Abstract
The 4,4-difluoro-4-bora-3a,4a-diaza-s-indacene fluorescent dyes (BODIPYs) were first synthesized almost 50 years ago; however, the exploration of their technological application has only begun in the last 20 years. These dyes possess interesting photophysical properties, increasing interest in their application as fluorescent markers and/or dyes. Herein, we report the synthesis of tetramethyl BODIPY and four meso-substituted dyes (2-thienyl, 4-pyridinyl, 4-fluorophenyl and 4-nitrophenyl derivatives). Their photophysical characterization (absorption spectra, emission spectra, fluorescence quantum yields and time-resolved fluorescence) and solvatochromic behavior were studied. Absorption and emission were barely affected by substituents, with a slightly higher stokes shift observed in the substituted dyes. Substitutions could be associated with a shorter fluorescence lifetime and lower quantum yields. Good correlations were observed between the Catalán solvent descriptors and the photophysical parameters. Also, better correlation was observed between the solvent polarizability descriptor (SP) and photophysical parameters. Overall, only slight solvatochromism was observed. The 4-pyridinyl derivative was the subject of a relatively significant solvatochromism regarding the wavelengths of the emission spectra, with the observation of a bathochromically shifted emission in methanol. The fluorescence quantum yield of the 4-nitrophenyl substituted BODIPY was approximately 30 times higher in hexane, which may be of interest for practical applications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
An increased interest for fluorescent small molecules has inspired the development of a large variety of fluorescence-based spectroscopic and imaging techniques. These fluorescent compounds, also known as fluorochromes or fluorophores, are used as dyes and/or markers and are fundamental for several scientific experimental procedures; these procedures can encompass biological, chemical or physical interests. Though a diverse set of fluorophores are commercially available, several research groups are engaged in the synthesis of novel, more selective and efficient compounds. The 4,4-difluoro-4-bora-3a,4a-diaza-s-indacene (BODIPY) group of fluorescent dyes meet the criteria for a good fluorescent probe (high photostability and quantum yields) and have significant chemical and application versatility.
BODIPY dyes were first synthesized in 1968 by Treibs and Kreuzer [1] and the application and chemical modification of these dyes has dramatically risen since the late 1980’s [2]. BODIPYs are the product of complexation between a dipyrrin dye and a difluoroboryl unity, which compose a tricyclic flat structure with six resonating pairs of π electrons. These dyes are also usually associated with good photophysical parameters, easy chemical manipulation, high stability and tunable emission, making them highly interesting to the scientific community [3].
Photophysical processes are closely associated with the intermolecular interactions between a dye and its immediate environment. Fluorescence intensity, absorption and emission spectra, as well as the lifetime of the excited state are usually influenced by the interaction of the dye with the solvent and other solutes; this process is known as solvatochromism. This environmental effect is the result of changes in the fluorophore’s dipole due to the interaction between the dye and solvent molecules. This effect is usually associated with solvent polarity, solvent viscosity and solvent relaxation, among other solvent parameters [4]. The influence of the molecular environment around fluorochromes is important due to the potential analytical applications of solvent-dependent fluorescent dyes for obtaining information regarding a microenvironment in a cell or macromolecule [5–7].
Some research groups have published articles pertaining to the solvatochromic effects on BODIPY dyes, and in the present study we aimed to gather new data on this subject by comparing the behavior of four meso-substituted tetramethyl BODIPY dyes and an unsubstituted tetramethyl BODIPY dye in different solvents varying in polarity. For this analysis, the steady-state absorption and fluorescence emission spectra, fluorescence quantum yields and the time-resolved fluorescence profiles were collected in six solvents: hexane, dichloromethane, acetonitrile, ethanol, methanol and dimethyl sulfoxide.
Experimental
Synthesis
Reagents were obtained from Sigma-Aldrich Brasil Ltda. (São Paulo, SP—Brazil) and were readily used in the synthetic procedures. Solvents were obtained from local suppliers and treated according to established purification protocols. The structures of the BODIPYs synthesized herein were determined by 125 MHz 13C-NMR and 500 MHz 1H-NMR using a Bruker DRX 500-MHz NMR system from Bruker Daltonics® (Billerica, MA, USA), infrared spectroscopy (IR) using a Shimadzu IR-Prestige 21 system from Shimadzu (Kyoto, Japan), and a high-resolution electrospray mass spectrometer (HRMS-ESI) using the ultrOTOFQ—ESI-TOF system from Bruker Daltonics ® (Billerica, MA, USA).
Synthesis of 2
To a stirring solution of 1 (123 mg, 1 mmol) in CH2Cl2 at 0 °C under inert atmosphere, POCl3 (92 μL, 1 mmol) was slowly added. After 3 h at room temperature, the starting material was completely consumed. Subsequently, DIPEA (800 μL, ≈5 mmol) and BF3.OEt2 (650 μL, ≈5 mmol) were added to the reaction, and the mixture was stirred for 1 h. The fluorescent solution was washed with water (3 times) and dried with Na2SO4. The solvent was removed by distillation under reduced pressure and the oily residue was purified by flash column chromatography (230–400 mesh, hexane/ethyl acetate 9:1) to yield 53.9 mg (0.217 mmol) of 2 (43.5 %). 1 H NMR (400 MHz, CDCl 3 ) δH: 7.04 (s, 1H), 6.04 (s, 2H), 2.53 (s, 6H), 2.24 (s, 6H). 13 C NMR (101 MHz, CDCl 3 ) δC: 156.83, 141.34, 133.52, 120.21, 119.14, 77.16, 14.80, 11.41. HRMS-ESI: [M + H]+ calculated for C13H16BF2N2 249.1369; found, 249.1343.
Synthesis of 8
To a stirring solution of 3 (570 mg, 6 mmol) and 4 (300 mg, ≈2.7 mmol) in CH2Cl2 at room temperature under inert atmosphere, three drops of TFA were added. After 1 h of stirring under these conditions, DDQ (613 mg, 2.7 mmol) was added, and the mixture was stirred for another 6 h. The mixture was washed 3 times with 0.1 M NaOH(aq), dried with Na2SO4, filtered, and combined with TEA (3.2 mL, 20 mmol) and BF3.OEt2 (2.6 mL, 20 mmol) at room temperature. The solution was washed with water (3 times) and dried under Na2SO4. The solvent was removed by distillation under reduced pressure and the oily residue was purified by flash column chromatography (230–400 mesh, hexane/ethyl acetate 3:1) to yield 135.7 mg (0.411 mmol) of 8 (15.2 %). 1 H NMR (500 MHz, CDCl 3 ) δH: 7.50 (d, J = 5.0 Hz, 1H), 7.13 (dd, J = 5.0, 3.5 Hz, 1H), 6.99 (d, J = 3.5 Hz, 1H), 6.00 (s, 2H), 2.55 (s, 6H), 1.58 (s, 6H). 13 C NMR (125 MHz, CDCl 3 ) δC: 156.50, 143.89, 135.09, 132.82, 128.24, 127.98, 127.79, 125.91, 121.90, 15.01, 13.91 HRMS-ESI: [M + H]+ calculated for C17H18BF2N2S 331.1246; found, 331.1254. IR (ν - cm −1 ): 2923; 1544; 1303; 1243; 1172; 1078; 971; 806; 753; 474.
Synthesis of 9
To a stirring solution of 3 (864 mg, 8.4 mmol) and 5 (428 mg, ≈4 mmol) in CH2Cl2 at room temperature under inert atmosphere, three drops of TFA were added. After 3 h of stirring under these conditions, a solution of DDQ (907 mg, 4 mmol) in CH2Cl2 was added to the reaction, and the mixture was stirred for another 12 h. The mixture was washed 3 times with 0.1 M NaOH(aq), dried under Na2SO4, filtered, and combined TEA (4.8 mL, 30 mmol) and BF3.OEt2 (3.9 mL, 30 mmol) at room temperature. The solution was washed with water (3 times) and dried under Na2SO4. The solvent was removed by distillation under reduced pressure and the oily residue was purified by flash column chromatography (230–400 mesh, hexane/ethyl acetate/TEA 75:23:2) to yield 14480 mg (0.446 mmol) of 9(11.1 %). 1 H NMR (500 MHz, CDCl 3 ) δH: 8.78 (d, J = 5.3 Hz, 1H), 7.30 (d, J = 5.3 Hz, 1H), 6.00 (s, 1H), 2.55 (s, 3H), 1.40 (s, 3H). 13 C NMR (125 MHz, CDCl 3 ) δC: 156.59, 150.70, 150.04, 143.78, 142.77, 137.71, 130.44, 123.45, 121.93, 113.83, 77.16, 14.77, 14.75. HRMS-ESI: [M + H]+ calculated for C18H19BF2N3 326.1635; found, 326.1643. IR (ν - cm −1 ): 2918; 1654; 1508; 1466; 1410; 1306; 1156; 1120; 1076; 980; 812; 720.
Synthesis of 10
To a stirring solution of 3 (570 mg, 6 mmol) and 6 (181 mg, ≈3 mmol) in CH2Cl2 at room temperature under inert atmosphere, three drops of TFA were added. After 3 h of stirring under these conditions, a solution of DDQ (680 mg, 3 mmol) in CH2Cl2 was added to the reaction, and the mixture was stirred for another 6 h. The mixture was washed 3 times with NaOH(aq) 0.1 M, dried under Na2SO4, filtered, and reacted at room temperature with TEA (3.2 mL, 20 mmol) and BF3.OEt2 (2.6 mL, 20 mmol). The solution was washed with water (3 times) and dried under Na2SO4. The solvent was distilled off under reduced pressure and the oily residue was purified by flash column chromatography (230–400 mesh, hexane/ethyl acetate 3:1) to yield 183.0 mg (0.535 mmol) of 10 (17.8 %).: 1 H NMR (500 MHz, CDCl 3 ) δH: 7.27 (t, J = 7.6 Hz, 1H), 7.20 (t, J = 8.4 Hz, 1H), 5.99 (s, 1H), 2.55 (s, 3H), 1.40 (s, 3H). 13 C NMR (125 MHz, CDCl 3 ) δC: 155.94, 143.11, 143.08, 140.60, 131.70, 131.69, 131.66, 131.09, 131.06, 130.15, 130.08, 121.55, 121.53, 116.61, 116.43, 77.16, 14.72, 14.65. HRMS-ESI: [M + H]+ calculated for C19H19BF3N2 343.1588; found, 343.1599. IR (ν - cm −1 ): 2909; 1654; 1508; 1466; 1410; 1306; 1156; 1120; 1076; 980; 812; 720.
Synthesis of 11
To a stirring solution of 3 (200 mg, 2.1 mmol) and 7 (151 mg, 1 mmol) in CH2Cl2 at room temperature under inert atmosphere, three drops of TFA were added. After 1 h of stirring under these conditions, a solution of DDQ (227 mg, 1 mmol) in CH2Cl2 was added to the reaction, the mixture was stirred for another 2 h. The mixture was washed 3 times with 0.1 M NaOH(aq), dried under Na2SO4, filtered, and reacted with TEA (1.6 mL, 10 mmol) and BF3.OEt2 (1.3 mL, 10 mmol) at room temperature. The solution was washed with water (3 times) and dried under Na2SO4. The solvent was distilled off under reduced pressure and the oily residue was purified by flash column chromatography (230–400 mesh, hexane/ethyl acetate 3:1) to yield 159.3 mg (0.431 mmol) of 10 (43.1 %). 1 H NMR (500 MHz, CDCl 3 ) δH: 8.39 (d, J = 8.7 Hz, 1H), 7.54 (d, J = 8.7 Hz, 1H), 6.02 (s, 1H), 2.56 (s, 3H), 1.36 (s, 3H). 13 C NMR (125 MHz, CDCl 3 ) δC: 156.82, 148.45, 142.67, 142.11, 138.45, 130.75, 129.78, 124.52, 122.01, 77.16, 14.85, 14.82. HRMS-ESI: [M + H]+ calculated for C19H19BF2N3O2 370.1533; found, 370.1517. IR (ν - cm −1 ): 2924; 1734; 1543; 1512; 1462; 1261; 1076; 1028; 804.
Photophysical Parameters
Absorption spectra were obtained on an Agilent 8453 UV-visible spectrophotometer at room temperature in the solvents described above. Steady state fluorescence spectra were obtained on a Shimadzu RF5301PC spectrofluorimeter with a xenon arc lamp as the light source while using an excitation wavelength (λexc) of 470 nm. Molar extinction coefficients (ε) were obtained in ethanol. The absorbance of five solutions of known concentration of 2, 8, 9, 10 or 11 were obtained and plotted against the concentration, and ε was calculated from the slope of the regression analyses of the plotted data.
The EasyLife™ V (Optical Building Blocks) fluorescence lifetime system was used to obtain the time-resolved fluorescence spectroscopy. A dilute solution of colloidal silica was used to obtain the instrument response function. Fluorescence lifetimes were calculated using the EasyLife™ V software package by fitting an exponential decay curve to the obtained data. Chi-square statistics (χ 2), Durbin-Watson statistics (DW) and Z statistics (Z) were calculated, and every fitted curve showed results within reasonable statistical limits: 0.9 < χ 2 < 1.2; DW > 1.7; Z > −1.96.
Quantum yields were obtained by a comparative method [8] using fluorescein in 0.1 M NaOH(aq) as the standard (φ = 0.91, λexc = 470 nm) [9]. The emission spectra from five samples of each fluorophore (absorbance between 0.1 and 0.01 at the excitation wavelength −470 nm) were obtained. The results were plotted with the integrated fluorescence intensity vs. absorbance to obtain the slope of the curve. A curve was obtained for each tested compound and the standard. The quantum yield of the tested compound (ϕ x ) was calculated using formula 1, where ϕ st is the quantum yield of the standard, m x and m st are the slopes for the test compound and standard compound, and n x and n st are the refractive indexes of the solvents.
The influence of solvent parameters and descriptors over BODIPYs’ characteristics was analyzed via a simple linear regression and multilinear regression analyses. Statistical calculations were made using Bioestat 5.0 free software [10], and spectra were constructed and manipulated by plotting raw data with Spekwin32 free software [11].
Results and Discussion
Synthesis
The synthetic routes used to obtain the five BODIPY derivatives for this work are shown in Fig. 1. Non-meso-substituted tetramethyl-BODIPY (2) was obtained in a 43.5 % yield from the self-condensation of 3,5-dimethyl-2-carbaldehyde (1) in the presence of POCl3, followed by complexation with BF3.OEt2 using diisopropylethylamine (DIPEA) [12]. The meso-substituted BODIPY dyes 8, 9, 10 and 11 were obtained as red solids with variable green fluorescence when in solution (Fig. 2), from the reaction of 2,4-dimethyl pyrrole (3) with thiophene-2-carbaldehyde (4), pyridine-4-carbaldehyde (5), 4-fluorobenzaldehyde (6) or 4-nitrobenzaldehyde (7), respectively. For these compounds, we applied the trifluoroacetic acid method, as described by Lindsey, for meso-substituted dipyrromethanes [13] followed by oxidation with DDQ and complexation with BF3.OEt2 in triethylamine (TEA).
Photophysical Properties
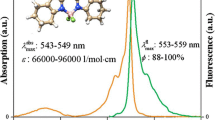
Photophysical properties of the synthesized BODIPYs were tested in six solvents, which were varied in polarity and were either protic or aprotic: ethanol (EtOH), hexane (HEXN), dichloromethane (DCM), acetonitrile (MeCN), dimethyl sulfoxide (DMSO) and methanol (MeOH). In Fig. 3a, normalized absorption spectra representing the average of the six spectra for each of the five compounds are shown (for complete absorption and emission data for each compound, please see the supplementary material). The shapes of the absorption spectra are similar to those previously collected from other BODIPY derivatives in the literature [14–16]. Commonly, a strong relatively narrow peak near 500 nm (attributed to the S0-S1 transition), a shoulder near 475 nm (resulting from the 0–1 vibrational transition), and a weak broad band near 350 nm (attributed to the S0-S2 transition) are observed [16–18]. The averaged normalized emission spectra of 2, 8, 9, 10, and 11 are shown in Fig. 3b. Due to the unusual shape of the emission spectrum collected from 11 in dichloromethane, which is likely the result of some specific effects of this solvent on 11, it was excluded from the averaging and was shown as a separate curve. While a relatively narrow emission peak near 510 nm was observed for compound 2, peak broadening was observed for 8, 9, 10, and 11. Compounds 8, 9 and 11 correlated with a weak bathochromic shift in the emission, which can be observed by the slight different green fluorescence of compound 9 in Fig. 2.
Table 1 shows detailed results regarding absorption and emission of the synthesized compounds. The maximum wavelength for the main absorption band (λabs) of the unsubstituted BODIPY 2 goes from 500 nm in MeCN to 506 nm in hexane. Compound 8 showed a bathochromic shift (6–9 nm) in the absorption maxima while compound 10 showed a weak hypsochromic shift (2–5 nm) with respect to 2. No significant difference was observed among the λabs of meso-substituted dyes 9 and 11. The effects of the aromatic substituents over the emission spectra were more evident. No significant shift of the wavelength at emission maxima (λem) was observed for compound 10; however, a red-shifted emission was observed in compounds 8 (15–20 nm), 9 (6–25 nm) and 11 (9–16 nm). The Stokes shift (\( \varDelta \overline{v} \)), which was calculated from the wavenumber of maximum absorption and emission intensities, was invariably increased with the addition of the aromatic substituents to the BODIPY core. Compounds 8, 9 and 11 showed a more remarkable increase in \( \varDelta \overline{v} \) than compound 10.
The subtle effects of meso-substitution over photophysical parameters of BODIPYs can be attributed to the lack of resonance interaction between the aromatic system of BODIPY core and the aromatic substituents. The presence of two methyl substituents near the central position is associated with a nearly perpendicular configuration of meso-substituted tetramethyl BODIPYs; this arrangement precludes the resonance interaction [19]. Bathochromic effects observed in the emission or absorption of some meso-substituted dyes can be explained by the increase in LUMO stabilization due to the aromatic substituents [16, 19]. Electron withdrawing substituents at central position of the BODIPY core have already been associated with bathochromism [16], and the four substituents varied in this work seem to having the same effect.
In Table 2 the results of time-resolved fluorescence spectroscopy and the quantum yields are shown. The fluorescence lifetime (τ) of 2 was in the range of 5 to 6 ns, which was in agreement with previous publications [20]. In the literature the fluorescence quantum yield of 2 ranges from 0.85 to ~1, with our results also being near unity. This discrepancy among the results found in the literature is due to inherent error within the comparative method of fluorescence quantum yield calculation. The substituted BODIPYs invariably showed shorter lifetimes and lower quantum yields than 2. The rate of non-radiative decay (Knr) was also significantly increased among the substituted compounds, especially for compounds 8 and 11. The rate of radiative decay (Kf) was relatively less influenced, except in the case of 11, where a significant lowering was observed. The results suggest that the insertion of aromatic moieties at the central position of 2 result in higher energy dissipation via non-radiative pathways.
Solvatochromic Effects
Tables 1 and 2 show the results of photophysical profile of the BODIPY derivatives in six solvents, which enabled the study of solvatochromism among the synthesized compounds. Table 3 shows the values of the dipole moment, the dielectric constant (ε r ) and the Dimroth-Reichardt solvent ionizing power index (E N T ), which were collected to rationalize the obtained results. Solvent descriptors proposed by Kamlet-Taft and by Catalán, which were used for multilinear regression analysis, are also shown in Table 3 and are going to be discussed ahead.
The effect of solvent polarity on the broadening of the absorption peak is easily observed; peak narrowing occurred for every compound when measured in hexane. The linear regression of the plot of FWHMabs against the polarity parameters (Fig. 4) highlights this relationship, which was especially intense for 2. In the case of 2, the slope was approximately 3 times higher than the observed for the other compounds (a list of linear regression parameters can be found at supplementary material). The broadening of FWHMem upon solvent polarity increase was usually less evident but was also observed, except in the case of 11. In this excepted case, the opposite effect was observed. The slightly positive slopes in the linear regressions of 2, 8, 9 and 10 and the negative slope for 11 corroborate this observation. The inverse trend of 11 results from the unusual shape of its emission peak in dichloromethane, which increases the FWHMem in this low polarity solvent and influenced the regression analysis. In the linear regression analysis of absorption and emission peak broadening of compounds 2, 8, 10, and 11, higher correlation coefficients and slopes were obtained for the dipole moment parameter, while for compound 9 this occurred for the Dimroth-Reichardt solvent polarity parameter. Polar solvents are commonly related to broader peaks due to the enhanced interaction of the solvent molecules with the transition dipole moment of the fluorophores.
The absorption spectrum maxima (λabs) were barely affected by solvent polarity; only slight solvatochromic effects were observed. This weak trend can be rationalized by the absence of significant differences between the dipole moment of the S0 and S1 states of the synthesized molecules [21]. It was observed that there was a negative relationship between solvent polarity and λabs. Moreover, methanol and acetonitrile were observed to be analogous, with shorter absorption wavelengths, while dichloromethane and hexane were similarly related, with longer absorption wavelengths. Additionally, λem was not highly influenced by the choice of solvent and the relationship of λem with solvent polarity was not as clear. The different shape of the absorption spectra of 11 in DCM and, additionally, the relatively significant increase of λem and \( \varDelta \overline{v} \) observed for compound 9 in methanol are worth noting in terms of solvatochromism.
To better understand the solvatochromism of these systems, the relationship between solvent parameters, emission maxima (\( {\overline{v}}_{em} \)), stokes shift (\( \varDelta \overline{v} \)) and wavenumber (cm−1) of absorption maxima (\( {\overline{v}}_{abs} \)) were analyzed via a multilinear regression. This type of analysis allows the simultaneous study of the effect of several solvent parameters through the following equation:
where a, b and c are solvent descriptors; C a, Cb and Cc are the coefficient for each descriptor; y is the predicted value of the photophysical property under study; and y 0 is the interception, which would be the value for y in gas phase, where C a, Cb and Cc are zero.
Two solvent sets of descriptors were chosen to perform the multilinear regression: the Kamlet-Taft solvent parameters [22–24] (Table 3), which are solvent acidity (α), solvent basicity (β), and solvent polarity/polarizability (π*), and the Catalán solvent parameters [25], which are solvent acidity (SA), basicity (SB), dipolarity (SdP), and polarizability (SP). Each approach was realized by applying the following equations.
The analysis using the Catalán solvent parameters resulted in a better correlation coefficient (R) thus reflecting the most reliable results obtained by this method (Table 4). It is interesting to note that the Catalán parameters were reviewed in 2009 with the substitution of the old polarity/polarizability parameter (SPP) for two new parameters (SP and SdP). Multilinear regression analyses using the old parameters were also performed in this work, and the correlation coefficients, which are shown in Table 4, were not as reliable as those obtained with the new parameters. To our knowledge, only a few studies addressed the novel Catalán descriptors to analyze solvatochromism in BODIPY derivatives, and excellent fits were unambiguously obtained when using this set of descriptors [26–29]. Due to these better fits, the results will be discussed based on the coefficients C SA, CSB, CSdP, and CSP from the analysis using the Catalán solvent descriptors, while results obtained using other descriptors can be found in the supplemental material.
In the analysis of \( {\overline{v}}_{abs} \) and \( {\overline{v}}_{em} \), high negative values of Csp were observed, which was similar to a recent study applying the same descriptors to analyze solvatochromism in BODIPY dyes [26]. This result indicates that the polarizability of the environment surrounding the fluorophore is the main factor influencing its absorption and emission maxima. Because both the absorption and emission maxima are highly influenced by solvent polarizability, the effects over \( \varDelta \overline{v} \) are not as prominent. Another interesting set of observations are the high negative values of C SA and the high positive values of CSB obtained in the analysis of \( {\overline{v}}_{em} \) and \( \varDelta \overline{v} \) of compound 9. This can be rationalized by the presence of a pyridinyl nitrogen within the π-conjugated system, whose protonation/deprotonation process directly influences electron distribution throughout the molecule.
The high quantum yield values collected for compound 2 were not greatly influenced by solvents nor was a significant relationship between polarity and quantum yield observed (Fig. 5). Compound 11 yielded very low quantum yield values, a situation usually associated with high analytical error, and we found it prudent not to include this compound in the solvatochromism study of this property and related coefficients (Kf and Knr). The most striking observation was the quantum yield of 11 in hexane, which was at least 30 times higher than the quantum yield in the other solvents, and the fluorescence could be easily observed under black light (Fig. 6). In the linear regression analysis, the solvent dipole moment was related to slightly higher slopes and regular correlation coefficients, especially for 9 and 10; these data indicate that the solvent dipole moment is most likely related to fluorescence quantum yields. The relatively good correlation coefficients observed using the dielectric constant also corroborate the existence of solvatochromic effects; however, the low slopes indicate that only subtle variations of quantum yield with polarity can be expected. As for the Dimroth-Reichardt solvent polarity parameter, good correlation was obtained only for compound 2, while values near zero were observed for this coefficient with the other compounds. Interestingly, the highest fluorescence quantum yields were observed for 8, 9 and 10 in DMSO, and this effect was specially observed in 9, where the quantum yield in DMSO was 3 times higher than methanol. Higher quantum yields in polar solvents are related to the better interaction of the polar solvent with the π system of the dyes, which makes the molecule less flexible and better able to avoid non-radiative decay [28].
Linear regressions of fluorescence lifetime are also shown in Fig. 5. Fluorescence lifetimes of 2 and 8 were hardly influenced by solvent parameters, as can be observed graphically by noting the low correlation coefficients and slopes. The relatively higher correlation coefficients obtained for 9 and 10 demonstrated a stronger relation between this parameter and solvent properties, mainly when the dielectric constant and dipole moment were used in the calculations. It is worth noting that the effect of DCM in the emission of 11 (which was observed as a change in the shape of emission spectra) was also observed in the time-resolved fluorescence analysis; the fluorescence lifetime was approximately two times longer in this solvent. Similarly to what was observed for fluorescence quantum yields, the use of DMSO was also related to higher fluorescence lifetimes in 8, 9 and 10.
We reasoned that the effect of DMSO in the enhancement of the fluorescence quantum yield and fluorescence lifetime might result from the effect of solvent viscosity. Relatively good correlation coefficients and high slopes were obtained from the linear regression analysis of solvent viscosity [30] versus quantum yield and fluorescence lifetimes of 8, 9, and 10 (please see the supplementary material for details). This result suggests an important effect of viscosity over the photophysical parameters. Viscosity slows molecular rotation and/or twisting, possibly serving as a barrier to processes of non-radiative decay and thus influencing both quantum yield and lifetime [31]. This hypothesis is supported by the smaller values of Knr obtained in DMSO. In compound 2, no dependence on solvent viscosity was observed, which can be explained by its lower molecular volume and the absence of non-radiative processes so that it is less subject to viscosity effects.
The multilinear regression approach was also applied for the analysis of Kf and Knr. The recent set of descriptors proposed by Catalán (SA, SB, SP, and SdP) resulted in superior fits for almost every analysis when compared to the results found when using Kamlet-Taft descriptors or the old Catalán descriptors (SA, SB, SPP). In the analysis of Knr, CSP was the highest (negative) coefficient obtained for the substituted BODIPYs. The negative CSP obtained for substituted dyes indicates that solvent polarizability leads to a decrease of the non-radiative deactivation, supporting the concept of polar solvent effects in non-radiative decay discussed earlier. Similar results were recently obtained with other BODIPYs [28]. It is interesting to note that for compound 9, values of C SA, CSB were near CSP, suggesting an important influence of solvent acidity and basicity on photophysical parameters of this compound. The reasoning behind this observation was discussed earlier in the text and involves the proton-accepting pyridinic nitrogen. Regarding Kf, none of the coefficients obtained in each solvent were significantly higher than the others, ruling out any significant influence of a particular solvent parameter over Kf. This result corroborates the very small variation of Kf observed at Table 2.
Conclusions
Overall, we observed only slight effects of meso-substitutions on the absorption and emission spectra of 2; however, the addition of aromatic substituents seems to invariably lead to a new path of non-radiative decay, which then influences the fluorescence quantum yield and fluorescence lifetime. Regarding solvatochromism, the emission and fluorescence spectra were not highly influenced by solvent, except for the unusual shape of the absorption spectra observed in dichloromethane for compound 11 and higher λem observed in methanol for compound 9. Additionally, compound 11 generally exhibited higher quantum yields in hexane and may be applied as a polarity fluorescent marker. With regard to the compounds synthesized in this work, Catalán solvent parameters served as excellent descriptors in the multilinear regression analysis of solvatochromism, and solvent polarizability seemed to be the most important parameter influencing the photophysical characteristics of the BODIPYs.
References
Treibs A, Kreuzer FH (1968) Difluoboryl-komplexe von di- and tripyrrylmethen. Liebigs Ann Chem 718:208–223
Ulrich G, Ziessel R, Harriman A (2008) The chemistry of fluorescent bodipy dyes: versatility unsurpassed. Angew Chem Int Ed 7(7):1184–1201
Loudet A, Burgess K (2007) BODIPY dyes and their derivatives: syntheses and spectroscopic properties. Chem Rev 107(11):4891–4932
Zakerhamidi MS, Moghadam M, Ghanadzadeh A, Hosseini S (2012) Anisotropic and isotropic solvent effects on the dipole moment and photophysical properties of rhodamine dyes. J Lumin 132(4):931–937
Toutchkine A, Kraynov V, Hahn K (2003) Solvent-sensitive dyes to report protein conformational changes in living cells. J Am Chem Soc 125(14):4132–4145
Bergstrom F, Hagglof P, Karolin J, Ny T, Johansson LBA (1999) The use of site-directed fluorophore labeling and donor-donor energy migration to investigate solution structure and dynamics in proteins. Proc Natl Acad Sci U S A 96(22):12477–12481
Karolin J, Johansson LBA, Strandberg L, Ny T (1994) Fluorescence and absorption spectroscopic properties of dipyrrometheneboron difluoride (bodipy) derivatives in liquids, lipid-membranes, and proteins. J Am Chem Soc 116(17):7801–7806
Williams ATR, Winfield SA, Miller JN (1983) Relative fluorescence quantum yields using a computer-controlled luminescence spectrometer. Analyst 108(1290):1067–1071
Resch-Genger U, Derose PC (2010) Fluorescence standards: classification, terminology, and recommendations on their selection, use, and production (IUPAC Technical Report). Pure Appl Chem 82(12):2315–2335
Ayres M, Ayres-Júnior M, Ayres DL, Santos AS (2007) BioEstat 5.0-Aplicações Estatísticas nas Áreas das Ciências Biológicas e Médicas. MCT; IDSM; CNPq, Belém
Spekwin32—optical spectroscopy software. Version 1.71.6.1, 2012, http://www.effemm2.de/spekwin/. Accessed 23 March 2013
Wu L, Burgess K (2008) A new synthesis of symmetric boraindacene (BODIPY) dyes. Chem Commun 2008(40):4933–4935
Littler BJ, Miller MA, Hung CH, Wagner RW, O’Shea DF, Boyle PD et al (1999) Refined synthesis of 5-substituted dipyrromethanes. J Org Chem 64(4):1391–1396
Bura T, Retailleau P, Ulrich G, Ziessel R (2011) Highly substituted bodipy dyes with spectroscopic features sensitive to the environment. J Org Chem 76(4):1109–1117
Leen V, Miscoria D, Yin S, Filarowski A, Ngongo JM, Van der Auweraer M et al (2011) 1,7-Disubstituted boron dipyrromethene (BODIPY) dyes: synthesis and spectroscopic properties. J Org Chem 76(20):8168–8176
Qin WW, Baruah M, Van der Auweraer M, De Schryver FC, Boens N (2005) Photophysical properties of borondipyrromethene analogues in solution. J Phys Chem A 109(33):7371–7384
Baruah M, Qin WW, Flors C, Hofkens J, Vallee RAL, Beljonne D et al (2006) Solvent and pH dependent fluorescent properties of a dimethylaminostyryl borondipyrromethene dye in solution. J Phys Chem A 110(18):5998–6009
Pardoen JA, Lugtenburg J, Canters GW (1985) Optical properties of pyrromethene derivatives. Possible excited-state deactivation through proton tunneling. J Phys Chem 89(20):4272–4277
Prieto JB, Arbeloa FL, Martinez VM, Lopez TA, Amat-Guerri F, Liras M et al (2004) Photophysical properties of a new 8-phenyl analogue of the laser dye PM567 in different solvents: internal conversion mechanisms. Chem Phys Lett 385(1–2):29–35
Johnson ID, Kang HC, Haugland RP (1991) Fluorescent membrane probes incorporating dipyrrometheneboron difluoride fluorophores. Anal Biochem 198:228–237
Arbeloa FL, Prieto JB, Martinez VM, Lopez TA, Arbeloa IL (2004) Intramolecular charge transfer in pyrromethene laser dyes: photophysical behaviour of PM650. Chemphyschem 5(11):1762–1771
Kamlet MJ, Taft RW (1976) The solvatochromic comparison method. I. The β-scale of solvent hydrogen-bond acceptor (HBA) basicities. J Am Chem Soc 98(2):377–383
Kamlet MJ, Abboud JL, Taft RW (1977) The solvatochromic comparison method. 6. The π* scale of solvent polarities. J Am Chem Soc 99(18):6027–6038
Taft RW, Kamlet MJ (1976) The Solvatochromic Comparison Method. 2. The α-scale of Solvent Hydrogen-Bond Donor (HBD) Acidities. J Am Chem Soc 98(10):2886–2894
Catalan J (2009) Toward a generalized treatment of the solvent effect based on four empirical scales: dipolarity (SdP, a new scale), polarizability (SP), acidity (SA), and basicity (SB) of the medium. J Phys Chem B 113(17):5951–5960
Leen V, Qin W, Yang W, Cui J, Xu C, Tang X et al (2010) Synthesis, spectroscopy, crystal structure determination, and quantum chemical calculations of BODIPY dyes with increasing conformational restriction and concomitant Red-shifted visible absorption and fluorescence spectra. Chem-Asian J 5(9):2016–2026
Galangau O, Dumas-Verdes C, Meallet-Renault R, Clavier G (2010) Rational design of visible and NIR distyryl-BODIPY dyes from a novel fluorinated platform. Org Biomol Chem 8(20):4546–4553
Banuelos-Prieto J, Agarrabeitia AR, Garcia-Moreno I, Lopez-Arbeloa I, Costela A, Infantes L et al (2010) Controlling optical properties and function of BODIPY by using asymmetric substitution effects. Chem Eur J 16(47):14094–14105. doi:10.1002/chem.201002095
Boens N, Leen V, Dehaen W, Wang L, Robeyns K, Qin W et al (2012) Visible absorption and fluorescence spectroscopy of conformationally constrained, annulated BODIPY dyes. J Phys Chem A 116(39):9621–9631
Lide DR (2004) CRC Handbook of Chemistry and Physics. CRC Press, Boca Raton
Kuimova MK, Yahioglu G, Levitt JA, Suhling K (2008) Molecular rotor measures viscosity of live cells via fluorescence lifetime imaging. J Am Chem Soc 130(21):6672–6673. doi:10.1021/ja800570d
Acknowledgments
This work was financed by São Paulo Research Foundation (FAPESP– grant #2011/23342-9), NAP-FTO—USP, INCT-IF. We are grateful to prof. Roberto Santana da Silva and the analytical centre of the institution.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 807 kb)
Rights and permissions
About this article
Cite this article
Cunha Dias de Rezende, L., Menezes Vaidergorn, M., Biazzotto Moraes, J.C. et al. Synthesis, Photophysical Properties and Solvatochromism of Meso-Substituted Tetramethyl BODIPY Dyes. J Fluoresc 24, 257–266 (2014). https://doi.org/10.1007/s10895-013-1293-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-013-1293-8