Abstract
The relationship between the number of BODIPY in a compound and the increase on its fluorescence has been established such as an aggregation induced by multiple BODIPY. We aimed to determine the influence of an electron donor substituent in the BODIPY-triazine system. In this sense, as a first step, we collected data such as photophysical characteristics about BODIPY without substituent and meso-triazine-BODIPY system. Then, three more meso-triazine-BODIPY were synthetized by Lyndsey method. In addition, absorption and emission spectra, fluorescence quantum yields and time-resolved fluorescence data were obtained. Furthermore, solvatochromism was determined by solvent descriptors and photophysical parameters. Finally, the results showed that the triazine core stabilized the system and we observed that the number of BODIPY increased fluorescence mainly in polar solvents. While electron donation maintained the conjugation that reduced the influence of the solvent on the photophysical characteristics.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fluorescent dyes have been intensively studied by the scientific community in multidisciplinary areas, like protein analysis [1], gene expression [2], mark subcellular organelles [3] and sensors [4,5,6]. The technological interest of these dyes has allowed their successful application as active media of tunable lasers, in the development of photoelectronic devices, such as fluorescent probes and chemical sensor, or monitoring the physicochemical characteristics of the surrounding ambiences [7].
Among the available classes of fluorescent dyes, BODIPY (acronym for boron-dipyrromethene, IUPAC name, 4,4-Difluoro-5,7-Dimethyl-4-Bora−3a,4a-Diaza-s-Indacene) have received special attention, due to their potential application in optoelectronics, medicine and biology [8]. Also, core such as 1,3,5-triazines, which has a high electron mobility and structural characteristics such as π conjugation, planarity, thermal and photochemical stability, has recently become a promissory structure for the synthesis of photochemical materials. [9].
The number of units of BODIPY are directly related with the enhancement of molecule fluorescence [10]. Indeed, hybrid compounds with two BODIPY units exhibited a higher fluorescence emission when compared with a hybrid compound linked to a single BODIPY [11]. However, there are not any studies about how the number of BODIPY, the presence of triazine core and an electron donor group in the final structure affect solvatochromism. Thus, we sought to synthesize 1,3,5-triazine derivatives linked to both BODIPY and electron donor groups to compare these with previously described compounds such as 2 and 3 which were synthetized by different synthetic approaches, which have in their structure the 1,3,5-triazine-core linked to BODIPY system with one unit of BODIPY in 2 and two units in 3, nevertheless in both molecules without the presence of electron donating groups.
Experimental
Synthesis
Reagents were obtained from Sigma-Aldrich Brasil Ltd. (São Paulo, SP—Brazil) and were readily used in the synthetic procedures. Solvents were obtained from local suppliers and treated according to established purification protocols. The structures of the BODIPYs synthesized herein were determined by 125 MHz 13C-NMR and 500 MHz 1H-NMR using a Bruker DRX 500-MHz NMR system from Bruker Daltonics® (Billerica, MA, USA), using a Shimadzu IR-Prestige 21 system from Shimadzu (Kyoto, Japan), and a high-resolution electrospray mass spectrometer (HRMS-ESI) using the ultrOTOFQ—ESI-TOF system from Bruker Daltonics ® (Billerica, MA, USA).
Synthesis of 6. This is a cyanuric Chloride derivative used as starting material to obtain 8 in better yield. In this way, to a solution of dichloromethane (15 mL) and cyanuric chloride 4 (368 mg, 2.2 mmol) in a ice bath at 0 °C was added dropwise a solution of valine 5 (289 mg, 1.9 mmol) and triethylamine (TEA) (310 μL, 2.2 mmol) in dichloromethane which was also at 0 °C. Product formation was monitored using thin layer chromatography (TLC), the end of the reaction was noted as soon as the starting material was consumed. The product was recrystallized using ethyl acetate and ether. Product was used in the next step.
Synthesis of 8 To a stirring solution of dimethylpyrrole 7 (273 mg, 0.66 mmol) and 6 (428 mg, ≈4 mmol) in CH2Cl2 at room temperature under inert atmosphere, three drops of trifluoroacetic acid (TFA) were added. After 3 h of stirring under these conditions, a solution of 2,3-dichloro-5,6-dicyano-p-benzoquinone (DDQ) (150 mg, 0.66 mmol) in was added to the reaction, and the mixture was stirred for another 4 h. The mixture was washed 3 times with 0.1 M NaOH(aq), dried under Na2SO4, filtered, and combined TEA (2.4 mL, 15 mmol) and Boron trifluoride ethyl etherate (BF3.OEt2) (2 mL, 15 mmol) at room temperature. The solution was washed with water (3 times) and dried under Na2SO4. The solvent was removed by distillation under reduced pressure and the oily residue was purified by flash column chromatography (230–400 mesh, hexane/ethyl acetate/TEA 75:23:2) to yield 110 mg (0.129 mmol) of 8 (40%). 1H NMR (400 MHz, CDCl3) δ 7.30 (d, J = 7.9 Hz, 1H), 6.97–6.90 (m, 4H), 6.05–5.97 (m, 4H), 3.79 (s, 6H), 2.56 (s, 12H), 1.55 (s, 12H), (125 MHz, CDCl3) δ 173.9, 156.3, 152.6, 143.6, 142.0, 140.8, 134.2, 131.8, 123.7, 121.8, 121.1, 113.4, 77.1, 56.9, 15.0, HRMS-ESI: [M + H]+ calculated for C43H40ClF4N7O4+: 852,3025; found: 852.3036.
Synthesis of 9: To a stirring solution of dimethylpyrrole 7 (200 mg, 0.6 mmol) and valine 5 (300 μL, ≈2 mmol) in CH2Cl2 at room temperature under inert atmosphere, three drops of TFA were added. After 3 h of stirring under these conditions, a solution of DDQ (150 mg, 0.66 mmol) in CH2Cl2 was added to the reaction, and the mixture was stirred for another 4 h. The mixture was washed 3 times with 0.1 M NaOH(aq), dried under Na2SO4, filtered, and combined TEA (2.4 mL, 15 mmol) and BF3.OEt2 (2 mL, 15 mmol) at room temperature. The solution was washed with water (3 times) and dried under Na2SO4. The solvent was removed by distillation under reduced pressure and the oily residue was purified by flash column chromatography (230–400 mesh, hexane/ethyl acetate/TEA 75:23:2) to yield 204 mg (0.39 mmol) (65.0%). 1H NMR (300 MHz, CDCl3) δ 7.04 (d. J = 6.8 Hz. 1H). 6.79 (dd. J = 6.4. 1.7 Hz. 2H). 6.00 (s. 2H). 5.80 (s. 2H). 3.88 (s. 3H). 2.57 (s. 6H). 1.50 (s. 6H) 13CNMR (75 MHz, CDCl3) δ 155.4, 147.4, 146.,2, 143.1, 141.6, 131.8, 126.,6, 121.1, 1152, 110.5, 56.2, 14.5.
Synthesis of 10: To a solution of dichloromethane (15 mL) and cyanuric chloride 4 (40 mg, 2 mmol) in an ice bath at 0 °C was added dropwise a solution of 9 (80 mg, 0.2 mmol) and TEA (70 μL, 0.5 mmol) in dichloromethane. After the addition, the system was left under stirring at 0 °C for 1 h, the formation of the product was monitored using thin layer chromatography. Product was purified using (230–400 mesh, hexane/ethyl acetate/95:5). The product is a solid orange. 30 mg. 29% yield. 1H NMR (300 MHz, CDCl3) δ 7.29 (dd. J = 6.6. 2.1 Hz. 1H). 6.99 (d. J = 2.1 Hz. 2H). 6.04 (s. 2H). 3.79 (s. 3H). 2.58 (s. 6H). 1.55 (s. 6H 13C NMR (75 MHz, CDCl3) δ 173.8, 155.9, 152.1, 143.1, 141.5, 140.6, 133.8, 131.4, 123.3, 121.5, 120.7, 113.0, 77.2, 56.4, 15.4, 14.7. HRMS-ESI: [M + H]+ calculated for C23H20BCl2F2N5O2 518.1128; found: 518.1127.
Photophysical Parameters
Absorption spectra were obtained on an Agilent 8453 UV-Visible spectrophotometer at room temperature in the solvents described above. Steady state fluorescence spectra were obtained on a Shimadzu RF5301PC spectrofluorimeter with a xenon arc lamp as the light source while using an excitation wavelength (λexc) of 470 nm.
The MicroTime 100 Upright Time-resolved Fluorescence Microscope™, was used to obtain the time-resolved fluorescence spectroscopy. One drop of sample was placed in Upright microscope BX43 from Olympus, data acquisition was made based on the method of Time-Correlated Single Photon Counting (TCSPC) in the unique Time-Tagged Time Resolved (TTTR) measurement mode Simultaneous data acquisition of up to two channels. The system software ®SymPhoTime64, was used by fitting an exponential decay curve to the obtained data, Fluorescence Lifetime Correlation Spectroscopy (FLCS).
Quantum yields were obtained by a comparative method [12] using fluorescein in 0.1 M NaOH(aq) as the standard (φ = 0.91, λexc = 470 nm) [13]. The quantum yield of the tested compound (φx) was calculated using Eqs. (1), where φst is the quantum yield of the standard, mx and mst are the slopes for the test compound and standard compound, and ƞx and ƞst are the refractive indexes of the solvents.

Nonradiative (κnr) and radiative (κr) were calculated from experimentally measured fluorescence quantum yield φ and calculated fluorescence lifetime τ according to Eqs. (2) and (3) [14].
The influence of solvent parameters and descriptors over 1,3,5-triazina-BODIPYs’ characteristics was analyzed via a simple linear regression and multilinear regression analyses. Statistical calculations were done using R free software.
Results and Discussion
Synthesis
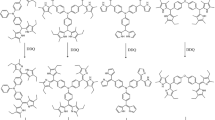
The synthetic routes used to obtain the three BODIPY derivatives for this work are shown in Figs. 1 and 2. The reaction between 5 and 4 in dichloromethane at room temperature produced the 1.3.5-triazine derivative 6. For obtaining 8, we applied the trifluoroacetic acid method, as described by Lindsey for meso-substituted dipyrromethanes [15]. Thus, reaction between 6 and 7, followed by oxidation with DDQ and complexation with BF3.OEt2 in triethylamine (TEA) produced 8. In addition, this procedure was used to synthesize meso-dihydroxyphenil BODIPY 9. Meanwhile, reaction between 9 and 4, in dichloromethane at 0 °C produced 10 (Fig. 3).
Photophysical Properties
Photophysical properties of the synthesized BODIPYs were tested in five solvents with different polarities: Methanol (MeOH), acetonitrile (MeCN), tetrahydrofurane (THF) dichloromethane (DCM) and hexane (HEX). To compare with BODIPY without meso substitution we used data reported by Rezende et al. [16], and to compare with meso-1,3,5-triazina-BODIPY we used data reported by zhou et al. [11]. These data are summarized together with our experimental datas in Tables 1, 2 and 3.
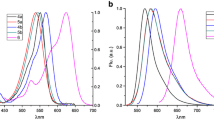
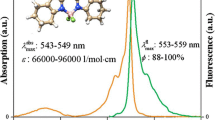
The photophysical properties of compounds 8, 9 and 10 were investigated by UV–Vis spectra and fluorescence spectra as shown in Fig. 4. All of them exhibited the maximum absorption at around 500 nm, due to strong S0-S1 transition [17] and which were similar to that of compound 1, 2 and 3.
We observed that for all solvents, molecules 8 and 10 had a hypsochromic shift for λabs and a bathochromic shift for λem, when compared with 1. Similar effect was found when the λabs of MeOH and λem of DCM in 2 and 3 were compared with 1. This result showed that the presence of 1,3,5-triazine core is responsible for hypsochromic shifts. Which is due to π - π* transition that frequently cause a hypsochromic shift [18].
When hydroxyl-methoxyphenyl group was added (9) on the BODIPY, there was a hypsochromic shift in λabs in all solvents. Just with DCM the λem wavelength had a hypsochromic shift. Blue-shift absorption bands were observed due to the electron-donating character of this type of group, which generally increases the LUMO energy and increases the HOMO−LUMO gap [19,20,21]. Regarding the number of BODIPY units, there was no observed significant shift when 2 and 3 were compared with 1.
Figure 5 shows a comparative intensity of compounds 8, 9 and 10. In polar solvents, intensity of absorption and emission were greater for 8 and 10 than 9. This differences is due to the high electron mobility and π conjugation of 1,3,5-triazine core that increase the intensity of energy emitted causing the intramolecular charge transfer and mesomeric dipole moment thus, increasing the intensity of emission. [9].
Figure 6 showed that the presence of 1,3,5-triazine decreases the quantum yield. Value for 1 is φ = 0.95 and values of molecules 2 and 3 are about φ = 0.1; addition of methoxy group caused two times increase in the φ values for all solvents about φ = 0.3. This showed that 1,3,5-triazine linked by phenyl to the core of BODIPY causes the loss of the ability to dissipate energy by rotational and vibrational relaxation [22]. In addition, values of the non-radiative deactivation constants are in an order of magnitude higher than the radiative ones that confirms the hypothesis; the results leads us to think that the presence of triazine core causes the energy to be dissipated by other pathways [23]. The Stokes shift (Δv), which was calculated from the wavenumber of maximum absorption and emission intensities, was variably increased with the addition of meso-substituent, 9 was two times higher than 1. In spite of the presence of 1,3,5-triazine core and methoxy group in the system to increase the values but we did not find a significant increment.
Solvatochromic Effects
The Dimroth-Reichardt solvent ionizing power index (ETN) [13], the dielectric constant (εr) and dipolar moment [24], of binary solvent systems were collected to rationalize the obtained results. The solvent polarity measured like ETN affects significantly the photophysical properties, in 8 and 9, in the regression analysis with heightened polarity of solvents the FWHMabs value decreased, except for values obtained with DCM. Already the differences obtained in analysis of solvatochromism in other studies had been reported [16]. The inverse trend was observed in the regression analysis of ETN, DM and εr for 10 had a positive slope which was three times higher than 8 and 9. Emission charts, all the compounds present a slight positive slope, and in the fluorescence, the non-polar solvents present the greatest difference between the analyzed molecules. Our results agree with the literature which said that Polar solvents are commonly related to broader peaks due to the enhanced interaction of the solvent molecules with the transition dipole moment of the fluorophores [25].
When we did the comparison with the values reported by Rezende et al. who did the same lineal regression for BODIPYS meso-subsituited. we found that FMWH values were 2 times higher than values reported for BODIPY 1 [16]. That is, because the electronic excitation that is connected with a reduction in dipole moment in agreement with a considerable charge transfer and the introduction of substituents in the acceptor part, that increased its electron affinity and led to the result obtained [26].
We decided to perform the analysis of solvent parameters and emission maxima (vem), stokes shift (Δv) and wavenumber (cm − 1) of absorption maxima (vabs), through a multilinear regression. This kind of analysis allows the simultaneous study of the effect of several solvent parameters. For the analysis we used the catalán solvents parameter [27], solvent acidity (SA), basicity (SB), dipolarity (SdP) and polarizability (SP) because it was reported for BODIPY that, catalán solvents parameters resulted in a better correlation [16].
The results showed that there is a marked stabilizing effect due to the presence of the 1,3,5-triazine ring, due to the large differences shown between 1 and 8, 10, 2 and 3. When comparing the obtained value for polarizability (SP) we find that the value obtained for 2 and 3 in magnitude is less than 1, the addition of the methoxy group to the system further decreased the value. For dipolarity (SdP) we found that the values of 2 and 3 are close to zero, presenting an average difference of 150 units. However, when introducing the methoxy group into the system, the influence of the dipolarity of the solvent is greater, the values observed for 8 and 10 are 5 times larger than the values of 2 and 3, this result is related to the high dispersions observed in Fig. 7. Values for 8, 9, 10, 2 and 3, are positives, indicating blue shifts with increasing SdP. This positive value of SdP suggests that the excited state dipole moment is smaller than that of the ground state [28].
The introduction of the 1,3,5-triazine ring decreased the effect of the acidity and the basicity of the solvent on the absorption of the BODIPY-1,3,5-triazine system; however, the differences are smaller when compared with the other variables. The introduction of the methyl group, the differences between the values of 2 and 3 increased with 8 and 10, which showed that in the addition of the methyl group, in more basic pH, the photophysical properties can be maintained almost unaltered. Positive values of SB and SA indicated the existence of a destabilized excited state in polar and basic solvents, which decreased upon excitation. Our results showed that for BODIPY-1,3,5-triazine solvent dipolarity is not the sole factor affecting the position of the absorption maxima and that solvent basicity (SB) and polarizability (SP) are additional, although minor contributors (Tables 4 and 5).
Conclusion
In conclusion, the presence of a single core BODIPY and electron donor in derivatives of 1,3,5-triazine generate a stabilization effect by electronic conjugation doing to molecule be less susceptible to changes due to physicochemical characteristics of solvent however, the addition of more BODIPY units reduce the electronic stability. Here, we reported the first study of salvatochromism phenomena using a novel BODIPY-triazine compound and how its structure do not alter photophysical properties of 1,3,5 traizine derivatives.
References
Hawe A, Sutter M, Jiskoot W (2008) Extrinsic fluorescent dyes as tools for protein characterization. Pharm Res 25:1487–1499
Clegg RM (1992) [18] Fluorescence resonance energy transfer and nucleic acids. In: Methods in enzymology. Elsevier, pp 353–388
Terasaki M, Loew L, Lippincott-Schwartz J, Zaal K (1998) Fluorescent staining of subcellular organelles: ER, Golgi complex, and mitochondria. Curr Protoc cell Biol 4
Ueno T, Nagano T (2011) Fluorescent probes for sensing and imaging. Nat Methods 8:642–645
Sun H, Scharff-Poulsen AM, Gu H, Almdal K (2006) Synthesis and characterization of ratiometric, pH sensing nanoparticles with covalently attached fluorescent dyes. Chem Mater 18:3381–3384
Wolfbeis OS (2005) Materials for fluorescence-based optical chemical sensors. J Mater Chem 15:2657–2669
Bañuelos J, Arbeloa FL, Arbeloa T, et al (2012) BODIPY laser dyes applied in sensing and monitoring environmental properties. Appl Sci Innov Pvt Ltd
Yang Y, Zhang L, Gao C, Xu L, Bai S, Liu X (2014) Pyrene-based BODIPY: synthesis, photophysics and lasing properties under UV-pumping radiation. RSC Adv 4:38119–38123
Padalkar VS, Patil VS, Sekar N (2011) Synthesis and photo-physical properties of fluorescent 1, 3, 5-triazine styryl derivatives. Chem Cent J 5:77
Feng H, Geng X, Lin J, Guo H, Yang F (2018) Novel fluorescent liquid crystals: synthesis, mesomorphism and fluorescence of triphenylene-Bodipy derivatives based on 1,3,5-triazine core. Liq Cryst 45:1470–1476. https://doi.org/10.1080/02678292.2018.1446554
Zhou W, Guo H, Lin J, Yang F (2018) Multiple BODIPY derivatives with 1, 3, 5-triazine as core: balance between fluorescence and numbers of BODIPY units. J Iran Chem Soc 15:2559–2566
Williams ATR, Winfield SA, Miller JN (1983) Relative fluorescence quantum yields using a computer-controlled luminescence spectrometer. Analyst 108:1067–1071
Resch-Genger U, DeRose PC (2010) Fluorescence standards: classification, terminology, and recommendations on their selection, use, and production (IUPAC technical report). Pure Appl Chem 82:2315–2335
Marfin YS, Merkushev DA, Usoltsev SD, Shipalova MV, Rumyantsev EV (2015) Fluorescent properties of 8-substituted BODIPY dyes: influence of solvent effects. J Fluoresc 25:1517–1526. https://doi.org/10.1007/s10895-015-1643-9
Littler BJ, Miller MA, Hung C-H, Wagner RW, O'Shea DF, Boyle PD, Lindsey JS (1999) Refined synthesis of 5-substituted dipyrromethanes. J Org Chem 64:1391–1396
de Rezende LCD, Vaidergorn MM, Moraes JCB, da Silva Emery F (2014) Synthesis, photophysical properties and solvatochromism of meso-substituted tetramethyl BODIPY dyes. J Fluoresc 24:257–266
Atkins P, de Paula J (2006) Molecular spectroscopy: Electronic transitions In: Atkins’ Physical Chemistry pp 496–500
Bohle M, Borzilleri RM, Döpp D, et al (2014) Science of synthesis: Houben-Weyl methods of molecular transformations Vol. 17: Six-membered Hetarenes with two unlike or more than two heteroatoms and fully unsaturated larger-ring heterocycles. Thieme
Swavey S, Quinn J, Coladipietro M, Cox KG, Brennaman MK (2017) Tuning the photophysical properties of BODIPY dyes through extended aromatic pyrroles. RSC Adv 7:173–179. https://doi.org/10.1039/c6ra26331c
Di Carlo G, Caramori S, Casarin L et al (2017) Charge transfer dynamics in β- and Meso-substituted Dithienylethylene porphyrins. J Phys Chem C 121:18385–18400. https://doi.org/10.1021/acs.jpcc.7b05823
Zhao N, Xuan S, Fronczek FR, Smith KM, Vicente MGH (2017) Enhanced Hypsochromic shifts, quantum yield, and π–π interactions in a meso,β-Heteroaryl-fused BODIPY. J Org Chem 82:3880–3885. https://doi.org/10.1021/acs.joc.6b02981
Liao J, Zhao H, Xu Y, Zhou W, Peng F, Wang Y, Fang Y (2017) Novel BODIPY dyes with electron donor variety for dye-sensitized solar cells. RSC Adv 7:33975–33985. https://doi.org/10.1039/C7RA04402J
Curwiel VB (1997) Regulation of photosynthesis and energy dissipation in triazine-resistant and susceptible Chenopodium album. Curwiel
Morgan E (1990) Vogel’s textbook of practical organic chemistry. 5th edn. Endeavour 14:148. https://doi.org/10.1016/0160-9327(90)90017-L
Yang X, Zhang X-F, Lu X, Yu C, Jiao L (2015) Red fluorescent monobenzo-BODIPY dyes: solvent effects on spectra and efficient fluorescence quenching by quinones and phenols. J Photochem Photobiol A Chem 297:39–44. https://doi.org/10.1016/j.jphotochem.2014.10.013
Reichardt C (1994) Solvatochromic dyes as solvent polarity indicators. Chem Rev 94:2319–2358. https://doi.org/10.1021/cr00032a005
Catalán J (2009) Toward a generalized treatment of the solvent effect based on four empirical scales: dipolarity (SdP, a new scale), polarizability (SP), acidity (SA), and basicity (SB) of the medium. J Phys Chem B 113:5951–5960
Boens N, Wang L, Leen V, Yuan P, Verbelen B, Dehaen W, van der Auweraer M, de Borggraeve WD, van Meervelt L, Jacobs J, Beljonne D, Tonnelé C, Lazzaroni R, Ruedas-Rama MJ, Orte A, Crovetto L, Talavera EM, Alvarez-Pez JM (2014) 8-HaloBODIPYs and their 8-(C, N, O, S) substituted analogues: solvent dependent UV–vis spectroscopy, variable temperature NMR, crystal structure determination, and quantum chemical calculations. J Phys Chem A 118:1576–1594
Acknowledgements
The authors gratefully acknowledge the financial support of Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, Grants numbers 165417/2014-5 and 168073/2017-0) and CAPES (This work was carried out with the support of the Coordination of Improvement of Higher Education Personnel - Brazil (CAPES)- Financing code 001). We also would like to acknowledge the Analytical Centre of NPPNS (FCFRP-USP, Brazil) for the Mass Spectrometry analysis.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
González, M.T.P., de Mello, S.M.G. & da Silva Emery, F. Influence of 1,3,5-triazine Core and Electron Donor Group in Photophysical Properties of BODIPY Dyes. J Fluoresc 29, 845–852 (2019). https://doi.org/10.1007/s10895-019-02389-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-019-02389-x