:81
Rao RP (1996) Solid State Commun 99:439
Chen L, Deng X, Xue S, Bahader A, Zhao E, Mu Y, Tian H, Lu S, Yu K, Jiang Y, Chen S, Tao Y, Zhang W (2014) J Lumin 149:144–149
Acknowledgments
The authors thank to NIT Hamirpur for providing the financial assistance to carry out the present work. The author (Shashi Thakur) send gratitude to the Department of Higher Education, Government of Himachal Pradesh for granting the study leave.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Thakur, S., Gathania, A.K. Fluorescence Study of Eu-YVO4 Nano-Phosphors as a Function of Calcination Temperature and Excitation Wavelengths. J Fluoresc 25, 657–661 (2015). https://doi.org/10.1007/s10895-015-1551-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-015-1551-z