Abstract
In this study a new fluorescent Schiff base; 1,1′-(4,4′-oxybis(4,1-phenylene)bis(azan-1-yl-1-ylidene))bis(methan-1-yl-1-ylidene)dinaphthalen-2-ol (2-HNA) was synthesized and characterized by FT-IR, UV-vis, and 1H and 13C-NMR techniques. Photoluminescent properties of 2-HNA were investigated in different solvents including methanol, THF, DMF, DMSO, acetone, acetonitrile, and dichloromethane. 2-HNA was found to have higher emission intensity and Stoke’s shift value (∆λST) in methanol solution. Relative emission intensity changes (I0−I/I0) of 2-HNA in methanol/water mixtures depending on different Cu+2 ion concentrations were determined and a linearized plot was obtained. Possible interference of some other transition metal ions was also determined. Sensitivity limit of the new sensor was found to be higher than 5 × 10−7 mol/L. 2-HNA has quite high selectivity against Cu+2 ion and, thus, can be used as a new fluorescence Cu+2 ion sensor in practice.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Detection of heavy metals in any aqueous sample has been a significant matter in Analytical Chemistry. Various analytical methods including atomic absorption spectroscopy (AAS), inductively coupled plasma atomic emission or mass spectroscopy (ICP-AES, ICP-MS) have been widely used for detection of heavy metals even at very low concentrations. However, these methods are relatively expensive and difficult to be applied. To solve this problem a series of fluorescence sensors have been developed so far [1–5]. Fluorescence signaling supplies high sensitivity and can be directly used for chemosensors with fiber optic systems [6, 7]. Also, a significantly advantage of this sensing method is easy handling as well as cheaper to use than the other methods mentioned above. So, developing of new generation fluorescent agents containing chelating groups can help to detect heavy metals more efficiently. Polymeric compounds were also used in this field to obtain more selective-sensitive sensors [8, 9].
Copper is one of the important trace elements existing in human body, plants, and animals. However, high amount of this element can cause serious health problems including irritation of nose and throat, nausea, vomiting, and diarrhea as well as damage to liver and kidneys [10]. In the view of the biological and environmental importance a considerable attention has been focused on detection of Cu(II) ion [11, 12]. The studies developed in this area have been usually depended on quenching of fluorescence intensity of the employed sensing material [13–16]. This is because of the paramagnetic nature of Cu(II) ion. A series of spectrofluorometric Cu(II) selective sensors have been investigated with their selectivity, sensitivity, and also interference of other possible heavy metal kinds [17, 18].
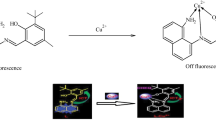
In this study a newly fluorescence Schiff base, 1,1′-(4,4′-oxybis(4,1-phenylene)bis(azan-1-yl-1-ylidene))bis(methan-1-yl-1-ylidene)dinaphthalen-2-ol (2-HNA), was synthesized and developed as a possible Cu(II)-selective fluorescence sensor. An important advantage of the proposed Cu(II) sensor is easy production (only one step reaction) with fine sensing properties. It was observed that 2-HNA is capable of selective complexation with Cu(II) ion which causes great quenching in the emission intensity. Selectivity study of the new Cu(II) sensor was investigated and a considerable selectivity was obtained according to relative intensity change values. Also, sensitivity of the present Cu(II) sensor was found quite high above 10−6 mol/L.
Experimental
Materials
2-Hydroxy-1-naphtaldehyde, bis(4-aminophenyl)ether, methanol, acetonitrile, acetone, dichloromethane, dimethylformamide (DMF), dimethylsulfoxide (DMSO), tetrahydrofurane (THF), Pb(CH3COO)2.3H2O, and Mn(CH3COO)2.H2O were supplied from Merck Chemical Co. (Germany), Cu(CH3COO)2.H2O, Zn(CH3COO)2.2H2O, Co(CH3COO)2.4H2O, Cd(CH3COO)2.2H2O, and Ni(CH3COO)2.4H2O were from Fluka, and ZrCl4 and CrCl3 were from Riedel Dehaen and they were used as received.
Synthesis of 1,1′-(4,4′-oxybis(4,1-phenylene)bis(azan-1-yl-1-ylidene))bis(methan-1-yl-1-ylidene)dinaphthalen-2-ol (2-HNA)
2-HNA was synthesized by the condensation reaction of bis(4-aminophenyl)ether with 2-hydroxy-1-naphtaldehyde. Schiff bases with similar structures have been previously synthesized and reported in the literature [19, 20]. Reaction was made as follow: Bis(4-aminophenyl)ether (0.2 g, 0.001 mol) was placed into a 250 ml three-necked round-bottom flask which was fitted with condenser, thermometer and magnetic stirrer and 60 ml methanol was added into the flask as a solvent. Reaction mixture was heated at boiling temperature of methanol and bis(4-aminophenyl)ether was solved. A solution of 2-hydroxy-1-naphtaldehyde (0.516 g, 0.003 mol) in 20 ml methanol was added into the flask. Reaction was maintained for 3 h under reflux (Scheme 1). The precipitated Schiff base filtered, recrystallized from acetonitrile and dried in a vacuum desiccator (yield: 87%).
Characterization techniques
The solubility tests were done in different solvents by using 1 mg sample and 1 ml solvent at 25 °C. The infrared and ultraviolet-visible spectra were measured by Perkin Elmer FT-IR Spectrum one and Perkin Elmer Lambda 25, respectively. The absorption spectrum was recorded in methanol at 25 °C. The optical band gap (E g ) value of 2-HNA was also calculated from its absorption edge [21]. The FT-IR spectrum was recorded using universal ATR sampling accessory (4,000−550 cm−1). 1H and 13C-NMR spectra (Bruker AC FT-NMR spectrometer operating at 400 and 100.6 MHz, respectively) were obtained by using deuterated DMSO-d6 as a solvent at 25 °C. The tetramethylsilane was used as internal standard.
Florescence measurements
A Shimadzu RF-5301PC spectrofluorophotometer was used in florescence measurements. Emission spectra of 2-HNA were obtained in different solvents with the concentration of 2 × 10−4 M 2-HNA. The optimal emission and excitation wavelengths in each solvent were determined. The effects of transition metal ions on quenching of emission spectra were investigated in 1:1 (v:v) MeOH / deionized water solutions each of which containing 5 × 10−2 M metal ion and 4.91 × 10−4 M 2-HNA. The changes of florescence intensities depending on the concentration of Cu+2 ion were determined using 4.50 × 10−4 M 2-HNA and Cu+2 ion with the concentration range of 5 × 10−2–5 × 10−8 M in 1:1 (v:v) MeOH / deionized water mixtures.
Results and discussion
Solubility and structure of 2-HNA
The synthesized Schiff base has yellow colored-powder form. 2-HNA is soluble in MeOH, EtOH, THF, DMF, DMSO, acetonitrile, acetone, and chlorinated hydrocarbons such as chloroform and dichloromethane. But it’s insoluble in water, benzene, toluene, hexane, and heptane. The FT-IR spectrum of 2-HNA is shown in Fig. 1. This spectrum confirms the proposed structure. After the condensation reaction the new peak at 1,618 cm−1 indicating imine (CH=N) bond appears. Also, -NH2 peaks of bis(4-aminophenyl)ether and –C=O peak of 2-hydroxy-1-naphtaldehyde disappear in the FT-IR spectrum of 2-HNA. The peak values of –OH, aromatic C-H, aromatic C=C, and C-O-C vibrations are seen at 3,290, 3,043, 1,542-1,493, and 1,248 cm−1, respectively. These values agree with the literature data published for similar structures [19].
The UV-vis spectrum of 2-HNA exhibits absorbances (λmax) at 258, 324, 337, 392, 444, and 466 nm (Fig. 2). The optical band gap value of 2-HNA is found as 2.34 eV. This is a smaller value than the previously reported similar structured bis(4-aminophenyl)ether Schiff bases [19]. The smaller band gap indicates easy electronic transitions from bulk to vacuum energy level of the compound and also high electrical conductivity as well as fluorescence property.
1H-NMR and 13C-NMR spectra of 2-HNA are given in Fig. 3a and b, respectively. The structure of 2-HNA is also confirmed by these spectra. NMR spectral data are summarized as follows:
-
1H-NMR (DMSO): 15.84 (s, 2H, -OH), 9.69 (s, 2H, -CH=N-), 8.52 (d, 2H, -Hb), 7.94 (d, 2H, -Hf), 7.81 (d, 2H, -Hc), 7.72 (d, 4H, -Hg), 7.56 (t, 2H, -Hd), 7.36 (t, 2H, -He), 7.19 (d, 4H, -Hi), 7.05 (d, 2H, -Ha).
-
13C-NMR (DMSO): 169.49 (C1-ipso), 156.31 (C15-ipso), 155.72 (C11-H), 140.65 (C12-ipso), 136.87 (C9-ipso), 133.50 (C3-H), 129.45 (C4-ipso), 128.49 (C5-H), 127.23 (C7-H), 123.93 (C6-H), 122.95 (C13-H), 122.15 (C2-H), 120.89 (C8-H), 120.11 (C14-H), 109.15 (C10-ipso).
Florescence characteristics of 2-HNA
The optimal emission and excitation wavelengths of 2-HNA in different solvents are determined and shown in Table 1. 2-HNA has no florescence effect in acetonitrile and dichloromethane. However it has remarkable emission and excitation intensities in the other solvents, as seen in Table 1. According to these results 2-HNA has the maximal emission intensity in methanol solution. Also, the Stoke’s shift values (∆λST) in all employed solvents are calculated as in the literature [3] and shown in Table 1. Stoke’s shift is important for a fluorescence sensor. The higher Stoke’s shift value supplies very low background signals and resultantly allows the usage of the material in construction of a fluorescence sensor. As seen in Table 1, in comparison with the other solvents methanol solution of 2-HNA has quite higher Stoke’s shift with the value of 37 nm, while in the other employed solvents it has ∆λST values between 13 and 16 nm. This indicates that the usage of 2-HNA as an ion selective sensor is useful in methanol solution. The emission spectra of 2-HNA in different solvents are also shown in Fig. 4.
It’s experimentally seen that absence of Cu+2 ion in MeOH-water solution of 2-HNA causes considerable quenching in fluorescence intensity. The changes in emission spectrum of 2-HNA solution in 1:1 (v:v) methanol/water mixture depending on different Cu+2 concentrations are also given in Fig. 5. According to these spectra when the Cu+2 concentrations are 5 × 10−2 and 5 × 10−7 mol/L the relative intensity changes (I0−I/I0) are 0.981 and 0.204, respectively.
Emission spectra of 2-HNA in the presence Cu+2 ion with different concentrations (mol/L, from bottom to top): 5 × 10−2, 2.5 × 10−2, 1.25 × 10−2, 5 × 10−3, 2.5 × 10−3, 1.25 × 10−3, 5 × 10−4, 2.5 × 10−4, 1.25 × 10−4, 5 × 10−5, 5 × 10−6, 5 × 10−7, and 0. Conditions: Slit: λEx 5 nm, λEm 5 nm; λEx 280 nm, λEm 531 nm; concentration of 2-HNA: 4.5 × 10−4 mol/L
The quenching in fluorescence of 2-HNA during exposure to Cu+2 cation indicates the complexation of 2-HNA with Cu+2. The possible structures of the Schiff base complexes and its polymeric forms derived from bis(4-aminophenyl)ether have been previously published [19, 22]. According to the proposed structures 2-HNA has bidentate sides in both ends of the molecule. As a result the structure of the forming Cu+2 complex is expected to be like in the Scheme 2.
The dependence of relative intensity change with the concentration of Cu+2 ion is shown in Fig. 6. According to Fig. 6 when the concentration of Cu+2 ion increased from 5 × 10−2 mol/L to 5 × 10−8 mol/L the relative intensity change linearly decreases. The linear regression equation is as follow:
where I0 is the emission intensity of Cu+2 free 2-HNA solution and I is the intensity of Cu+2 containing 2-HNA solution. The correlation coefficient is 0.99076. According to obtained results 2-HNA can be used as a new florescence sensor to detect the quantity of Cu+2 ion in any sample solution depending on the relative intensity change.
Selectivity studies
In order to determine the selectivity of the new Cu+2 sensor the influence of a number of transition metal cations were investigated. The experiments were performed in 5 × 10−2 mol/L Pb+2, Mn+2, Cu+2, Zn+2, Co+2, Cd+2, Ni+2, Zr+4, and Cr+3 separate solutions each of which contain 4.91 × 10−4 M 2-HNA in 1:1 (v:v) methanol/deionized water mixture (Fig. 7). The results show that 2-HNA has quite florescence quenching in absence of Cu+2 ion. Yet, there is no remarkable spectral change on exposure to the other cations, which are the same concentrated. The relative intensity changes with the tested ions are also shown in Fig. 8.
The selectivity results show that 2-HNA can be easily complex with Cu+2 ion. Also, as seen in Figs. 7 and 8, the intensity of 2-HNA relatively decreases in presence of some other ions such as Cr+3, Co+2, and Ni+2. These decreases may be affect the quantitative measurement of Cu+2 ion in any sample if they presence with too high concentrations. However, the known samples like sea water usually contain little concentration of these cations those aren’t expected to affect the quantitative measurement of Cu+2 ion. As a result, 2-HNA can be used as an alternative Cu+2 sensor by using the fluorescence technique.
Conclusion
A new tetradentate Schiff base 2-HNA was synthesized as a Cu+2 ion selective fluorescence sensor. Characterization of 2-HNA was made by FT-IR, UV-vis, and NMR techniques. Fluorescence intensities of 2-HNA in different solvents were determined. Stoke’s shift values in the employed solvents showed that methanol was the best solvent to use 2-HNA as a Cu+2 sensor. The relative emission intensity changes (I0−I/I0) of 2-HNA were determined in the presence of Cu+2 ion over the concentration range of 5 × 10−8 to 5 × 10−2. A linearized change with a regression coefficient of 0.99076 was obtained. Selectivity studies were carried out with some other transition metal cations those have the same concentrations. It was found that 2-HNA has quite high selectivity to Cu+2 ion. As a result, 2-HNA can be used in quantity determination of Cu+2 ion in any aqueous solution such as sea or lake water using relative fluorescence change.
References
Wang YW, Shi YT, Peng Y, Zhang AJ, Ma TH, Dou W, Zheng JR (2009) Fluorescent sensors for Ca+2 and Pb+2 based on binaphthyl derivatives. Spectrochim Acta A 72:322–326
Singh N, Kaur N, Dunn J, MacKay M, Callan JF (2009) A new fluorescent chemosensor for iron(III) based on the b-aminobisulfonate receptor. Tetrahedron Lett 50:953–956
Oter O, Ertekin K, Kılınçarslan R, Ulusoy M, Çetinkaya B (2007) Photo characterization of a novel fluorescent Schiff base and investigation of its utility as an optical Fe+3 sensor in PVC matrix. Dyes Pigments 74:730–735
Guo L, Hong S, Lin X, Xie Z, Chen G (2008) An organically modified sol–gel membrane for detection of lead ion by using 2-hydroxy-1-naphthaldehydene-8-aminoquinoline as fluorescence probe. Sens Actuators B 130:789–794
Farrugia G, Iotti S, Prodi L, Zaccheroni N, Montalti M, Savage PB, Andreani G, Trapani V, Wolf FI (2009) A simple spectrofluorometric assay to measure total intracellular magnesium by a hydroxyquinoline derivative. J Fluoresc 19:11–19
Kramer R (1998) Fluorescent chemosensors for Cu(II) ions: fast, selective, and highly sensitive. Angew Chem Int Ed 37:772–773
Derinkuyu S, Ertekin K, Oter O, Denizaltı S, Çetinkaya E (2007) Fiber optic pH sensing with long wavelength excitable Schiff bases in the pH range of 7.0–12.0. Anal Chim Acta 588:42–49
Adhikari B, Majmdar S (2004) Polymers in sensor applications. Prog Polym Sci 29:699–766
Li N, Xu Q, Xia X, Wang L, Lu J, Wen X (2009) A polymeric chemosensor for Fe+3 based on fluorescence quenching of polymer with quinoline derivative in the side chain. Mater Chem Phys 114:339–343
Aksuner N, Henden E, Yılmaz İ, Çukurovalı A (2008) Selective optical sensing of copper(II) ions based on a novel cyclobutane-substituted Schiff base ligand embedded in polymer films. Sens Actuators B 134:510–515
Li GK, Xu ZX, Chen CF, Huang ZT (2008) A highly efficient and selective turn-on fluorescent sensor for Cu2+ ion based on calix[4]arene bearing four iminoquinoline subunits on the upper rim. Chem Commun 15:1774–1776
Dujols V, Ford F, Czarnik AW (1997) Along-wavelength fluorescent chemodosimeter selective for Cu(II) ion in water. J Am Chem Soc 119:7386–7387
Kim HJ, Park SY, Yoon S, Kim JS (2008) FRET-derived radiometric fluorescence sensor for Cu2+. Tetrahedron 64:1230–1294
Mu HL, Gong R, Ma Q, Sun YM, Fu EQ (2007) A novel colorimetric and fluorescent chemosensor: synthesis and selective detection for Cu+2 and Hg+2. Tetrahedron Lett 48:5525–5529
Xie J, Menand M, Maisonneuve S, Metivier R (2007) Synthesis of bispyrenyl sugaraza-crown ethers as new fluorescent molecular sensors for Cu(II). J Org Chem 72:5980–5985
Mei YJ, Bentley PA, Wang W (2006) A selective and sensitive chemosensor for Cu+2 based on 8-hydroxyquinoline. Tetrahedron Lett 47:2447–2449
Martinez R, Zapata F, Caballero A, Espinosa A, Tarraga A, Molina P (2006) 2-Aza-1, 3-butadiene derivatives featuring an anthracene or pyrene unit: highly selective colorimetric and fluorescent signaling of Cu+2 cation. Org Lett 8:3235–3238
Qi X, Jun EJ, Xu L, Kim SJ, Hong JSJ, Yoon YJ, Yoon J (2006) New bodipy derivatives as off-on fluorescent chemosensor and fluorescent chemodosimeter for Cu2+: cooperative selectivity enhancement toward Cu+2. J Org Chem 71:2881–2884
Kaya İ, Yıldırım M (2008) Synthesis and characterization of novel polyphenol species derived from bis(4-aminophenyl)ether: substituent effects on thermal behavior, electrical conductivity, solubility, and optical band gap. J Appl Polym Sci 110:539–549
Kaya İ, Yıldırım M, Kamacı M (2009) Synthesis and characterization of new polyphenols derived from o-dianisidine: the effect of substituent on solubility, thermal stability, and electrical conductivity, optical and electrochemical properties. Eur Polym J 45:1586–1598
Colladet K, Nicolas M, Goris L, Lutsen L, Vanderzande D (2004) Low-band gap polymers for photovoltaic applications. Thin Solid Films 451:7–11
Kaya İ, Yıldırım M (2008) Synthesis and changes of conductivities and thermal stabilities of 4, 4′-oxybis [N-(3, 4-dihydroxybenzilidene) aniline] chelate polymers. J Inorg Organomet Polym 18:325–333
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yıldırım, M., Kaya, İ. Synthesis of a Novel Fluorescent Schiff Base as a Possible Cu(II) Ion Selective Sensor. J Fluoresc 20, 771–777 (2010). https://doi.org/10.1007/s10895-010-0620-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-010-0620-6