Abstract
Red phosphors YVO4:Eu3+ with high efficiency photoluminescence have been successfully synthesized using the molten salt synthesis (MSS) method, in which (Mg(NO3)2 ⋅ 6H2O, Ca(NO3)2 ⋅ 4H2O, Sr(NO3)2, and Ba(NO3)2) were used as the molten salt. These phosphors were characterized by powder X‑ray diffraction (XRD), scanning electron microscopy (SEM), and photoluminescence (PL). The results indicated that all phosphors showed rare earth Eu3+ ion characteristic emissions in the YVO4 host. The influence of adding molten salt on photoluminescence properties of YVO4:Eu3+ phosphors has been discussed. The red/orange (R/O) intensity ratio 5D0–7F2 to 5D0–7F1 values of Eu3+ of phosphors doped with different molten salts decreases in the order of Mg, Ca, Ba, Sr, which indicates that the red color of the light emitted by the material prepared using Mg(NO3)2 ⋅ 6H2O is the most pure. The most pure red light could be achieved from YVO4:Eu3+ phosphor with chromaticity coordinates of (0.64, 0.34) under excitation at 275 nm.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Yttrium vanadate (YVO4) has been proven to be a useful rare earth ions host lattice to produce efficient phosphors with different color because of the high luminescence quantum yields of the f–f transitions [1]. YVO4:Eu3+ is a highly efficient red light-emitting material because of an effective energy transfer from \({\text{VO}}_{4}^{{3 - }}\) to Eu3+, but also the YVO4:Eu3+ red phosphor is widely applied in fluorescent lamps, plasma display panels (PDP) [2–5], and so on. At the same time, the luminescence properties YVO4:Dy3+ powders was also reported [6]. Thus, the YVO4:Eu3+ has attracted intensive attention. However, synthesis technique strongly affects the luminescence intensity [7]. It is important to improve particle uniformity and luminescence efficiency YVO4:Eu3+ phosphors. There are many ways to synthesize YVO4 matrix materials, such as solid-state reaction [8], hydrothermal process [9], sol–gel process [10], co-precipitation method [11], and so on.
It is well known that the different preparation methods may result unlike effects on the material’s microstructure as well as physical properties. Furthermore, various synthesis procedures are developed for preparing certain functional micrometer or nanometer materials. Molten salt method has been extensively applied in the fields of ceramic powders in electronics and some other inorganic functional materials [12]. The fundamental basis of molten salt reactions relies on the use of different types of inorganic molten salts as the reaction medium.
In the context, a simple molten salt synthesis (MSS) technology is employed in the synthesis of homogenous controlled size YVO4:Eu3+ nanosphere using alkaline earth metal nitrates (Mg(NO3)2 ⋅ 6H2O, Ca(NO3)2 ⋅ 4H2O, Sr(NO3)2, and Ba(NO3)2) as the molten salts. We prepared YVO4:Eu3+ by the MSS method and investigate the crystal structure and luminescence properties in order to achieve better properties for practical applications.
EXPERIMENTAL
In a typical MSS process, the Eu(NO3)3 and Y(NO3)3 were prepared by dissolving 0.1 mmol Eu2O3 and 0.01 × (1/2) mol Y2O3 in concentrated nitric acid (~63–65%), and evaporating the solvent in beaker firstly, then 0.01 mol NH4VO3, 20 mL deionized water and molten salts (M(NO3)2, M = Mg, Ca, Sr, Ba) were added at a certain rate under stirring, then the mixed solution was dried in an oven at 90°C. The final product YVO4:Eu3+ was obtained followed by further calcination at 900°C for 40 min. The as-synthesized powders are thoroughly washed with deionized water for several times to ensure complete removal of the molten salt. The obtained products are further dried at 90°C.
X-ray diffraction (XRD) patterns were measured using a BRUKERD8 FOCUS with CuKα radiation (λ = 0.15418 nm), at a scanning rate of 4.0 deg min–1. PL emission/excitation spectra were measured using an F-4500 fluorescence spectrometer (Hitachi). The sizes and morphologies of the as-synthesized samples were studied by JSM-6700F (JEOL) scanning electron microscope (SEM). All the measurements were carried out at room temperature.
RESULTS AND DISCUSSION
Morphology of Phosphors
Figure 1 shows SEM images of YVO4:Eu3+ phosphors synthesized by molten salt method with different molten salts (M(NO3)2, M = Mg, Ca, Sr, Ba), respectively, and YVO4:Eu3+ phosphors prepared with no added molten salt. It can be seen that the morphology of the samples did not show apparent changes except for difference in the crystallite sizes using different alkaline earth metal nitrates as molten salts. As morphology strongly depends on synthesis conditions, all the samples were synthesized at same synthesis conditions. In spite of this, these phosphors nanocrystals tend to differently aggregate with using different alkaline earth metal nitrates as molten salts. Packed density of these phosphors was apparent different due to aggregates. However, the packed particles can prevent the scattering of light, and further affect to yield the light output. Different light output will lead to difference of the emission intensity.
X-ray Diffraction (XRD) Patterns
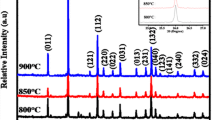
The X-ray diffraction (XRD) patterns of YVO4:Eu3+ phosphors synthesized by molten salt method with different molten salts (M(NO3)2, M = Mg, Ca, Sr, Ba), respectively, and as-prepared YVO4:Eu3+ phosphors no added molten salt are shown in Fig. 2. As can be seen, all the characteristic peaks can be indexed as a tetragonal phase, approaching the standard values for the bulk YVO4 (2θ = 25°, 33°, and 49°) [13]. No characteristic peaks are observed for foreign phases. The result demonstrates that the samples prepared using different alkaline earth metal nitrates as molten salts and Eu3+ do not remarkably change the crystal structure and lattice parameters of YVO4, which mean YVO4:Eu3+ phosphors can be obtained under the current synthesis conditions.
Photoluminescence Properties
The excitation spectra of YVO4:Eu3+ phosphors synthesized by molten salt method with different molten salts (M(NO3)2, M = Mg, Ca, Sr, Ba), respectively, and as-prepared YVO4:Eu3+ phosphors no added molten salt were shown in Fig. 3 (λem = 621 nm). In Fig. 3, the excitation spectra of the all samples have a similar shape and the respectively corresponding peaks locate at the same position except for difference in the intensity. The excitation spectra for these nanoparticles consisted of a broad band ranging from 200 to 350 nm with a maximum at about 275 nm in the spectra. This band is ascribed to a charge transfer from the oxygen ligands to the central vanadium atom inside the \({\text{VO}}_{4}^{{3 - }}\) ion, suggesting the emission center receives energy transfer from \({\text{VO}}_{4}^{{3 - }}\). But there seem two main excitation bands due to the absorptions by \({\text{VO}}_{4}^{{3 - }}\) groups. Xu et al. [14] also reported that the excitation spectrum of YVO4:Eu3+ powders represented a broad band ranging from 200 to 350 nm with a maximum peak at about 320 nm, a shoulder at 278 nm due to the absorption of \({\text{VO}}_{4}^{{3 - }}\) clusters. Using different molten salts (M(NO3)2, M = Mg, Ca, Sr, Ba) leads to difference of crystallite size, therefore, the excitation intensity significantly changes. Yang et al. [15] also found that size-induced variations in bulk/surface structures and their impact on photoluminescence properties of GdVO4:Eu3+ nanoparticles. Nuñez et al. [16] also thought that control of the size and shape of the nanoparticles was essential to achieve the desired luminescence properties. The emission spectra of YVO4:Eu3+ phosphors synthesized by molten salt method with different molten salts (M(NO3)2, M = Mg, Ca, Sr, Ba), respectively, and as-prepared YVO4:Eu3+ phosphors no added molten salt were shown in Fig. 4 (λex = 275 nm). As can be seen in Fig. 4, it is clear that the shapes of emission spectra using different molten salts (M(NO3)2, M = Mg, Ca, Sr, Ba) with same Eu3+ concentrations when annealed at 900°C are similar. The emissions spectra of Eu3+ (λex = 275 nm) such as 5D0–7F1 (596.6 nm), 5D0–7F2 (612.0, 617.2, and 621.2 nm) are observed, and the strongest red emission line is at 621.2 nm and the second strongest peak is at 617.2 nm. The 5D0–7F1 emission transition is mainly a magnetic-dipole transition, while 5D0–7F2 is hypersensitive forced electric-dipole transitions, being allowed only at low symmetries with no inversion center. The intensity ratio red/orange (R/O) of 5D0–7F2 to 5D0–7F1 depends strongly on the local symmetry of Eu3+ ions, and a lower symmetry of crystal field around Eu3+ ions will result in a higher R/O value. At the same time, the intensity ratio of 5D0–7F2 to 5D0–7F1 can be a measure of color purity. As the value of intensity ratio becomes higher, the color of phosphors is closer to pure red. The intensity ratio R/O values of Eu3+ using Mg(NO3)2 ⋅ 6H2O as the molten salt YVO4:Eu3+ phosphors was determined to be 5.97. The intensity ratio R/O values of Eu3+ using Ca(NO3)2 ⋅ 4H2O as the molten salt YVO4:Eu3+ phosphors was determined to be 5.65. The intensity ratio R/O values of Eu3+ using Sr(NO3)2 as the molten salt YVO4:Eu3+ phosphors was determined to be 5.36. The intensity ratio R/O values of Eu3+ using Ba(NO3)2 as the molten salt YVO4:Eu3+ phosphors was determined to be 5.44. The intensity ratio R/O values of the YVO4:Eu3+ particles without the molten salts was determined to be 5.13. From photoluminescence properties, one can see two striking differences in the emission spectra of Eu3+ (1) the intensity ratio 5D0–7F2 to 5D0–7F1 (R/O) values of Eu3+ is different, however, R/O values of all samples are more than 1, that is, the 5D0–7F2 electric dipole transition is dominant, Eu3+ is a site without an inversion center, (2) the intensity ratio 5D0–7F2 to 5D0–7F1 (R/O) values of Eu3+ using alkaline earth metal nitrates (Mg(NO3)2 ⋅ 6H2O, Ca(NO3)2 ⋅ 4H2O, Sr(NO3)2, and Ba(NO3)2) as the molten salts decreased according to the order of Mg, Ca, Ba, and Sr, which indicates the red color of the using Mg(NO3)2 ⋅ 6H2O as the molten salt samples is the most pure. CIE (Commission Internationale de L’Eclairage) parameters are important for the characterization of the color emission of the prepared samples. The ideal red chromaticity standard is (0.67, 0.33) as slated by National Television Standard Committee (NTSC), and Liu et al. [17] reported the CIE 1931 chromaticity coordinate of YVO4:1% Eu phosphor which was calculated based on the corresponding emission spectra was (0.67, 0.32). The CIE chromaticity color coordinates of the YVO4:Eu3+ phosphor prepared by using Mg(NO3)2 ⋅ 6H2O as the molten salt were calculated to be (0.64, 0.34), and the results is also the same as the ideal red chromaticity standard. Figure 5 shows the CIE 1931-chromaticity diagram of the YVO4:Eu3+ phosphor prepared by using Mg(NO3)2 ⋅ 6H2O as the molten salt.
CONCLUSIONS
In summary, YVO4:Eu3+ nanophosphors have been synthesized by a MSS technology using Mg(NO3)2 ⋅ 6H2O, Ca(NO3)2 ⋅ 4H2O, Sr(NO3)2, and Ba(NO3)2 as molten salts. After routine characterization by XRD and SEM, the photoluminescence properties of the phosphors were studied. The crystal structure of all samples shows a pure phase of YVO4. Under 275 nm light excitation, the strongest red emission line is at 621.2 nm and the second strongest peak is at 617.2 nm, both corresponding to the 5D0–7F2 transition of Eu3+ ion. The intensity ratio 5D0–7F2 to 5D0–7F1 (R/O) values of Eu3+ using alkaline earth metal nitrates (Mg(NO3)2 ⋅ 6H2O, Ca(NO3)2 ⋅ 4H2O, Sr(NO3)2, and Ba(NO3)2) as the molten salts decreased according to the order of Mg, Ca, Ba, and Sr, which indicates the red color of the using Mg(NO3)2 ⋅ 6H2O as the molten salt samples is the most pure, and the CIE chromaticity color coordinates of the YVO4:Eu3+ phosphor prepared by using Mg(NO3)2 ⋅ 6H2O as the molten salt were calculated to be (0.64, 0.34).
REFERENCES
P. Gerner, K. Kramer, and H. U. Gudel, J. Lumin. 102–103, 112 (2003).
H. K. Yang, H. Y. Choi, B. K. Moon, B. C. Choi, J. H. Jeong, J. H. Kim, and K. H. Kim, Solid State Sci. 12, 1445 (2010).
T. Jüstel, H. Nikol, and C. Ronda, Angew. Chem. Int. Ed. 37, 3084 (1998).
K. Riwotzki, and M. Haase, J. Phys. Chem. B 102, 10129 (1998).
G. H. Pan, H. W. Song, X. Bai, Z. X. Liu, H. Q. Yu, W. H. Di, S. W. Li, L. B. Fan, X. G. Ren, and S. Z. Lu, Chem. Mater. 18, 4526 (2006).
X. C. Zhou and X. J. Wang, Russ. J. Phys. Chem. A 91, 1138 (2017).
M. M. Yawalkar, G. B. Nair, G. D. Zade, and S. J. Dhoble, Mater. Chem. Phys. 189, 136 (2017).
J. G. Su, X. Y. Mi, J. C. Sun, L. X. Yang, C. L. Hui, L. P. Lu, Z. H. Bai, and X. Y. Zhang, J. Mater. Sci. 52, 782 (2017).
Y. L. Liu, C. M. Yang, H. L. Xiong, N. N. Zhang, Z. H. Leng, R. Q. Li, and S. C. Gan, Colloids Surf., A 502, 139 (2016).
Y. Cheng and K. N. Sun, J. Fluoresc. 28, 285 (2018).
J. Wang, Y. H. Xu, M. Hojamberdiev, Y. R. Cui, H. Liu, and G. Q. Zhu, J. Alloys Compd. 479, 772 (2009).
P. M. Rørvik, T. Lyngdal, R. Sæterli, A. T. J. van Helvoort, R. Holmestad, T. Grande, and M. A. Einarsrud, Inorg. Chem. 47, 3173 (2008).
M. Saltarelli, P. P. Luz, M. G. Matos, E. H. D. Faria, K. J. Ciuffi, P. S. Calefi, L. A. Rocha, and E. J. Nassar, J. Fluoresc. 22, 899 (2012).
J. Wang, M. Hojamberdiev, Y. H. Xu, and J. H. Peng, Mater. Chem. Phys. 125, 82 (2011).
L. S. Yang, L. P. Li, M. L. Zhao, and G. S. Li, Phys. Chem. Chem. Phys. 14, 9956 (2012).
N. Nuñez, J. Sabek, J. G. Sevillano, E. Cantelar, A. Escudero, and M. Ocaña, Eur. J. Inorg. Chem., 1301 (2013).
R. Liu, L. J. Liu, and Y. J. Liang, Opt. Mater. Express 8, 1686 (2018).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xiaochun Zhou, Xiaojun Wang Effect of Molten Salt on Synthesis and Luminescence Properties of YVO4:Eu3+ Red Phosphors. Russ. J. Phys. Chem. 94, 1078–1082 (2020). https://doi.org/10.1134/S0036024420050313
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036024420050313